

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12311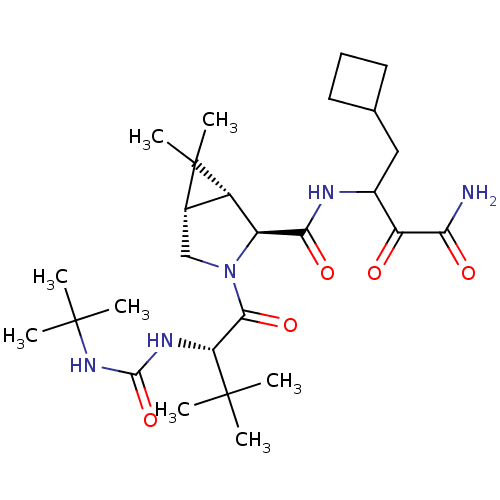 ((1R,5S)-N-[3-Amino-1-(cyclobutylmethyl)-2,3-dioxop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 14 | -45.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12287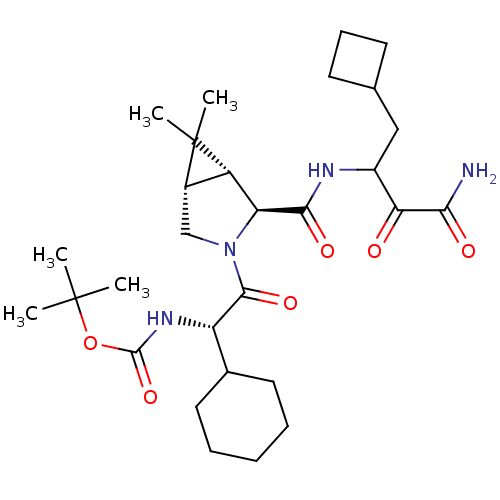 (SCH 491762 | tert-butyl N-[(1S)-2-[(1R,2S,5S)-2-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 15 | -45.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17886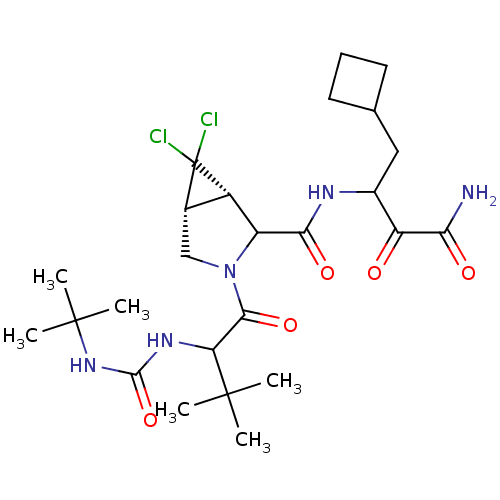 (3-{[(1S,5R)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17898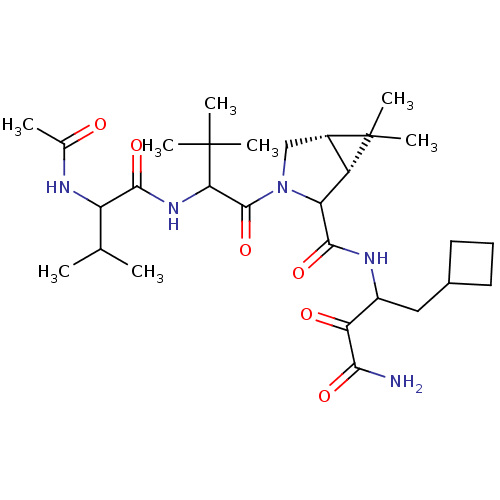 (Ketoamide inhibitor, 35 | N-{1-[(1R,5S)-2-[(1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17884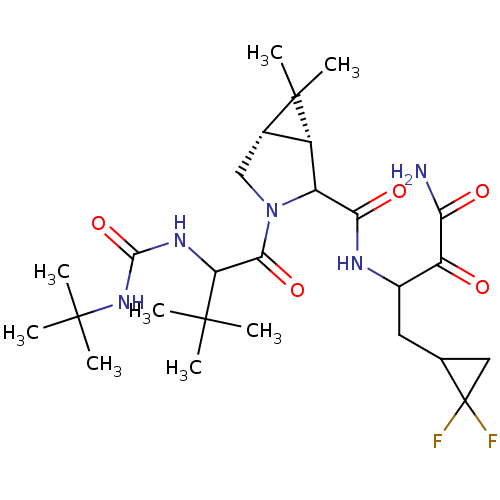 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17875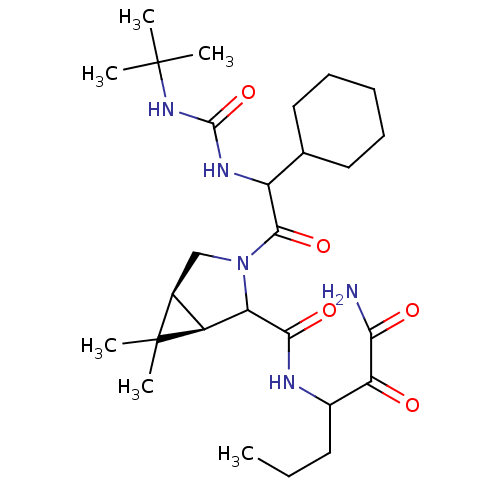 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-2-cy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 54 | -42.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM12297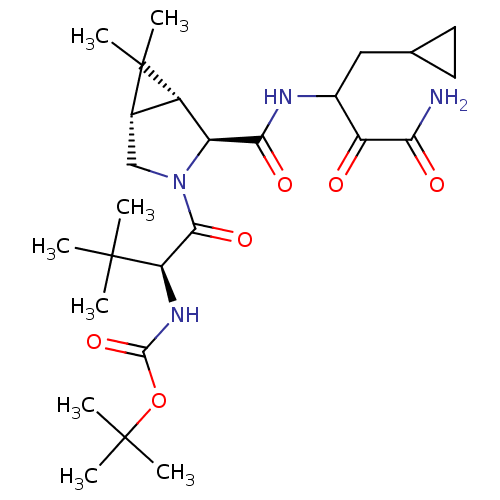 (1,1-Dimethylethyl-[1(S)-[[(1R,5S)-2(S)-[[[3-amino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 57 | -42.