 Found 1204 hits with Last Name = 'fisher' and Initial = 'j'
Found 1204 hits with Last Name = 'fisher' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086
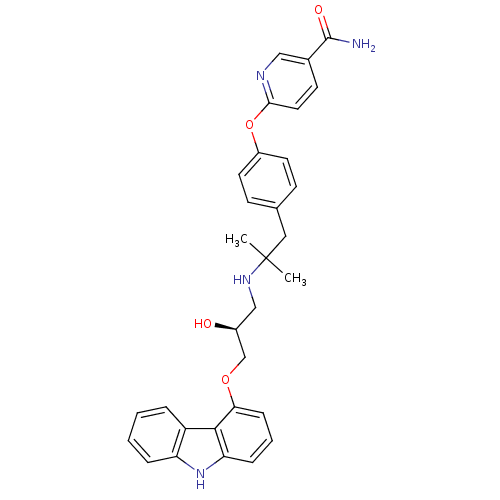
(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta2 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50379086

(CHEMBL2012521 | CHEMBL2012522 | LY-377604)Show SMILES CC(C)(Cc1ccc(Oc2ccc(cn2)C(N)=O)cc1)NC[C@H](O)COc1cccc2[nH]c3ccccc3c12 |r| Show InChI InChI=1S/C31H32N4O4/c1-31(2,16-20-10-13-23(14-11-20)39-28-15-12-21(17-33-28)30(32)37)34-18-22(36)19-38-27-9-5-8-26-29(27)24-6-3-4-7-25(24)35-26/h3-15,17,22,34-36H,16,18-19H2,1-2H3,(H2,32,37)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [125I]Iodocyanopindolol from human adrenergic beta1 receptor expressed in insect sf9 cells by scintillation counting |
ACS Med Chem Lett 2: 583-586 (2011)
Article DOI: 10.1021/ml200071k
BindingDB Entry DOI: 10.7270/Q20R9QDP |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144508

(4-Methyl-1-(5-phenyl-pentyl)-piperidine | CHEMBL30...)Show InChI InChI=1S/C17H27N/c1-16-11-14-18(15-12-16)13-7-3-6-10-17-8-4-2-5-9-17/h2,4-5,8-9,16H,3,6-7,10-15H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144518

(1-(5,5-Diphenyl-pentyl)-4-methyl-piperidine | CHEM...)Show InChI InChI=1S/C23H31N/c1-20-15-18-24(19-16-20)17-9-8-14-23(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-7,10-13,20,23H,8-9,14-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144512
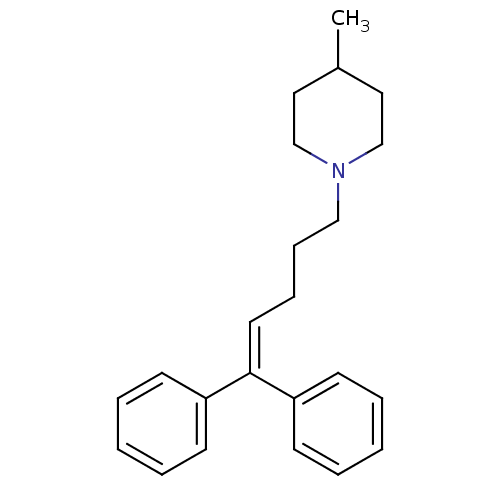
(1-(5,5-Diphenyl-pent-4-enyl)-4-methyl-piperidine |...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]-[#6]\[#6]=[#6](/c2ccccc2)-c2ccccc2)-[#6]-[#6]-1 Show InChI InChI=1S/C23H29N/c1-20-15-18-24(19-16-20)17-9-8-14-23(21-10-4-2-5-11-21)22-12-6-3-7-13-22/h2-7,10-14,20H,8-9,15-19H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041259
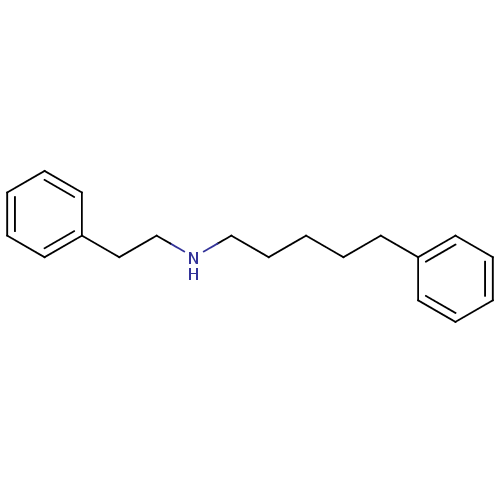
(CHEMBL19355 | Phenethyl-(5-phenyl-pentyl)-amine)Show InChI InChI=1S/C19H25N/c1-4-10-18(11-5-1)12-8-3-9-16-20-17-15-19-13-6-2-7-14-19/h1-2,4-7,10-11,13-14,20H,3,8-9,12,15-17H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50041246

(CHEMBL19544 | Methyl-phenethyl-(5-phenyl-pentyl)-a...)Show InChI InChI=1S/C20H27N/c1-21(18-16-20-14-7-3-8-15-20)17-10-4-9-13-19-11-5-2-6-12-19/h2-3,5-8,11-12,14-15H,4,9-10,13,16-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144511

((5,5-Diphenyl-pentyl)-methyl-phenethyl-amine | CHE...)Show InChI InChI=1S/C26H31N/c1-27(22-20-23-13-5-2-6-14-23)21-12-11-19-26(24-15-7-3-8-16-24)25-17-9-4-10-18-25/h2-10,13-18,26H,11-12,19-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM82122

