 Found 294 hits with Last Name = 'fitzgerald' and Initial = 'ce'
Found 294 hits with Last Name = 'fitzgerald' and Initial = 'ce' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM15241
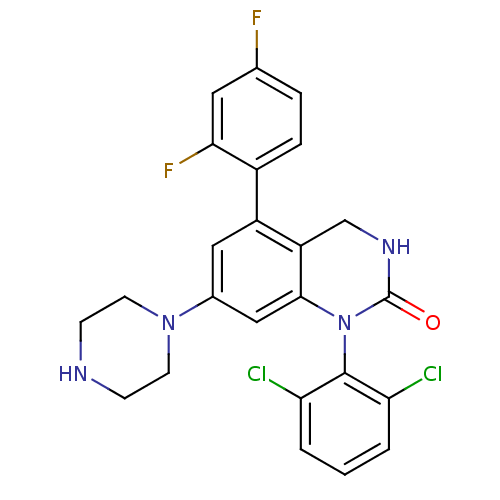
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)N1CCNCC1 Show InChI InChI=1S/C24H20Cl2F2N4O/c25-19-2-1-3-20(26)23(19)32-22-12-15(31-8-6-29-7-9-31)11-17(18(22)13-30-24(32)33)16-5-4-14(27)10-21(16)28/h1-5,10-12,29H,6-9,13H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50194461

(7-amino-5-(2,4-difluorophenyl)-1-(2,6-difluorophen...)Show SMILES Nc1cc2n(-c3c(F)cccc3F)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(15,-16.28,;13.66,-17.04,;12.34,-16.27,;11.01,-17.03,;9.67,-16.27,;9.67,-14.73,;11.01,-13.96,;12.35,-14.72,;11.01,-12.4,;9.67,-11.64,;8.34,-12.41,;8.34,-13.96,;7,-14.73,;8.34,-17.03,;7.01,-16.26,;8.34,-18.57,;9.67,-19.35,;11,-18.57,;12.32,-19.35,;13.66,-18.59,;12.31,-20.89,;10.97,-21.64,;10.96,-23.18,;12.29,-23.96,;12.28,-25.51,;13.63,-23.19,;13.64,-21.66,;14.97,-20.89,)| Show InChI InChI=1S/C20H11F4N3O/c21-10-4-5-11(15(24)8-10)19-12-6-7-18(28)27(16(12)9-17(25)26-19)20-13(22)2-1-3-14(20)23/h1-9H,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317587
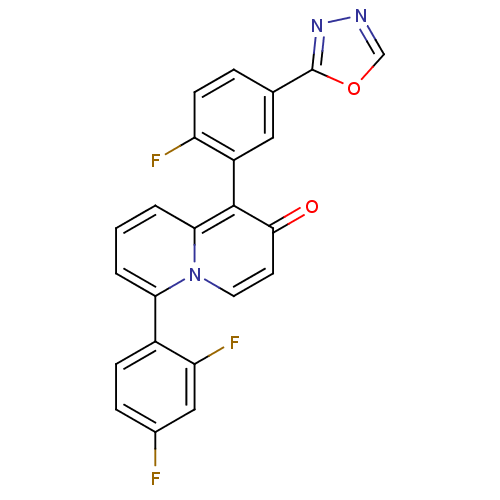
(6-(2,4-difluorophenyl)-1-(2-fluoro-5-(1,3,4-oxadia...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3cc(ccc3F)-c3nnco3)c(=O)ccn12 |(12.42,-9.58,;12.42,-8.04,;11.08,-7.27,;11.09,-5.73,;12.43,-4.96,;13.76,-5.73,;15.09,-4.95,;13.76,-7.26,;12.43,-3.42,;13.76,-2.65,;13.75,-1.11,;12.42,-.35,;11.1,-1.11,;9.77,-.33,;9.77,1.21,;11.11,1.97,;11.11,3.51,;9.77,4.29,;8.43,3.51,;8.44,1.97,;7.11,1.2,;12.44,4.28,;13.91,3.83,;14.8,5.09,;13.87,6.32,;12.42,5.83,;8.44,-1.11,;7.1,-.35,;8.44,-2.65,;9.77,-3.42,;11.1,-2.65,)| Show InChI InChI=1S/C23H12F3N3O2/c24-14-5-6-15(18(26)11-14)19-2-1-3-20-22(21(30)8-9-29(19)20)16-10-13(4-7-17(16)25)23-28-27-12-31-23/h1-12H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317588
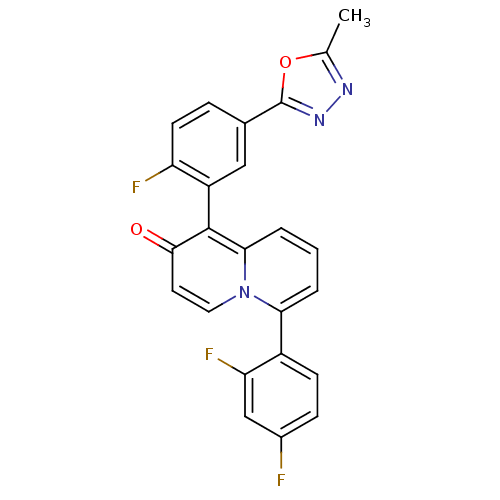