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17896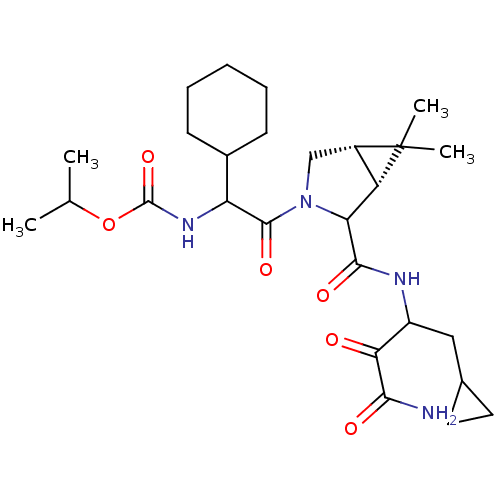 (Ketoamide inhibitor, 33 | propan-2-yl N-{2-[(1R,5S...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17897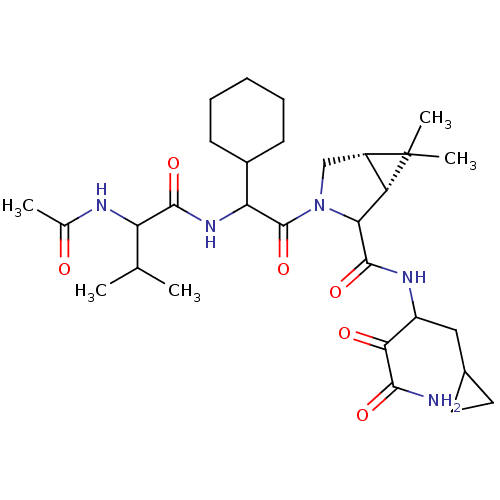 (Ketoamide inhibitor, 34 | N-{2-[(1R,5S)-2-[(1-carb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444921 (CHEMBL3099750) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444918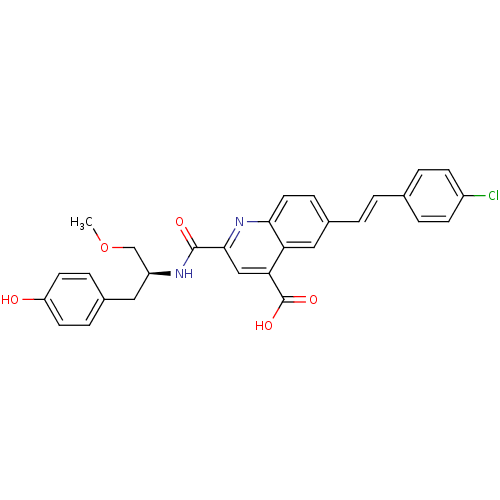 (CHEMBL3099753) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444919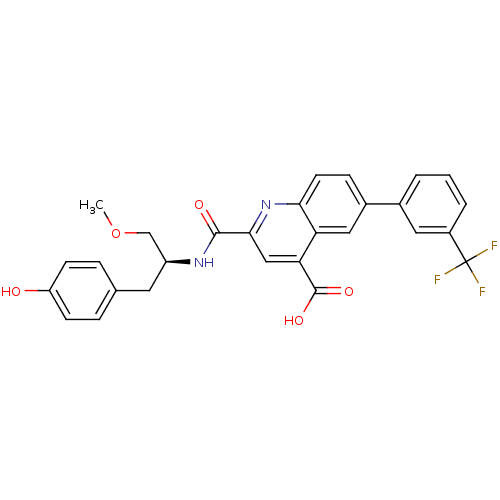 (CHEMBL3099752) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444935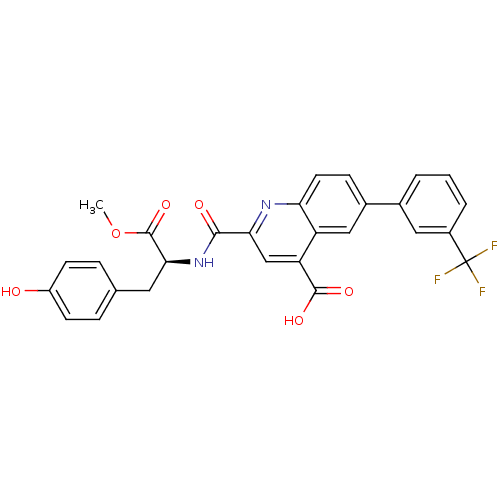 (CHEMBL3099761) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444934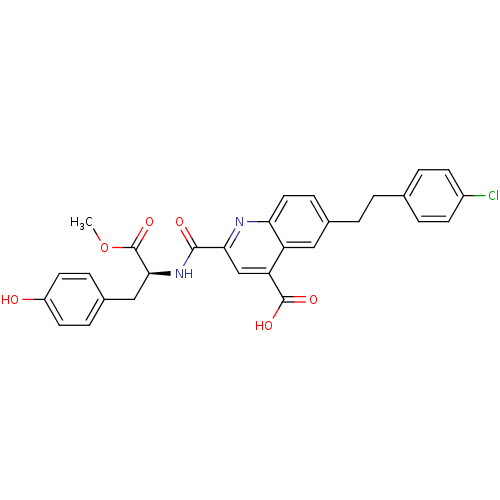 (CHEMBL3099762) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444932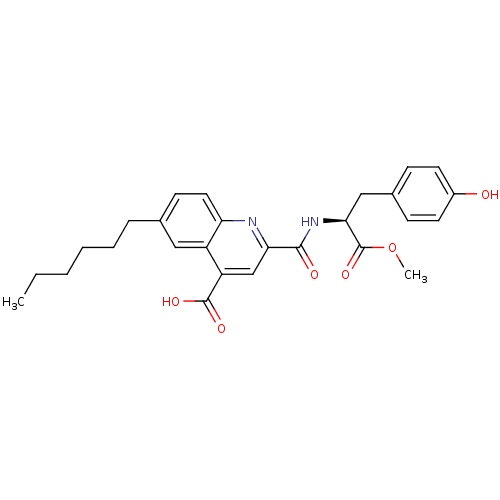 (CHEMBL3099764) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444933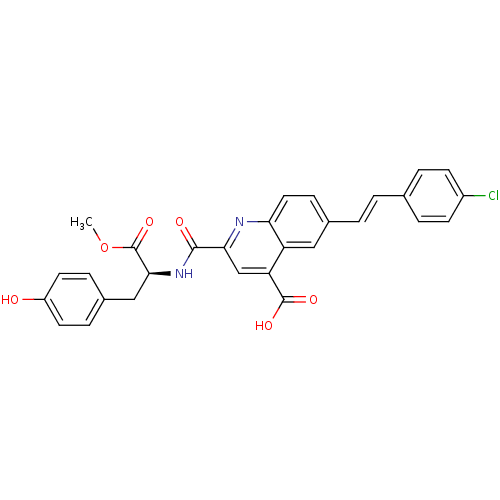 (CHEMBL3099763) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | <100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17893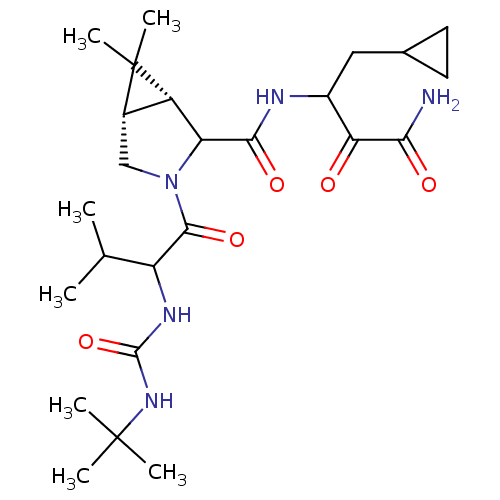 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3-me...