(Vinylsulfone, 7)Show InChI InChI=1S/C15H14O3S2/c16-20(17,12-4-11-19)15-9-7-14(8-10-15)18-13-5-2-1-3-6-13/h1-10,12,19H,11H2/b12-4+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Notre Dame
| Assay Description
Inhibition assay using matrix metalloproteinase (MMP-2). |
Chem Biol Drug Des 74: 527-34 (2009)
Checked by Author
Article DOI: 10.1111/j.1747-0285.2009.00881.x
BindingDB Entry DOI: 10.7270/Q20P0XJV |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144513

((5,5-Diphenyl-pentyl)-phenethyl-amine | CHEMBL3032...)Show InChI InChI=1S/C25H29N/c1-4-12-22(13-5-1)19-21-26-20-11-10-18-25(23-14-6-2-7-15-23)24-16-8-3-9-17-24/h1-9,12-17,25-26H,10-11,18-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144509
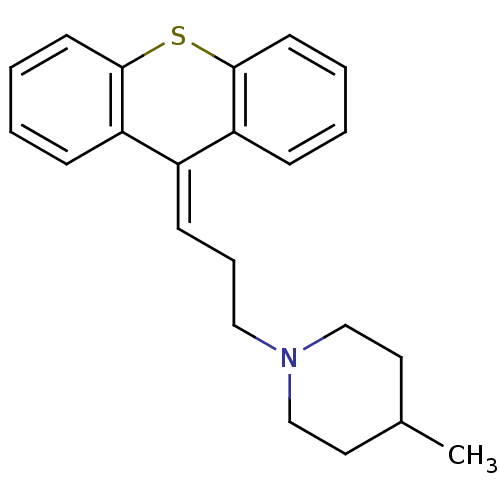
(4-Methyl-1-{3-[8aH-10lambda*4*-thioxanthen-(9E)-yl...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]\[#6]=[#6]-2/c3ccccc3-[#16]-c3ccccc-23)-[#6]-[#6]-1 Show InChI InChI=1S/C22H25NS/c1-17-12-15-23(16-13-17)14-6-9-18-19-7-2-4-10-21(19)24-22-11-5-3-8-20(18)22/h2-5,7-11,17H,6,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144507

((5,5-Diphenyl-pent-4-enyl)-methyl-phenethyl-amine ...)Show SMILES [#6]-[#7](-[#6]-[#6]-[#6]\[#6]=[#6](/c1ccccc1)-c1ccccc1)-[#6]-[#6]-c1ccccc1 Show InChI InChI=1S/C26H29N/c1-27(22-20-23-13-5-2-6-14-23)21-12-11-19-26(24-15-7-3-8-16-24)25-17-9-4-10-18-25/h2-10,13-19H,11-12,20-22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50212827
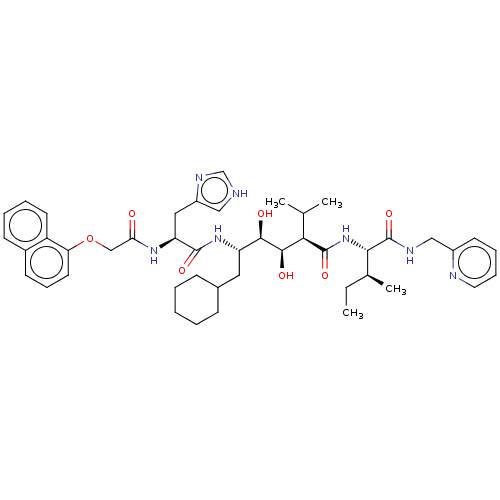
(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144515