(6-(2,4-difluorophenyl)-1-(2-fluoro-5-(5-methyl-1,3...)Show SMILES Cc1nnc(o1)-c1ccc(F)c(c1)-c1c2cccc(-c3ccc(F)cc3F)n2ccc1=O |(31.09,6.57,;30.64,5.09,;31.57,3.86,;30.68,2.6,;29.21,3.05,;29.18,4.59,;27.88,2.28,;26.54,3.06,;25.2,2.28,;25.21,.74,;23.87,-.03,;26.54,-.02,;27.87,.74,;26.54,-1.56,;27.87,-2.34,;29.19,-1.58,;30.52,-2.34,;30.52,-3.88,;29.19,-4.65,;29.19,-6.19,;27.85,-6.96,;27.85,-8.5,;29.19,-9.27,;29.19,-10.81,;30.53,-8.49,;30.52,-6.95,;31.85,-6.18,;27.86,-3.88,;26.54,-4.65,;25.21,-3.88,;25.21,-2.34,;23.87,-1.58,)| Show InChI InChI=1S/C24H14F3N3O2/c1-13-28-29-24(32-13)14-5-8-18(26)17(11-14)23-21-4-2-3-20(30(21)10-9-22(23)31)16-7-6-15(25)12-19(16)27/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50317579

(6-(2,4-difluorophenyl)-1-(2,6-difluorophenyl)-2H-q...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3c(F)cccc3F)c(=O)ccn12 |(29.65,-6.7,;29.65,-5.16,;28.31,-4.39,;28.31,-2.85,;29.65,-2.08,;30.98,-2.84,;32.31,-2.07,;30.99,-4.38,;29.65,-.54,;30.98,.23,;30.98,1.77,;29.65,2.53,;28.33,1.77,;27,2.55,;27,4.09,;28.33,4.85,;29.67,4.08,;28.33,6.39,;27,7.17,;25.66,6.39,;25.67,4.85,;24.33,4.08,;25.67,1.77,;24.33,2.53,;25.67,.23,;27,-.54,;28.32,.23,)| Show InChI InChI=1S/C21H11F4NO/c22-12-7-8-13(16(25)11-12)17-5-2-6-18-21(19(27)9-10-26(17)18)20-14(23)3-1-4-15(20)24/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of labeled MK-499 from human ERG in HEK293 cells |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14 [G110A]
(Mus musculus (mouse)) | BDBM15240
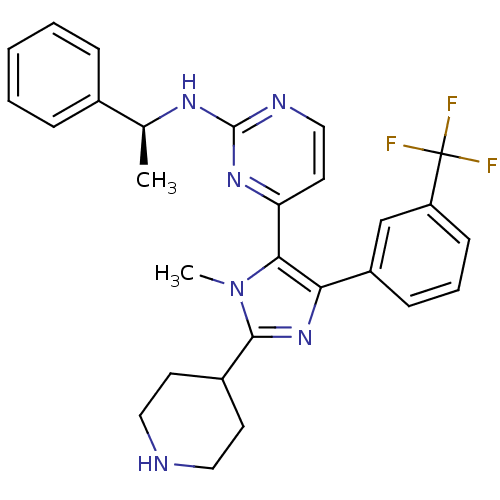
(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15240

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14 [G110D]
(Mus musculus (mouse)) | BDBM15240

(4-[1-methyl-2-(piperidin-4-yl)-4-[3-(trifluorometh...)Show SMILES C[C@H](Nc1nccc(n1)-c1c(nc(C2CCNCC2)n1C)-c1cccc(c1)C(F)(F)F)c1ccccc1 |r| Show InChI InChI=1S/C28H29F3N6/c1-18(19-7-4-3-5-8-19)34-27-33-16-13-23(35-27)25-24(21-9-6-10-22(17-21)28(29,30)31)36-26(37(25)2)20-11-14-32-15-12-20/h3-10,13,16-18,20,32H,11-12,14-15H2,1-2H3,(H,33,34,35)/t18-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421140