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17882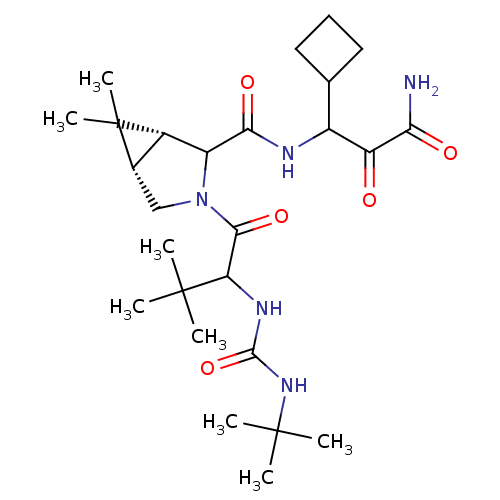 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 100 | -40.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17885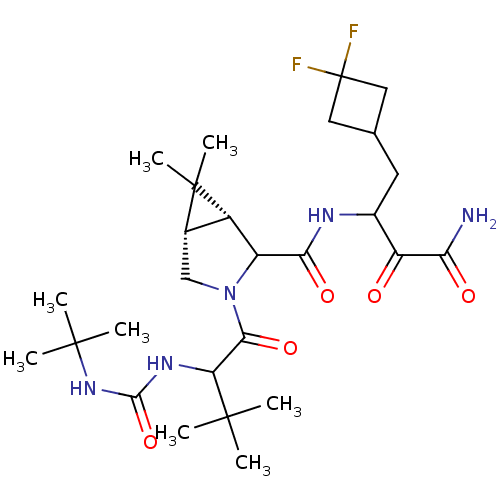 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50136003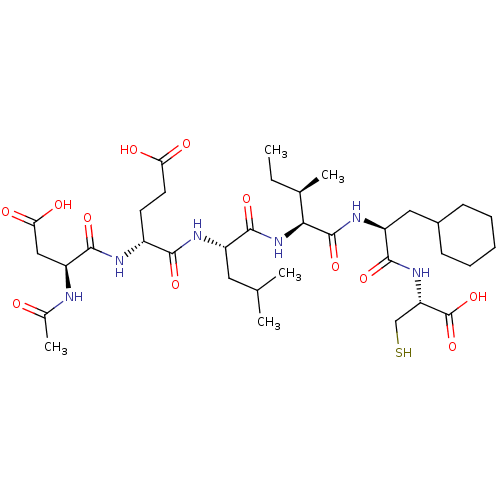 ((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding constant against hepatitis C virus (HCV) protease | J Med Chem 46: 5360-4 (2003) Article DOI: 10.1021/jm030040o BindingDB Entry DOI: 10.7270/Q20001J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50136004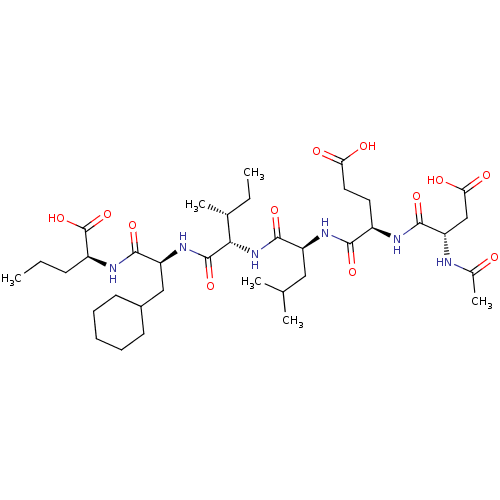 ((S)-2-[(S)-2-((R)-2-{(S)-2-[(R)-2-((S)-2-Acetylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding constant against hepatitis C virus (HCV) protease | J Med Chem 46: 5360-4 (2003) Article DOI: 10.1021/jm030040o BindingDB Entry DOI: 10.7270/Q20001J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444941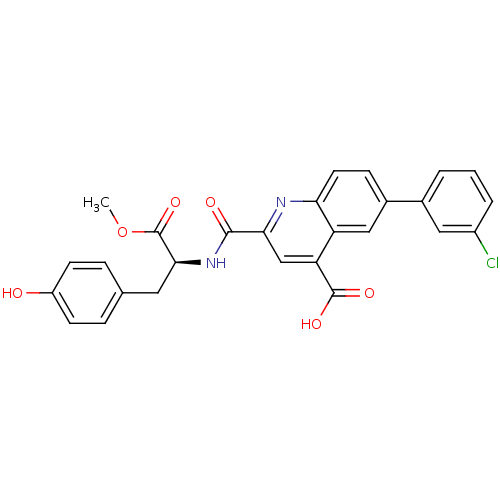 (CHEMBL3099755) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17887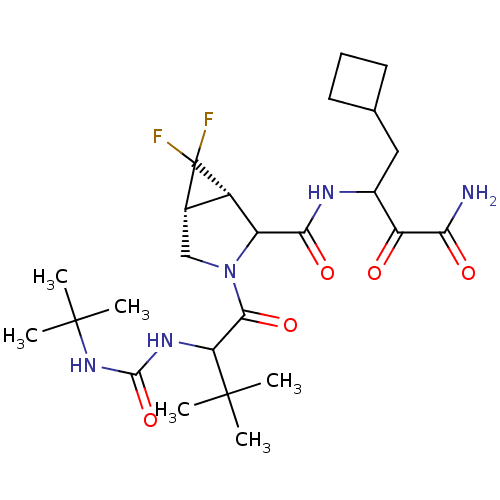 (3-{[(1S,5R)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444920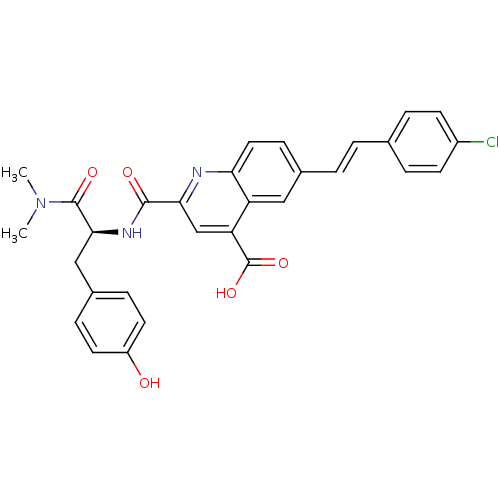 (CHEMBL3099751) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444938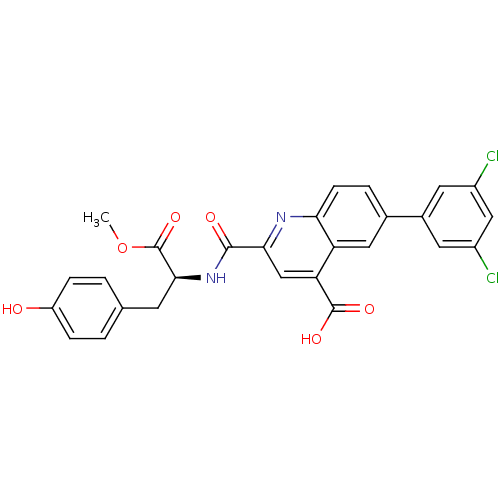 (CHEMBL3099758) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17876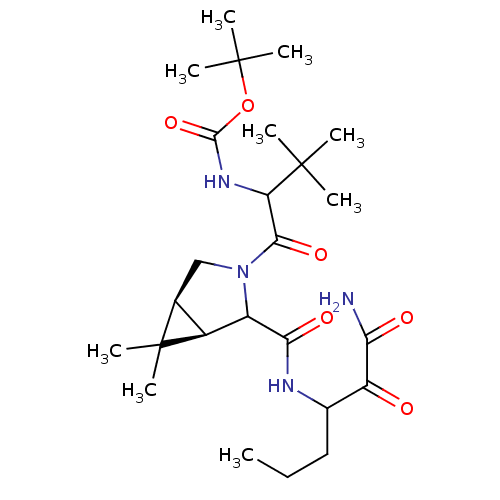 (Ketoamide inhibitor, 5 | tert-butyl N-{1-[(1R,5S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 220 | -38.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17895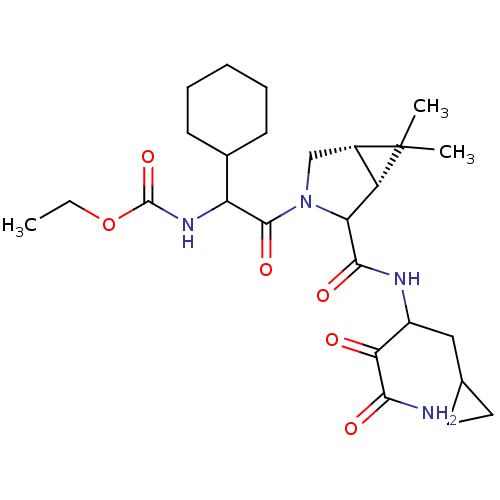 (Ketoamide inhibitor, 32 | ethyl N-{2-[(1R,5S)-2-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444922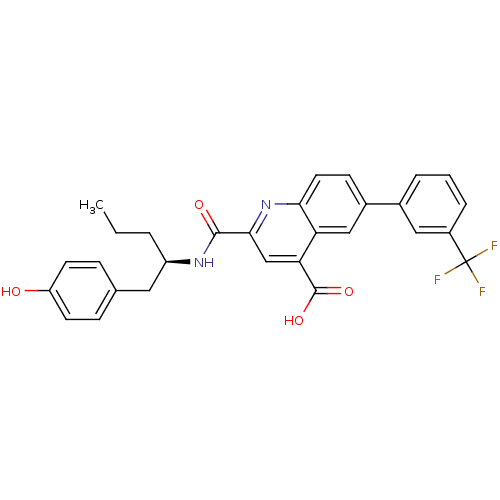 (CHEMBL3099749) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17877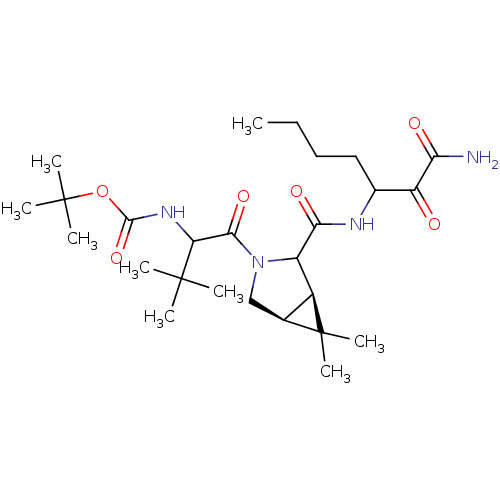 (Ketoamide inhibitor, 6 | tert-butyl N-{1-[(1R,5S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 360 | -37.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17878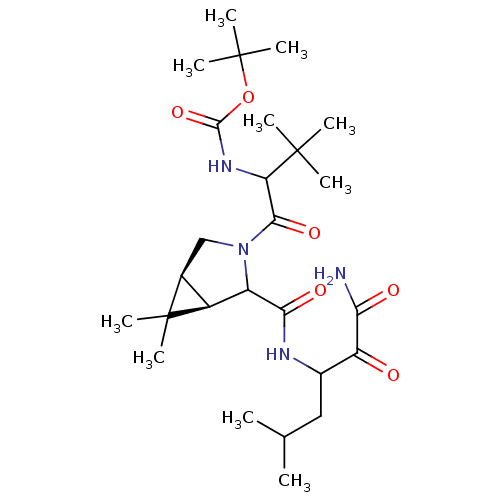 (Ketoamide inhibitor, 8 | tert-butyl N-{1-[(1R,5S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 480 | -36.