(1-(4,4-Diphenyl-butyl)-4-methyl-piperidine | CHEMB...)Show InChI InChI=1S/C22H29N/c1-19-14-17-23(18-15-19)16-8-13-22(20-9-4-2-5-10-20)21-11-6-3-7-12-21/h2-7,9-12,19,22H,8,13-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144514
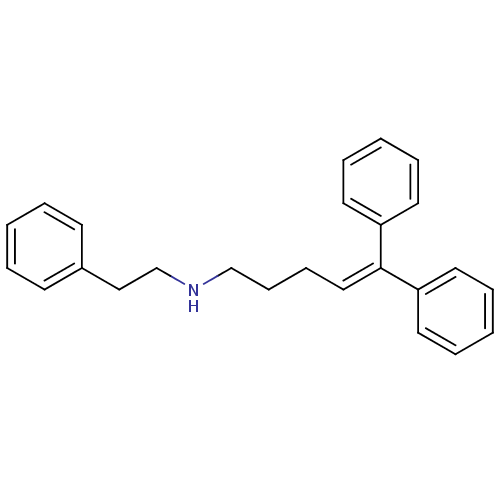
((5,5-Diphenyl-pent-4-enyl)-phenethyl-amine | CHEMB...)Show SMILES [#6](-[#6]-[#7]-[#6]-[#6]-c1ccccc1)-[#6]\[#6]=[#6](\c1ccccc1)-c1ccccc1 Show InChI InChI=1S/C25H27N/c1-4-12-22(13-5-1)19-21-26-20-11-10-18-25(23-14-6-2-7-15-23)24-16-8-3-9-17-24/h1-9,12-18,26H,10-11,19-21H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144503
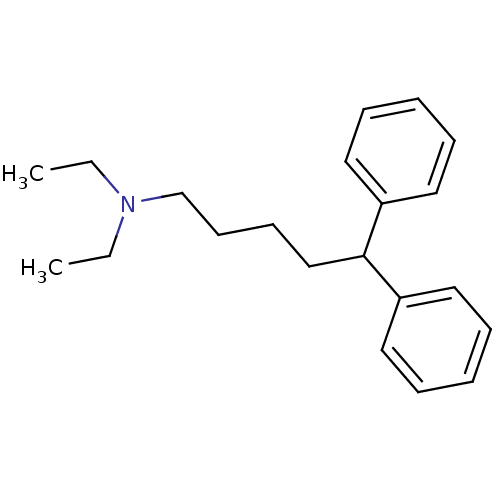
((5,5-Diphenyl-pentyl)-diethyl-amine | CHEMBL305461)Show InChI InChI=1S/C21H29N/c1-3-22(4-2)18-12-11-17-21(19-13-7-5-8-14-19)20-15-9-6-10-16-20/h5-10,13-16,21H,3-4,11-12,17-18H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Homo sapiens (Human)) | BDBM50144517
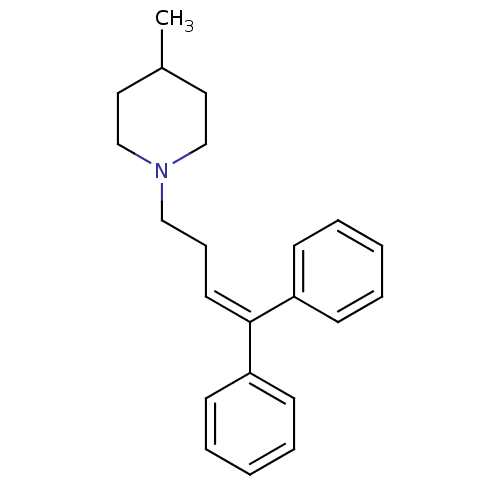
(1-(4,4-Diphenyl-but-3-enyl)-4-methyl-piperidine | ...)Show SMILES [#6]-[#6]-1-[#6]-[#6]-[#7](-[#6]-[#6]\[#6]=[#6](\c2ccccc2)-c2ccccc2)-[#6]-[#6]-1 Show InChI InChI=1S/C22H27N/c1-19-14-17-23(18-15-19)16-8-13-22(20-9-4-2-5-10-20)21-11-6-3-7-12-21/h2-7,9-13,19H,8,14-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Virginia Commonwealth University
Curated by ChEMBL
| Assay Description
Binding affinity for Sigma receptor type 1,using [3H]-(+)-pentazocine as radioligand |
Bioorg Med Chem Lett 14: 2217-20 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.018
BindingDB Entry DOI: 10.7270/Q2R210T8 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186127
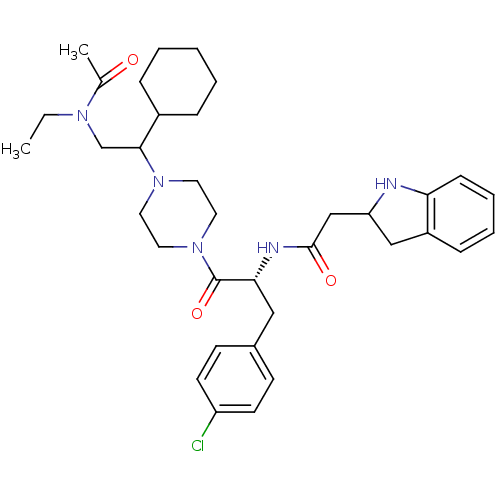
(CHEMBL378408 | N-[(R)-2-{4-[2-(acetyl-ethyl-amino)...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)C(C)=O Show InChI InChI=1S/C35H48ClN5O3/c1-3-39(25(2)42)24-33(27-9-5-4-6-10-27)40-17-19-41(20-18-40)35(44)32(21-26-13-15-29(36)16-14-26)38-34(43)23-30-22-28-11-7-8-12-31(28)37-30/h7-8,11-16,27,30,32-33,37H,3-6,9-10,17-24H2,1-2H3,(H,38,43)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Cathepsin D
(Homo sapiens (Human)) | BDBM50281636
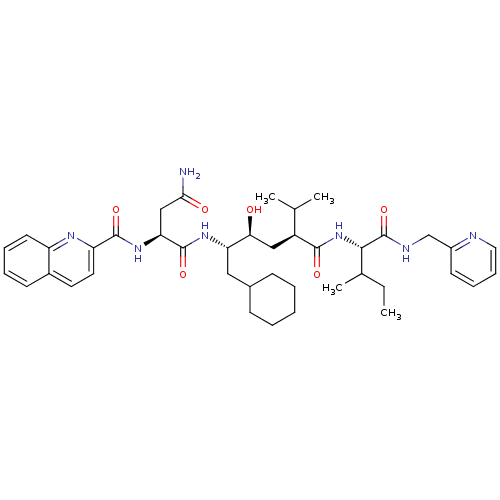
((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin D |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50281638
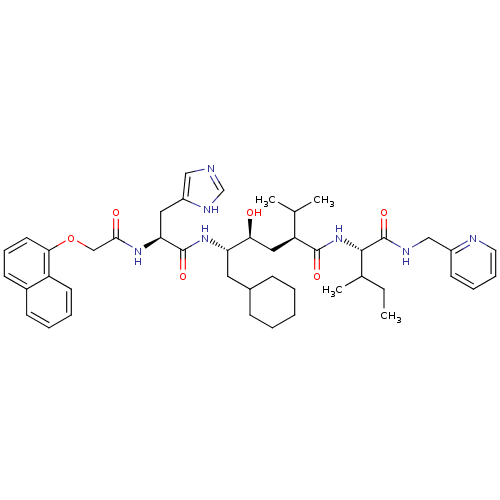
((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140256