(CHEMBL2088591)Show SMILES COc1ccc(cc1-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O)-c1nnc(C)o1 |(33.77,-46.54,;35.1,-47.32,;36.43,-46.55,;36.44,-45,;37.77,-44.23,;39.1,-45,;39.11,-46.55,;37.77,-47.32,;37.77,-48.85,;39.1,-49.62,;40.42,-48.85,;41.75,-49.61,;41.75,-51.14,;43.09,-51.91,;43.1,-53.45,;41.76,-54.22,;41.77,-55.76,;43.1,-56.53,;43.11,-58.07,;44.44,-55.74,;44.43,-54.21,;45.76,-53.43,;40.43,-51.91,;39.1,-51.15,;37.77,-51.93,;36.43,-51.16,;36.43,-49.62,;35.1,-48.85,;40.36,-44.1,;41.82,-44.58,;42.73,-43.34,;41.83,-42.09,;42.31,-40.63,;40.36,-42.56,)| Show InChI InChI=1S/C24H16F2N4O4/c1-13-27-28-24(33-13)14-3-6-20(32-2)16(11-14)23-18-5-8-22(29-30(18)10-9-19(23)31)34-21-7-4-15(25)12-17(21)26/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421141
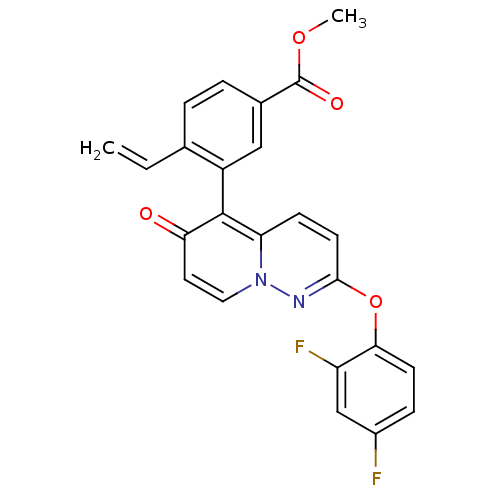
(CHEMBL2088578)Show SMILES COC(=O)c1ccc(C=C)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(46,-11.48,;44.67,-12.26,;43.33,-11.49,;43.33,-9.95,;42,-12.27,;40.67,-11.5,;39.34,-12.27,;39.34,-13.82,;38,-14.59,;36.67,-13.82,;40.67,-14.59,;42.01,-13.82,;40.67,-16.12,;42,-16.89,;43.32,-16.12,;44.65,-16.88,;44.65,-18.42,;45.99,-19.18,;46,-20.72,;44.66,-21.49,;44.67,-23.03,;46.01,-23.8,;46.01,-25.34,;47.34,-23.01,;47.33,-21.48,;48.66,-20.7,;43.33,-19.18,;42,-18.42,;40.67,-19.2,;39.33,-18.43,;39.34,-16.89,;38,-16.12,)| Show InChI InChI=1S/C24H16F2N2O4/c1-3-14-4-5-15(24(30)31-2)12-17(14)23-19-7-9-22(27-28(19)11-10-20(23)29)32-21-8-6-16(25)13-18(21)26/h3-13H,1H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421137
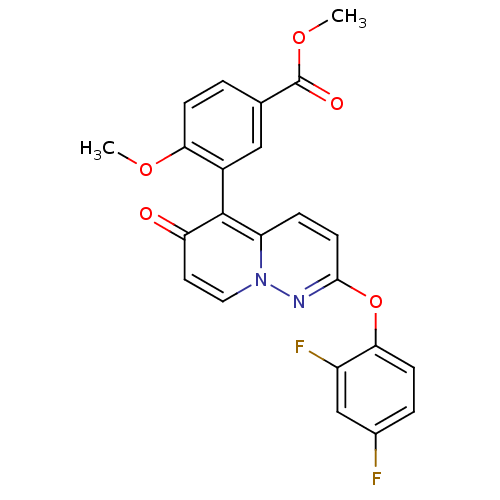