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50136007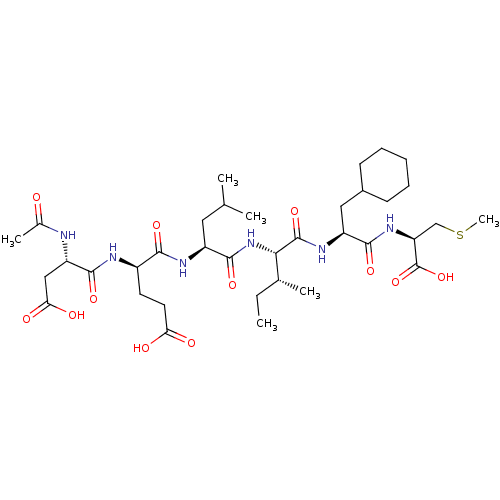 ((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding constant against hepatitis C virus (HCV) protease | J Med Chem 46: 5360-4 (2003) Article DOI: 10.1021/jm030040o BindingDB Entry DOI: 10.7270/Q20001J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17888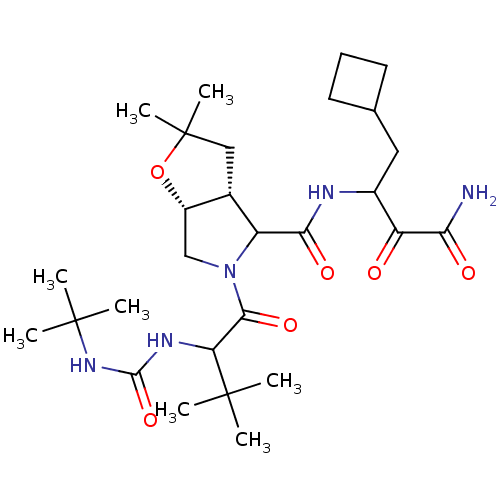 (3-{[(3aR,6aR)-5-{2-[(tert-butylcarbamoyl)amino]-3,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50136002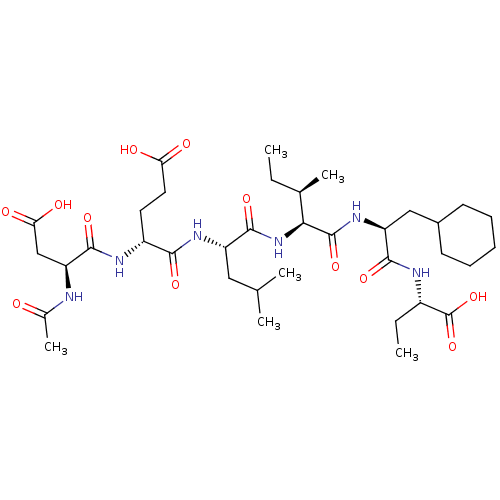 (2-[(S)-(S)-2-((R)-2-{(S)-2-[(R)-2-((S)-2-Acetylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding constant against hepatitis C virus (HCV) protease | J Med Chem 46: 5360-4 (2003) Article DOI: 10.1021/jm030040o BindingDB Entry DOI: 10.7270/Q20001J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444940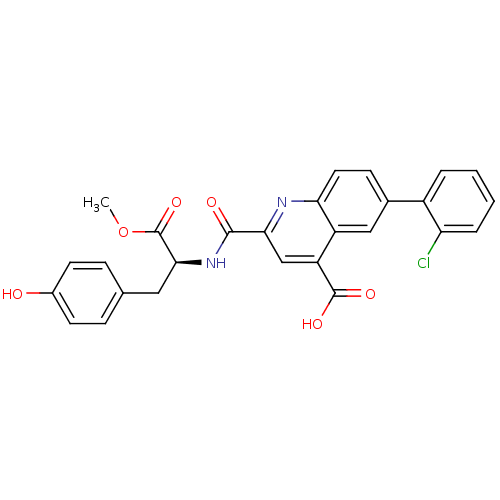 (CHEMBL3099756) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17894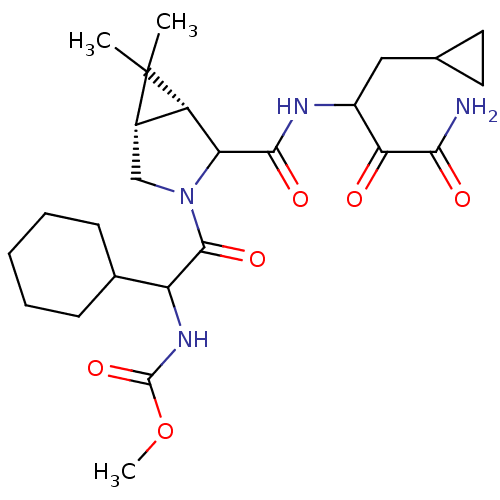 (Ketoamide inhibitor, 31 | methyl