(CHEMBL3740223)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(CO)c1 Show InChI InChI=1S/C27H23NO4/c1-16-10-11-22(27(31)32)17(2)25(16)28-26(30)24-14-21(13-20-7-3-4-9-23(20)24)19-8-5-6-18(12-19)15-29/h3-14,29H,15H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50212827

(CHEMBL3350189)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1c[nH]cn1)NC(=O)COc1cccc2ccccc12)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O7/c1-5-29(4)40(45(58)48-25-32-18-11-12-21-47-32)52-44(57)39(28(2)3)42(55)41(54)35(22-30-14-7-6-8-15-30)51-43(56)36(23-33-24-46-27-49-33)50-38(53)26-59-37-20-13-17-31-16-9-10-19-34(31)37/h9-13,16-21,24,27-30,35-36,39-42,54-55H,5-8,14-15,22-23,25-26H2,1-4H3,(H,46,49)(H,48,58)(H,50,53)(H,51,56)(H,52,57)/t29-,35-,36-,39+,40-,41+,42+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to renin |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186127

(CHEMBL378408 | N-[(R)-2-{4-[2-(acetyl-ethyl-amino)...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)C(C)=O Show InChI InChI=1S/C35H48ClN5O3/c1-3-39(25(2)42)24-33(27-9-5-4-6-10-27)40-17-19-41(20-18-40)35(44)32(21-26-13-15-29(36)16-14-26)38-34(43)23-30-22-28-11-7-8-12-31(28)37-30/h7-8,11-16,27,30,32-33,37H,3-6,9-10,17-24H2,1-2H3,(H,38,43)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Cathepsin E
(Homo sapiens (Human)) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for aspartyl protease inhibition selectivity relative to Cathepsin E |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186127

(CHEMBL378408 | N-[(R)-2-{4-[2-(acetyl-ethyl-amino)...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)C(C)=O Show InChI InChI=1S/C35H48ClN5O3/c1-3-39(25(2)42)24-33(27-9-5-4-6-10-27)40-17-19-41(20-18-40)35(44)32(21-26-13-15-29(36)16-14-26)38-34(43)23-30-22-28-11-7-8-12-31(28)37-30/h7-8,11-16,27,30,32-33,37H,3-6,9-10,17-24H2,1-2H3,(H,38,43)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50140258
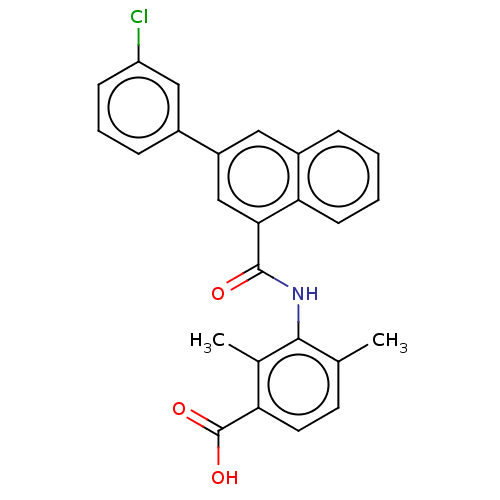
(CHEMBL3740325)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(Cl)c1 Show InChI InChI=1S/C26H20ClNO3/c1-15-10-11-21(26(30)31)16(2)24(15)28-25(29)23-14-19(17-7-5-8-20(27)13-17)12-18-6-3-4-9-22(18)23/h3-14H,1-2H3,(H,28,29)(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499955

(CHEMBL3741430)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(cc2ccccc12)-c1cccc(CO)c1)C(O)=O Show InChI InChI=1S/C27H23NO4/c1-16-10-22(27(31)32)11-17(2)25(16)28-26(30)24-14-21(13-20-7-3-4-9-23(20)24)19-8-5-6-18(12-19)15-29/h3-14,29H,15H2,1-2H3,(H,28,30)(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186131

((R)-N-((R)-3-(4-chlorophenyl)-1-(4-(1-cyclohexyl-2...)Show SMILES CCN(CC)CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C35H50ClN5O2/c1-3-39(4-2)25-33(27-10-6-5-7-11-27)40-18-20-41(21-19-40)35(43)32(22-26-14-16-30(36)17-15-26)38-34(42)31-23-28-12-8-9-13-29(28)24-37-31/h8-9,12-17,27,31-33,37H,3-7,10-11,18-25H2,1-2H3,(H,38,42)/t31-,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186121
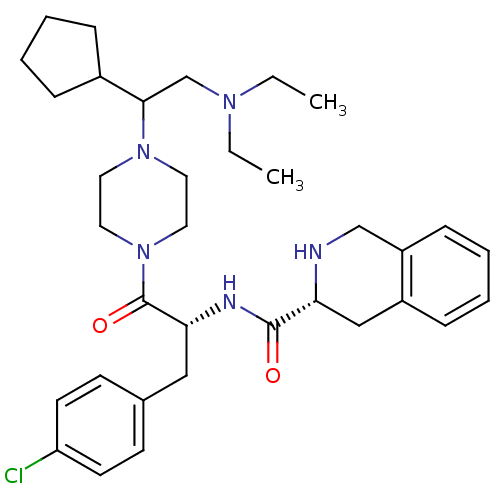
((R)-N-((R)-3-(4-chlorophenyl)-1-(4-(1-cyclopentyl-...)Show SMILES CCN(CC)CC(C1CCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C34H48ClN5O2/c1-3-38(4-2)24-32(26-9-5-6-10-26)39-17-19-40(20-18-39)34(42)31(21-25-13-15-29(35)16-14-25)37-33(41)30-22-27-11-7-8-12-28(27)23-36-30/h7-8,11-16,26,30-32,36H,3-6,9-10,17-24H2,1-2H3,(H,37,41)/t30-,31-,32?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50368890