(CHEMBL2088588)Show SMILES COC(=O)c1ccc(OC)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(7.25,-42.41,;5.92,-43.19,;4.58,-42.42,;4.58,-40.88,;3.25,-43.2,;1.92,-42.43,;.59,-43.21,;.59,-44.75,;-.75,-45.52,;-2.08,-44.75,;1.92,-45.52,;3.26,-44.75,;1.92,-47.06,;3.25,-47.82,;4.57,-47.05,;5.9,-47.81,;5.91,-49.35,;7.24,-50.11,;7.25,-51.65,;5.91,-52.42,;5.92,-53.96,;7.26,-54.73,;7.26,-56.27,;8.59,-53.95,;8.58,-52.41,;9.91,-51.63,;4.58,-50.12,;3.25,-49.35,;1.92,-50.14,;.59,-49.36,;.59,-47.83,;-.75,-47.05,)| Show InChI InChI=1S/C23H16F2N2O5/c1-30-19-6-3-13(23(29)31-2)11-15(19)22-17-5-8-21(26-27(17)10-9-18(22)28)32-20-7-4-14(24)12-16(20)25/h3-12H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222105
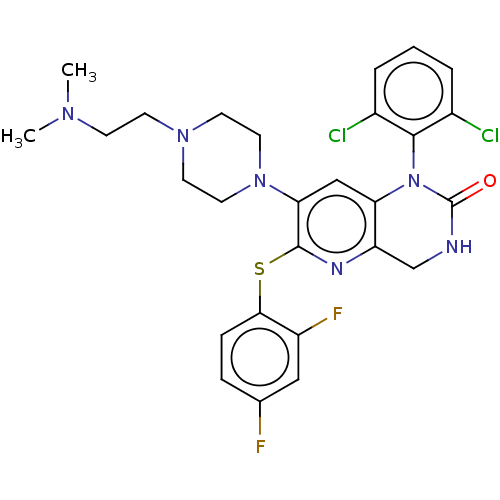
(CHEMBL92814)Show SMILES CN(C)CCN1CCN(CC1)c1cc2N(C(=O)NCc2nc1Sc1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C27H28Cl2F2N6OS/c1-34(2)8-9-35-10-12-36(13-11-35)23-15-22-21(33-26(23)39-24-7-6-17(30)14-20(24)31)16-32-27(38)37(22)25-18(28)4-3-5-19(25)29/h3-7,14-15H,8-13,16H2,1-2H3,(H,32,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50194461

(7-amino-5-(2,4-difluorophenyl)-1-(2,6-difluorophen...)Show SMILES Nc1cc2n(-c3c(F)cccc3F)c(=O)ccc2c(n1)-c1ccc(F)cc1F |(15,-16.28,;13.66,-17.04,;12.34,-16.27,;11.01,-17.03,;9.67,-16.27,;9.67,-14.73,;11.01,-13.96,;12.35,-14.72,;11.01,-12.4,;9.67,-11.64,;8.34,-12.41,;8.34,-13.96,;7,-14.73,;8.34,-17.03,;7.01,-16.26,;8.34,-18.57,;9.67,-19.35,;11,-18.57,;12.32,-19.35,;13.66,-18.59,;12.31,-20.89,;10.97,-21.64,;10.96,-23.18,;12.29,-23.96,;12.28,-25.51,;13.63,-23.19,;13.64,-21.66,;14.97,-20.89,)| Show InChI InChI=1S/C20H11F4N3O/c21-10-4-5-11(15(24)8-10)19-12-6-7-18(28)27(16(12)9-17(25)26-19)20-13(22)2-1-3-14(20)23/h1-9H,(H2,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38alpha after 3 hrs by SPA method |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222094

(CHEMBL96305)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CC3CC2CN3)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C24H19Cl2F2N5OS/c25-15-2-1-3-16(26)22(15)33-19-8-20(32-11-13-7-14(32)9-29-13)23(31-18(19)10-30-24(33)34)35-21-5-4-12(27)6-17(21)28/h1-6,8,13-14,29H,7,9-11H2,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222105