N-{2-[(1R,5S)-2-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444939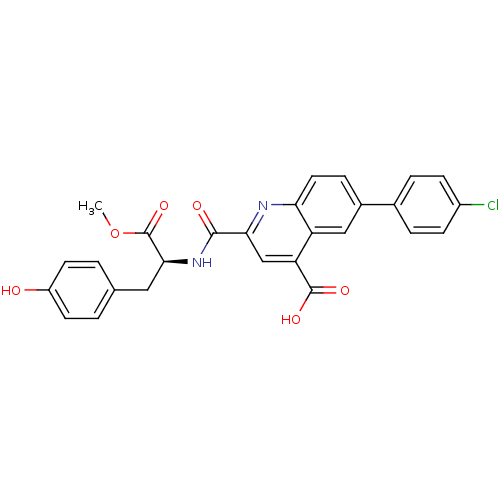 (CHEMBL3099757) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444942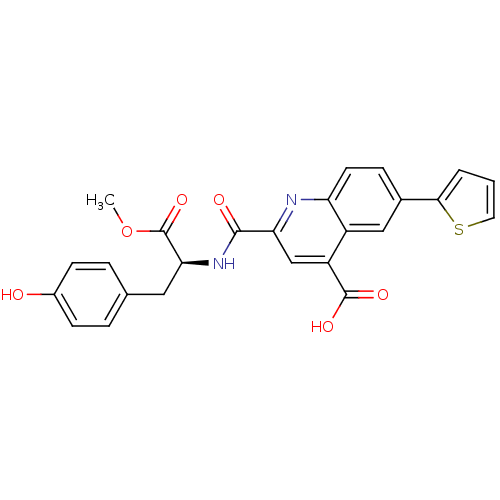 (CHEMBL3099754) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17890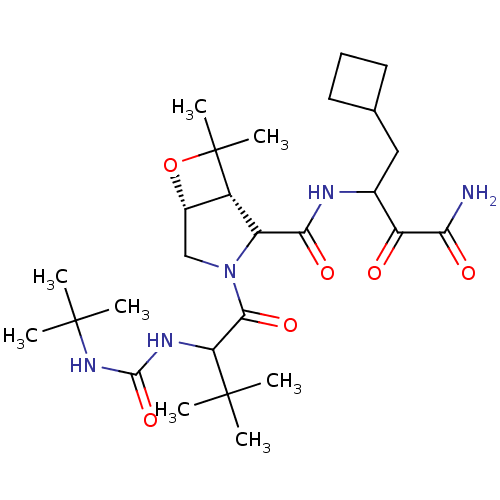 (3-{[(1R,5R)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 960 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17891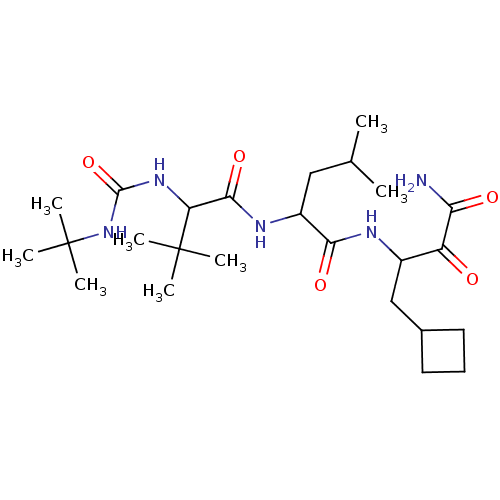 (2-{2-[(tert-butylcarbamoyl)amino]-3,3-dimethylbuta...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17874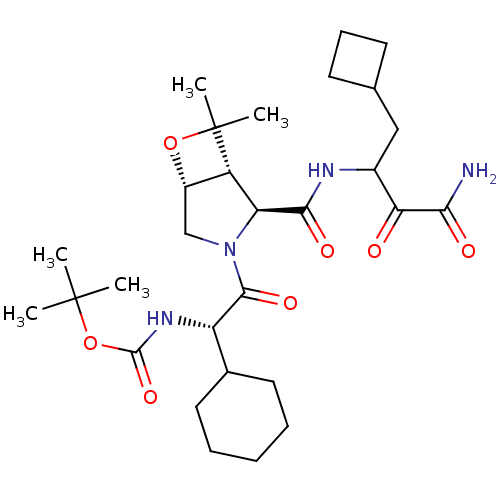 (Ketoamide inhibitor, 36 | SCH 571696 | tert-butyl ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 1.40E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17881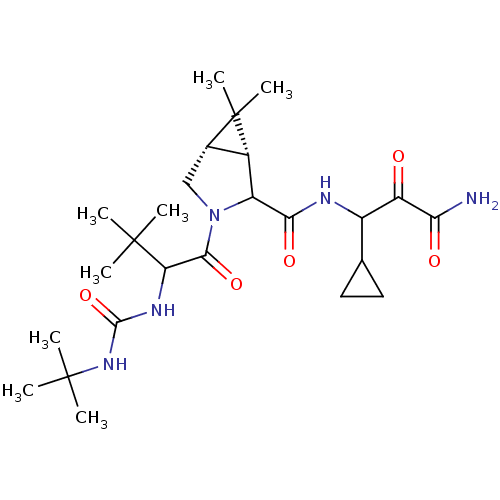 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17883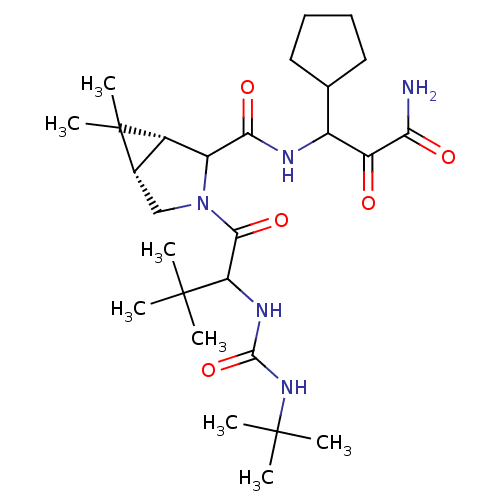 (3-{[(1R,5S)-3-{2-[(tert-butylcarbamoyl)amino]-3,3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.80E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50136001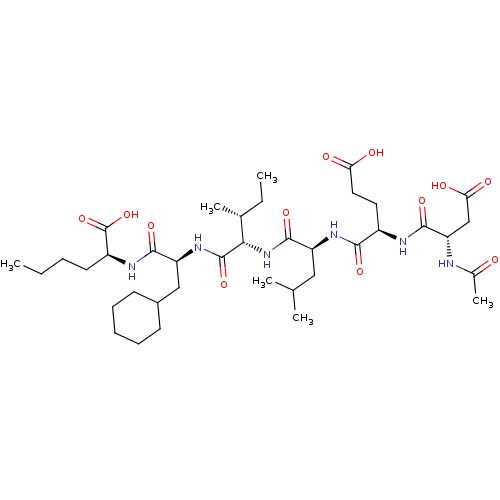 (2-[(S)-(S)-2-((R)-2-{(S)-2-[(R)-2-((S)-2-Acetylami...