(CHEMBL1790792)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@H](C(C)C)[C@@H](O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccc1SCCCCC[C@@H]1SCC2NC(=O)NC12)C(=O)NCCc1scnc1C Show InChI InChI=1S/C44H68N6O6S3/c1-6-27(4)37(43(55)45-21-20-33-28(5)46-25-59-33)49-42(54)36(26(2)3)40(52)39(51)31(23-29-15-9-7-10-16-29)47-41(53)30-17-12-13-18-34(30)57-22-14-8-11-19-35-38-32(24-58-35)48-44(56)50-38/h12-13,17-18,25-27,29,31-32,35-40,51-52H,6-11,14-16,19-24H2,1-5H3,(H,45,55)(H,47,53)(H,49,54)(H2,48,50,56)/t27-,31+,32?,35+,36-,37+,38?,39-,40-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Upjohn Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against biotinylated human HIV-1 protease |
J Med Chem 37: 293-304 (1994)
BindingDB Entry DOI: 10.7270/Q27S7PD1 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281641
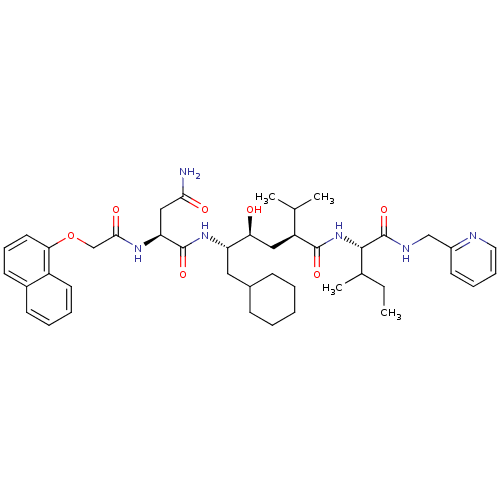
((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C43H60N6O7/c1-5-28(4)40(43(55)46-25-31-18-11-12-21-45-31)49-41(53)33(27(2)3)23-36(50)34(22-29-14-7-6-8-15-29)48-42(54)35(24-38(44)51)47-39(52)26-56-37-20-13-17-30-16-9-10-19-32(30)37/h9-13,16-21,27-29,33-36,40,50H,5-8,14-15,22-26H2,1-4H3,(H2,44,51)(H,46,55)(H,47,52)(H,48,54)(H,49,53)/t28?,33-,34-,35-,36-,40-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186863
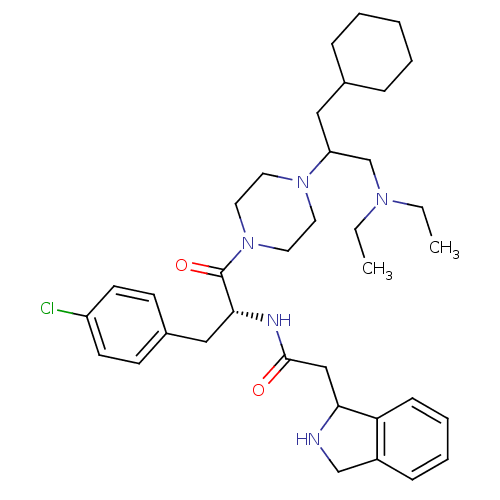
(CHEMBL379497 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-c...)Show SMILES CCN(CC)CC(CC1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1NCc2ccccc12 Show InChI InChI=1S/C36H52ClN5O2/c1-3-40(4-2)26-31(22-27-10-6-5-7-11-27)41-18-20-42(21-19-41)36(44)34(23-28-14-16-30(37)17-15-28)39-35(43)24-33-32-13-9-8-12-29(32)25-38-33/h8-9,12-17,27,31,33-34,38H,3-7,10-11,18-26H2,1-2H3,(H,39,43)/t31?,33?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human MC4 receptor transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3843-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.016
BindingDB Entry DOI: 10.7270/Q2SQ900D |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186128

(CHEMBL209990 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-c...)Show SMILES CCN(CC)CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1 Show InChI InChI=1S/C35H50ClN5O2/c1-3-39(4-2)25-33(27-10-6-5-7-11-27)40-18-20-41(21-19-40)35(43)32(22-26-14-16-29(36)17-15-26)38-34(42)24-30-23-28-12-8-9-13-31(28)37-30/h8-9,12-17,27,30,32-33,37H,3-7,10-11,18-25H2,1-2H3,(H,38,42)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186128