(CHEMBL92814)Show SMILES CN(C)CCN1CCN(CC1)c1cc2N(C(=O)NCc2nc1Sc1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C27H28Cl2F2N6OS/c1-34(2)8-9-35-10-12-36(13-11-35)23-15-22-21(33-26(23)39-24-7-6-17(30)14-20(24)31)16-32-27(38)37(22)25-18(28)4-3-5-19(25)29/h3-7,14-15H,8-13,16H2,1-2H3,(H,32,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15242
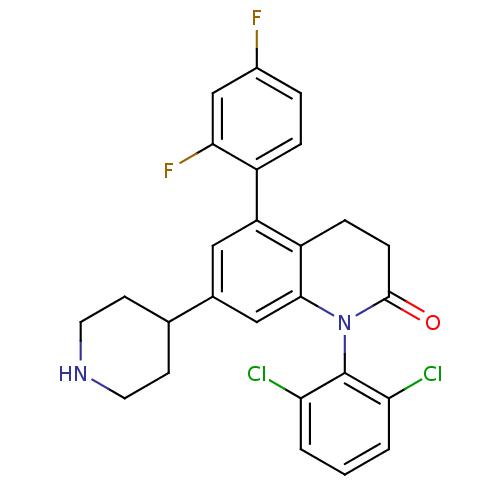
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(4...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)CCc12)c1c(Cl)cccc1Cl)C1CCNCC1 Show InChI InChI=1S/C26H22Cl2F2N2O/c27-21-2-1-3-22(28)26(21)32-24-13-16(15-8-10-31-11-9-15)12-20(19(24)6-7-25(32)33)18-5-4-17(29)14-23(18)30/h1-5,12-15,31H,6-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15244
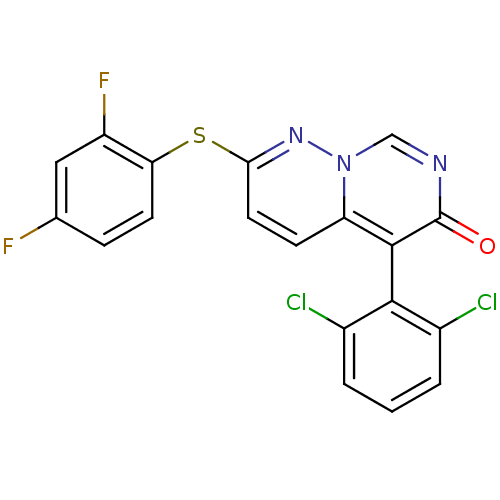
(5-(2,6-dichlorophenyl)-2-(2,4-difluorophenyl)sulfa...)Show SMILES Fc1ccc(Sc2ccc3c(-c4c(Cl)cccc4Cl)c(=O)ncn3n2)c(F)c1 |(-1.69,6.87,;-1.69,5.33,;-.36,4.56,;-.36,3.02,;-1.69,2.25,;-1.69,.71,;-3.03,-.06,;-3.03,-1.6,;-4.36,-2.37,;-5.75,-1.54,;-7.08,-2.31,;-7.08,-3.85,;-5.75,-4.62,;-4.42,-3.85,;-5.75,-6.16,;-7.08,-6.93,;-8.42,-6.16,;-8.42,-4.62,;-9.75,-3.85,;-8.42,-1.54,;-9.75,-2.31,;-8.42,,;-7.08,.77,;-5.75,,;-4.36,.71,;-3.03,3.02,;-4.36,2.25,;-3.03,4.56,)| Show InChI InChI=1S/C19H9Cl2F2N3OS/c20-11-2-1-3-12(21)17(11)18-14-5-7-16(25-26(14)9-24-19(18)27)28-15-6-4-10(22)8-13(15)23/h1-9H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222098
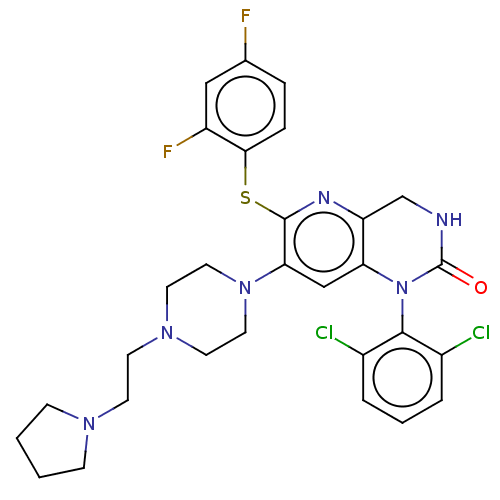
(CHEMBL93161)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CCN(CCN3CCCC3)CC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C29H30Cl2F2N6OS/c30-20-4-3-5-21(31)27(20)39-24-17-25(38-14-12-37(13-15-38)11-10-36-8-1-2-9-36)28(35-23(24)18-34-29(39)40)41-26-7-6-19(32)16-22(26)33/h3-7,16-17H,1-2,8-15,18H2,(H,34,40) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222096
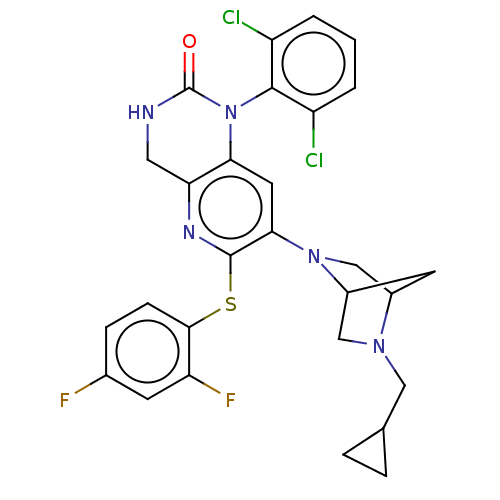