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding constant against hepatitis C virus (HCV) protease | J Med Chem 46: 5360-4 (2003) Article DOI: 10.1021/jm030040o BindingDB Entry DOI: 10.7270/Q20001J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444928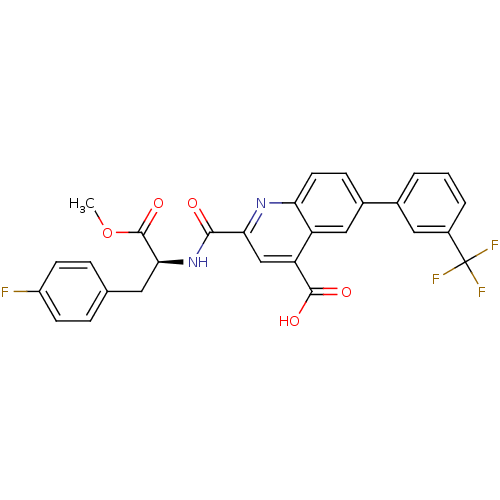 (CHEMBL3099743) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444923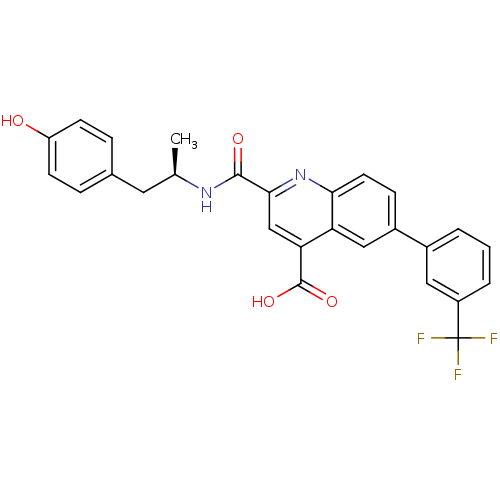 (CHEMBL3099748) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2 (Homo sapiens (Human)) | BDBM50444936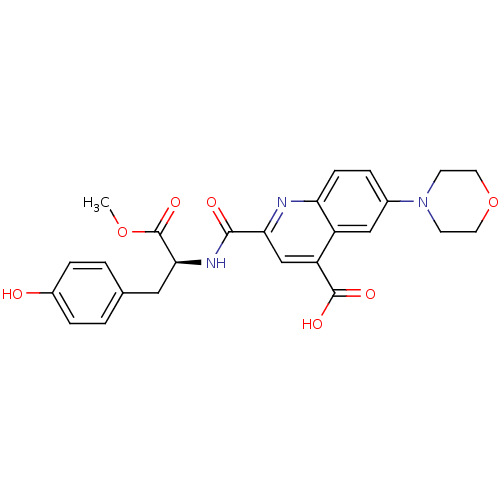 (CHEMBL3099760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of B-Alexa-Fluor647 from CDK2 (unknown origin) by fluorescence polarization assay | Bioorg Med Chem Lett 24: 199-203 (2013) Article DOI: 10.1016/j.bmcl.2013.11.041 BindingDB Entry DOI: 10.7270/Q2SN0BF8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17879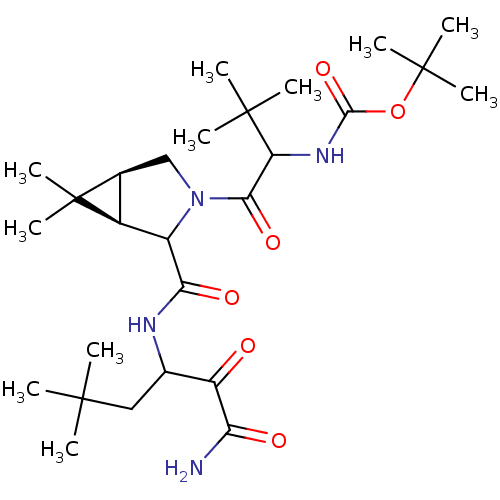 (Ketoamide inhibitor, 9 | tert-butyl N-{1-[(1R,5S)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >3.30E+3 | >-31.