(CHEMBL209990 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-c...)Show SMILES CCN(CC)CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1 Show InChI InChI=1S/C35H50ClN5O2/c1-3-39(4-2)25-33(27-10-6-5-7-11-27)40-18-20-41(21-19-40)35(43)32(22-26-14-16-29(36)17-15-26)38-34(42)24-30-23-28-12-8-9-13-31(28)37-30/h8-9,12-17,27,30,32-33,37H,3-7,10-11,18-25H2,1-2H3,(H,38,42)/t30?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186125
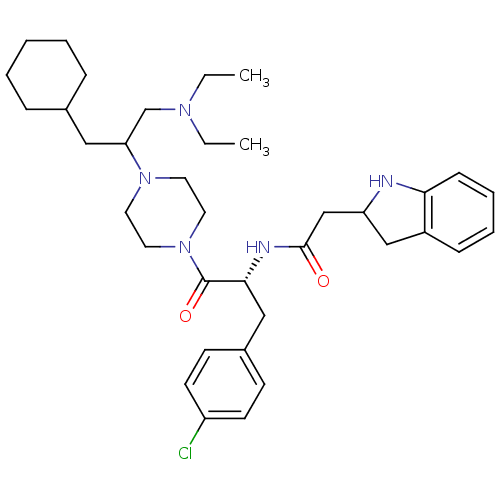
(CHEMBL377825 | N-((R)-3-(4-chlorophenyl)-1-(4-(3-c...)Show SMILES CCN(CC)CC(CC1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1 Show InChI InChI=1S/C36H52ClN5O2/c1-3-40(4-2)26-32(22-27-10-6-5-7-11-27)41-18-20-42(21-19-41)36(44)34(23-28-14-16-30(37)17-15-28)39-35(43)25-31-24-29-12-8-9-13-33(29)38-31/h8-9,12-17,27,31-32,34,38H,3-7,10-11,18-26H2,1-2H3,(H,39,43)/t31?,32?,34-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281638

((2S,4S,5S)-6-Cyclohexyl-4-hydroxy-5-{(S)-3-(1H-imi...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)COc1cccc2ccccc12)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C45H61N7O6/c1-5-30(4)42(45(57)48-26-33-18-11-12-21-47-33)52-43(55)36(29(2)3)24-39(53)37(22-31-14-7-6-8-15-31)51-44(56)38(23-34-25-46-28-49-34)50-41(54)27-58-40-20-13-17-32-16-9-10-19-35(32)40/h9-13,16-21,25,28-31,36-39,42,53H,5-8,14-15,22-24,26-27H2,1-4H3,(H,46,49)(H,48,57)(H,50,54)(H,51,56)(H,52,55)/t30?,36-,37-,38-,39-,42-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50177564

(CHEMBL380540 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...)Show SMILES CCN(CC)CC(N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)C1Cc2ccccc2C(C)(C)N1)c1ccccc1F Show InChI InChI=1S/C37H47ClFN5O2/c1-5-42(6-2)25-34(29-12-8-10-14-31(29)39)43-19-21-44(22-20-43)36(46)33(23-26-15-17-28(38)18-16-26)40-35(45)32-24-27-11-7-9-13-30(27)37(3,4)41-32/h7-18,32-34,41H,5-6,19-25H2,1-4H3,(H,40,45)/t32?,33-,34?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 2341-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.103
BindingDB Entry DOI: 10.7270/Q2NS0TF6 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186124

(CHEMBL410087 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-c...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)S(C)(=O)=O Show InChI InChI=1S/C34H48ClN5O4S/c1-3-40(45(2,43)44)24-32(26-9-5-4-6-10-26)38-17-19-39(20-18-38)34(42)31(21-25-13-15-28(35)16-14-25)37-33(41)23-29-22-27-11-7-8-12-30(27)36-29/h7-8,11-16,26,29,31-32,36H,3-6,9-10,17-24H2,1-2H3,(H,37,41)/t29?,31-,32?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186124

(CHEMBL410087 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-c...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1Cc2ccccc2N1)S(C)(=O)=O Show InChI InChI=1S/C34H48ClN5O4S/c1-3-40(45(2,43)44)24-32(26-9-5-4-6-10-26)38-17-19-39(20-18-38)34(42)31(21-25-13-15-28(35)16-14-25)37-33(41)23-29-22-27-11-7-8-12-30(27)36-29/h7-8,11-16,26,29,31-32,36H,3-6,9-10,17-24H2,1-2H3,(H,37,41)/t29?,31-,32?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50177560
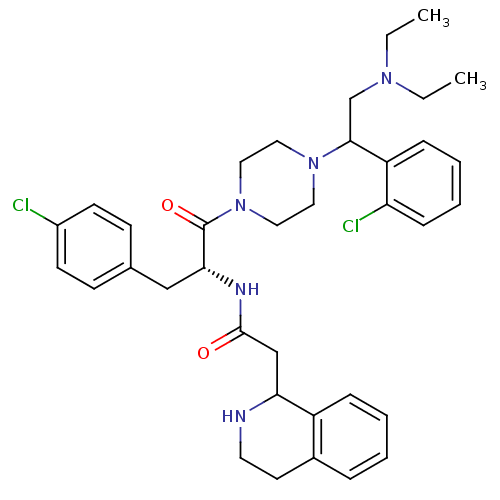
(CHEMBL206042 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-(...)Show SMILES CCN(CC)CC(N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1NCCc2ccccc12)c1ccccc1Cl Show InChI InChI=1S/C36H45Cl2N5O2/c1-3-41(4-2)25-34(30-11-7-8-12-31(30)38)42-19-21-43(22-20-42)36(45)33(23-26-13-15-28(37)16-14-26)40-35(44)24-32-29-10-6-5-9-27(29)17-18-39-32/h5-16,32-34,39H,3-4,17-25H2,1-2H3,(H,40,44)/t32?,33-,34?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 2341-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.103
BindingDB Entry DOI: 10.7270/Q2NS0TF6 |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50177560