(CHEMBL92648)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CC3CC2CN3CC2CC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C28H25Cl2F2N5OS/c29-19-2-1-3-20(30)26(19)37-23-10-24(36-14-17-9-18(36)13-35(17)12-15-4-5-15)27(34-22(23)11-33-28(37)38)39-25-7-6-16(31)8-21(25)32/h1-3,6-8,10,15,17-18H,4-5,9,11-14H2,(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222108

(CHEMBL93028)Show SMILES CCN1CC2CC1CN2c1cc2N(C(=O)NCc2nc1Sc1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C26H23Cl2F2N5OS/c1-2-33-12-16-9-15(33)13-34(16)22-10-21-20(32-25(22)37-23-7-6-14(29)8-19(23)30)11-31-26(36)35(21)24-17(27)4-3-5-18(24)28/h3-8,10,15-16H,2,9,11-13H2,1H3,(H,31,36) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421127

(CHEMBL2088582)Show SMILES COC(=O)c1ccc(F)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(41.55,3.13,;41.54,4.67,;40.21,5.43,;40.2,6.97,;38.87,4.66,;37.54,5.42,;36.21,4.65,;36.21,3.11,;34.87,2.34,;37.54,2.34,;38.88,3.11,;37.55,.8,;38.88,.04,;40.19,.8,;41.52,.05,;41.53,-1.49,;42.86,-2.26,;42.87,-3.8,;41.54,-4.57,;41.54,-6.11,;42.88,-6.87,;42.89,-8.41,;44.21,-6.09,;44.2,-4.55,;45.53,-3.78,;40.2,-2.26,;38.88,-1.5,;37.54,-2.28,;36.21,-1.51,;36.21,.03,;34.88,.8,)| Show InChI InChI=1S/C22H13F3N2O4/c1-30-22(29)12-2-4-15(24)14(10-12)21-17-5-7-20(26-27(17)9-8-18(21)28)31-19-6-3-13(23)11-16(19)25/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222094

(CHEMBL96305)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CC3CC2CN3)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C24H19Cl2F2N5OS/c25-15-2-1-3-16(26)22(15)33-19-8-20(32-11-13-7-14(32)9-29-13)23(31-18(19)10-30-24(33)34)35-21-5-4-12(27)6-17(21)28/h1-6,8,13-14,29H,7,9-11H2,(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50317582
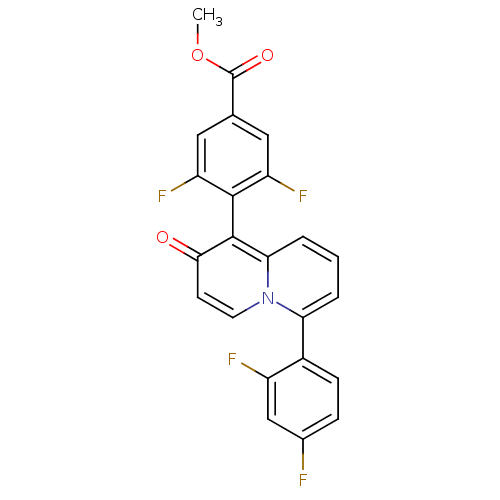
(CHEMBL1096830 | methyl 4-(6-(2,4-difluorophenyl)-2...)Show SMILES COC(=O)c1cc(F)c(c(F)c1)-c1c2cccc(-c3ccc(F)cc3F)n2ccc1=O |(27.64,-9.08,;27.64,-10.62,;26.31,-11.39,;24.97,-10.62,;26.31,-12.93,;27.65,-13.7,;27.65,-15.24,;28.98,-16.01,;26.31,-16.01,;24.98,-15.25,;23.65,-16.02,;24.97,-13.71,;26.31,-17.55,;27.64,-18.33,;28.96,-17.56,;30.29,-18.32,;30.29,-19.86,;28.96,-20.63,;28.96,-22.17,;27.62,-22.94,;27.62,-24.48,;28.96,-25.25,;28.96,-26.79,;30.29,-24.47,;30.29,-22.94,;31.62,-22.16,;27.63,-19.86,;26.31,-20.63,;24.98,-19.87,;24.98,-18.33,;23.64,-17.56,)| Show