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17873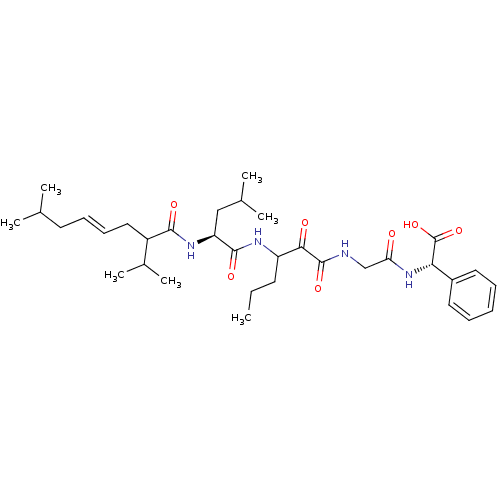 ((2S)-2-(2-{3-[(2S)-4-methyl-2-[(4E)-7-methyl-2-(pr...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 4.30E+3 | -31.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 30 |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50136005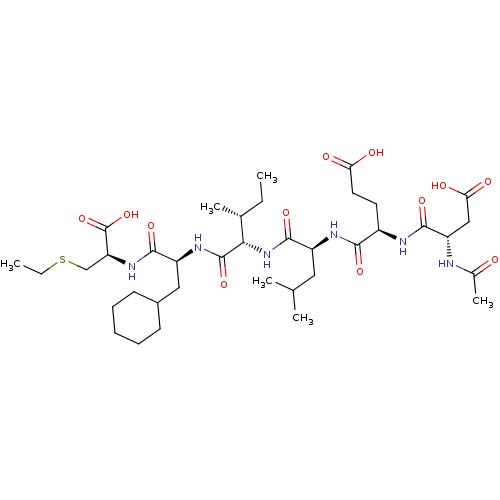 ((S)-4-((S)-2-Acetylamino-3-carboxy-propionylamino)...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Binding constant against hepatitis C virus (HCV) protease | J Med Chem 46: 5360-4 (2003) Article DOI: 10.1021/jm030040o BindingDB Entry DOI: 10.7270/Q20001J6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV genotype 1a, isolate H)) | BDBM17889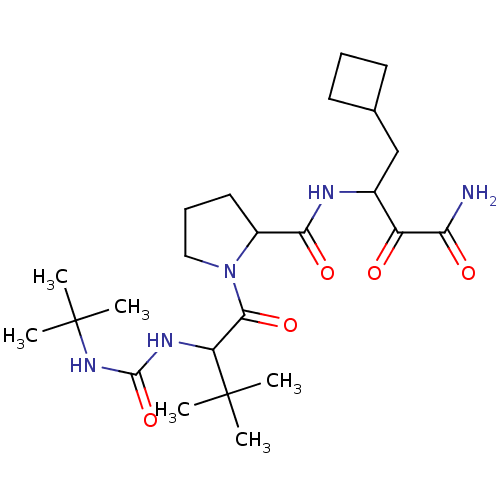 (3-[(1-{2-[(tert-butylcarbamoyl)amino]-3,3-dimethyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute | Assay Description Proteolytic cleavage of the ester linkage between the Nva (L-norvaline) and the chromophore (PAP) was monitored for change in absorbance at 370 nm. I... | J Med Chem 50: 2310-8 (2007) Article DOI: 10.1021/jm060173k BindingDB Entry DOI: 10.7270/Q27D2SDK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1922 total ) | Next | Last >> |