(CHEMBL206042 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-(...)Show SMILES CCN(CC)CC(N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1NCCc2ccccc12)c1ccccc1Cl Show InChI InChI=1S/C36H45Cl2N5O2/c1-3-41(4-2)25-34(30-11-7-8-12-31(30)38)42-19-21-43(22-20-42)36(45)33(23-26-13-15-28(37)16-14-26)40-35(44)24-32-29-10-6-5-9-27(29)17-18-39-32/h5-16,32-34,39H,3-4,17-25H2,1-2H3,(H,40,44)/t32?,33-,34?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 2341-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.103
BindingDB Entry DOI: 10.7270/Q2NS0TF6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499950
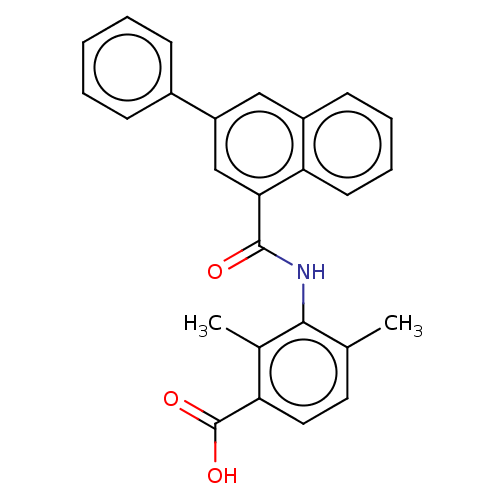
(CHEMBL3739435)Show SMILES Cc1ccc(C(O)=O)c(C)c1NC(=O)c1cc(cc2ccccc12)-c1ccccc1 Show InChI InChI=1S/C26H21NO3/c1-16-12-13-21(26(29)30)17(2)24(16)27-25(28)23-15-20(18-8-4-3-5-9-18)14-19-10-6-7-11-22(19)23/h3-15H,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186868
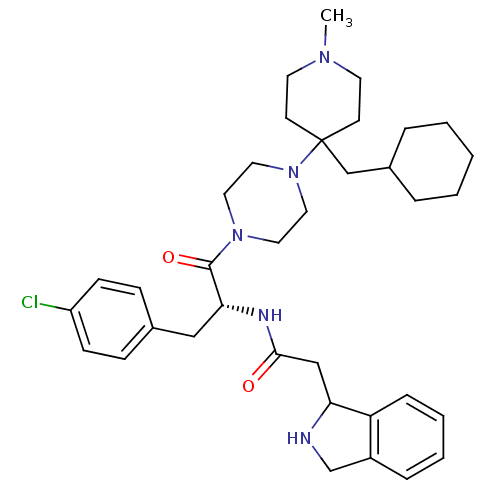
(CHEMBL213340 | N-{(R)-1-(4-chloro-benzyl)-2-[4-(4-...)Show SMILES CN1CCC(CC2CCCCC2)(CC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1NCc2ccccc12 Show InChI InChI=1S/C36H50ClN5O2/c1-40-17-15-36(16-18-40,25-28-7-3-2-4-8-28)42-21-19-41(20-22-42)35(44)33(23-27-11-13-30(37)14-12-27)39-34(43)24-32-31-10-6-5-9-29(31)26-38-32/h5-6,9-14,28,32-33,38H,2-4,7-8,15-26H2,1H3,(H,39,43)/t32?,33-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from human MC4 receptor transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3843-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.016
BindingDB Entry DOI: 10.7270/Q2SQ900D |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50186138

(CHEMBL425609 | N-((R)-3-(4-chlorophenyl)-1-(4-(1-c...)Show SMILES CCN(CC(C1CCCCC1)N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)n1cc2ccccc2c1)S(C)(=O)=O Show InChI InChI=1S/C33H44ClN5O4S/c1-3-39(44(2,42)43)24-31(26-9-5-4-6-10-26)36-17-19-37(20-18-36)32(40)30(21-25-13-15-29(34)16-14-25)35-33(41)38-22-27-11-7-8-12-28(27)23-38/h7-8,11-16,22-23,26,30-31H,3-6,9-10,17-21,24H2,1-2H3,(H,35,41)/t30-,31?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [125]I-NDP-alpha-MSH binding to human MC4R transfected in HEK293 cells |
Bioorg Med Chem Lett 16: 3449-53 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.002
BindingDB Entry DOI: 10.7270/Q2ST7PFS |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50177563

(CHEMBL380855 | N-((R)-3-(4-chlorophenyl)-1-(4-(2-(...)Show SMILES CCN(CC)CC(N1CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)CC1NCc2ccccc12)c1ccccc1F Show InChI InChI=1S/C35H43ClFN5O2/c1-3-40(4-2)24-33(29-11-7-8-12-30(29)37)41-17-19-42(20-18-41)35(44)32(21-25-13-15-27(36)16-14-25)39-34(43)22-31-28-10-6-5-9-26(28)23-38-31/h5-16,31-33,38H,3-4,17-24H2,1-2H3,(H,39,43)/t31?,32-,33?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [125I]NDP-alpha-MSH from cloned human MC4R expressed in HEK293 cells |
Bioorg Med Chem Lett 16: 2341-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.103
BindingDB Entry DOI: 10.7270/Q2NS0TF6 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50499949