InChI InChI=1S/C23H13F4NO3/c1-31-23(30)12-9-16(26)21(17(27)10-12)22-19-4-2-3-18(28(19)8-7-20(22)29)14-6-5-13(24)11-15(14)25/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38alpha after 3 hrs by SPA method |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50317580

(4-(6-(2,4-difluorophenyl)-2-oxo-2H-quinolizin-1-yl...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3c(F)cc(cc3F)C#N)c(=O)ccn12 |(-4.26,-27.22,;-4.26,-25.67,;-5.59,-24.9,;-5.59,-23.36,;-4.25,-22.59,;-2.92,-23.36,;-1.59,-22.59,;-2.92,-24.9,;-4.25,-21.05,;-2.92,-20.29,;-2.92,-18.74,;-4.25,-17.98,;-5.58,-18.75,;-6.91,-17.97,;-6.91,-16.43,;-5.57,-15.66,;-4.23,-16.43,;-5.57,-14.12,;-6.9,-13.35,;-8.24,-14.13,;-8.24,-15.66,;-9.57,-16.44,;-6.91,-11.8,;-6.91,-10.26,;-8.24,-18.75,;-9.57,-17.98,;-8.24,-20.29,;-6.91,-21.05,;-5.58,-20.29,)| Show InChI InChI=1S/C22H10F4N2O/c23-13-4-5-14(15(24)10-13)18-2-1-3-19-22(20(29)6-7-28(18)19)21-16(25)8-12(11-27)9-17(21)26/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38alpha after 3 hrs by SPA method |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222107
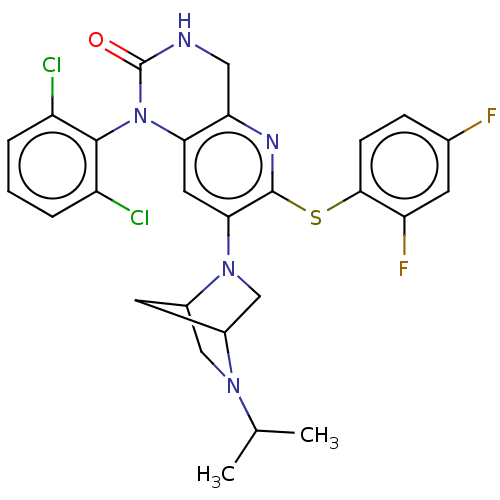
(CHEMBL93650)Show SMILES CC(C)N1CC2CC1CN2c1cc2N(C(=O)NCc2nc1Sc1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C27H25Cl2F2N5OS/c1-14(2)34-12-17-9-16(34)13-35(17)23-10-22-21(33-26(23)38-24-7-6-15(30)8-20(24)31)11-32-27(37)36(22)25-18(28)4-3-5-19(25)29/h3-8,10,14,16-17H,9,11-13H2,1-2H3,(H,32,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421138

(CHEMBL2088589)Show SMILES CCCNC(=O)c1ccc(OC)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(20.21,-42.85,;18.88,-43.63,;17.54,-42.86,;16.21,-43.64,;14.87,-42.87,;14.87,-41.33,;13.54,-43.65,;12.21,-42.89,;10.88,-43.66,;10.88,-45.2,;9.54,-45.97,;8.21,-45.2,;12.21,-45.97,;13.55,-45.2,;12.21,-47.51,;13.54,-48.27,;14.86,-47.51,;16.19,-48.26,;16.2,-49.8,;17.53,-50.56,;17.54,-52.1,;16.2,-52.88,;16.21,-54.41,;17.55,-55.18,;17.55,-56.72,;18.88,-54.4,;18.87,-52.86,;20.2,-52.08,;14.87,-50.57,;13.54,-49.81,;12.21,-50.59,;10.88,-49.81,;10.88,-48.28,;9.54,-47.5,)| Show