(CHEMBL3742015)Show SMILES Cc1cc(cc(C)c1NC(=O)c1cc(cc2ccccc12)-c1ccccc1)C(O)=O Show InChI InChI=1S/C26H21NO3/c1-16-12-21(26(29)30)13-17(2)24(16)27-25(28)23-15-20(18-8-4-3-5-9-18)14-19-10-6-7-11-22(19)23/h3-15H,1-2H3,(H,27,28)(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eli Lilly and Company
Curated by ChEMBL
| Assay Description
Antagonist activity at human EP4 receptor |
Bioorg Med Chem Lett 26: 105-9 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.023
BindingDB Entry DOI: 10.7270/Q2G73HRK |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281651
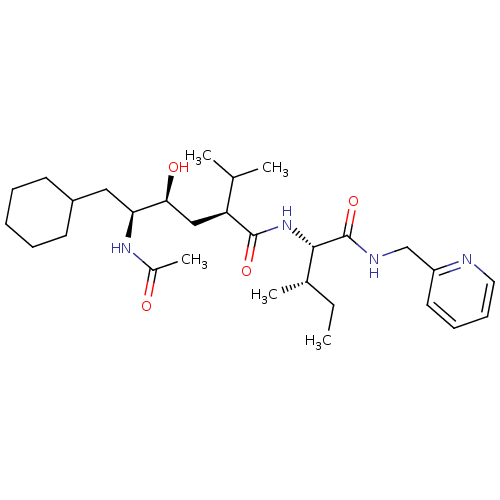
((2S,4S,5S)-5-Acetylamino-6-cyclohexyl-4-hydroxy-2-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(C)=O)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C29H48N4O4/c1-6-20(4)27(29(37)31-18-23-14-10-11-15-30-23)33-28(36)24(19(2)3)17-26(35)25(32-21(5)34)16-22-12-8-7-9-13-22/h10-11,14-15,19-20,22,24-27,35H,6-9,12-13,16-18H2,1-5H3,(H,31,37)(H,32,34)(H,33,36)/t20-,24-,25-,26-,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281642
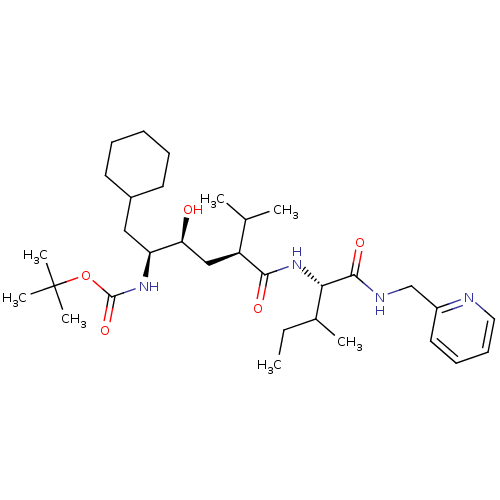
(((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-5-methyl-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)OC(C)(C)C)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C32H54N4O5/c1-8-22(4)28(30(39)34-20-24-16-12-13-17-33-24)36-29(38)25(21(2)3)19-27(37)26(18-23-14-10-9-11-15-23)35-31(40)41-32(5,6)7/h12-13,16-17,21-23,25-28,37H,8-11,14-15,18-20H2,1-7H3,(H,34,39)(H,35,40)(H,36,38)/t22?,25-,26-,27-,28-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281640
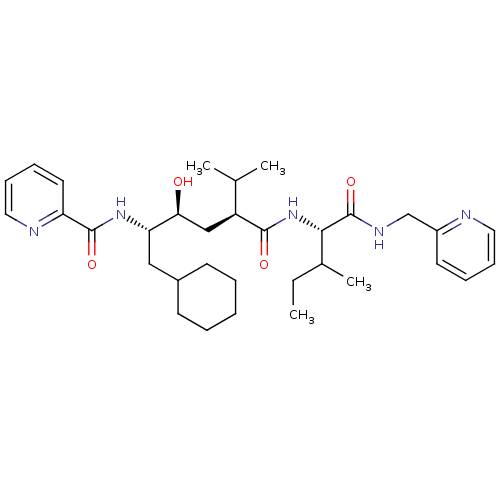
(CHEMBL433729 | Pyridine-2-carboxylic acid ((1S,2S,...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)c1ccccn1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C33H49N5O4/c1-5-23(4)30(33(42)36-21-25-15-9-11-17-34-25)38-31(40)26(22(2)3)20-29(39)28(19-24-13-7-6-8-14-24)37-32(41)27-16-10-12-18-35-27/h9-12,15-18,22-24,26,28-30,39H,5-8,13-14,19-21H2,1-4H3,(H,36,42)(H,37,41)(H,38,40)/t23?,26-,28-,29-,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50281636

((S)-N*1*-((1S,2S,4S)-1-Cyclohexylmethyl-2-hydroxy-...)Show SMILES CCC(C)[C@H](NC(=O)[C@@H](C[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(N)=O)NC(=O)c1ccc2ccccc2n1)C(C)C)C(=O)NCc1ccccn1 Show InChI InChI=1S/C41H57N7O6/c1-5-26(4)37(41(54)44-24-29-16-11-12-20-43-29)48-38(51)30(25(2)3)22-35(49)33(21-27-13-7-6-8-14-27)46-40(53)34(23-36(42)50)47-39(52)32-19-18-28-15-9-10-17-31(28)45-32/h9-12,15-20,25-27,30,33-35,37,49H,5-8,13-14,21-24H2,1-4H3,(H2,42,50)(H,44,54)(H,46,53)(H,47,52)(H,48,51)/t26?,30-,33-,34-,35-,37-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluated for inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 3: 819-824 (1993)
Article DOI: 10.1016/S0960-894X(00)80673-3
BindingDB Entry DOI: 10.7270/Q2NS0VCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data