InChI InChI=1S/C25H21F2N3O4/c1-3-11-28-25(32)15-4-7-21(33-2)17(13-15)24-19-6-9-23(29-30(19)12-10-20(24)31)34-22-8-5-16(26)14-18(22)27/h4-10,12-14H,3,11H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421139
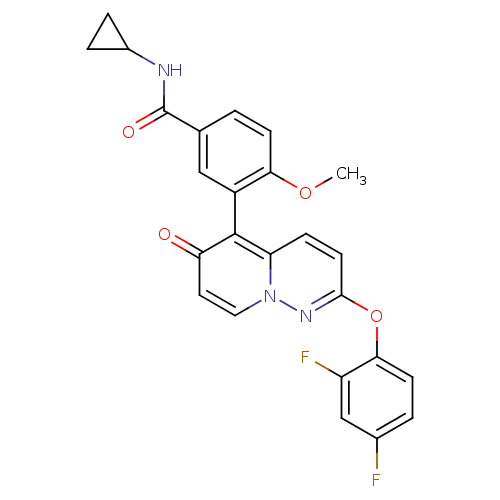
(CHEMBL2088590)Show SMILES COc1ccc(cc1-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O)C(=O)NC1CC1 |(18.95,-46.3,;20.28,-47.07,;21.62,-46.31,;21.62,-44.76,;22.95,-43.99,;24.28,-44.75,;24.29,-46.3,;22.95,-47.08,;22.95,-48.61,;24.28,-49.38,;25.6,-48.61,;26.93,-49.36,;26.94,-50.9,;28.27,-51.67,;28.28,-53.21,;26.94,-53.98,;26.95,-55.52,;28.29,-56.29,;28.3,-57.83,;29.62,-55.5,;29.61,-53.97,;30.94,-53.19,;25.61,-51.67,;24.29,-50.91,;22.95,-51.69,;21.62,-50.92,;21.62,-49.38,;20.29,-48.61,;25.61,-43.98,;25.61,-42.44,;26.95,-44.74,;28.28,-43.97,;29.82,-43.96,;29.04,-42.63,)| Show InChI InChI=1S/C25H19F2N3O4/c1-33-21-7-2-14(25(32)28-16-4-5-16)12-17(21)24-19-6-9-23(29-30(19)11-10-20(24)31)34-22-8-3-15(26)13-18(22)27/h2-3,6-13,16H,4-5H2,1H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222109

(CHEMBL93059)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CCNCC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C23H19Cl2F2N5OS/c24-14-2-1-3-15(25)21(14)32-18-11-19(31-8-6-28-7-9-31)22(30-17(18)12-29-23(32)33)34-20-5-4-13(26)10-16(20)27/h1-5,10-11,28H,6-9,12H2,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222107

(CHEMBL93650)Show SMILES CC(C)N1CC2CC1CN2c1cc2N(C(=O)NCc2nc1Sc1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C27H25Cl2F2N5OS/c1-14(2)34-12-17-9-16(34)13-35(17)23-10-22-21(33-26(23)38-24-7-6-15(30)8-20(24)31)11-32-27(37)36(22)25-18(28)4-3-5-19(25)29/h3-8,10,14,16-17H,9,11-13H2,1-2H3,(H,32,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Mus musculus (mouse)) | BDBM16530
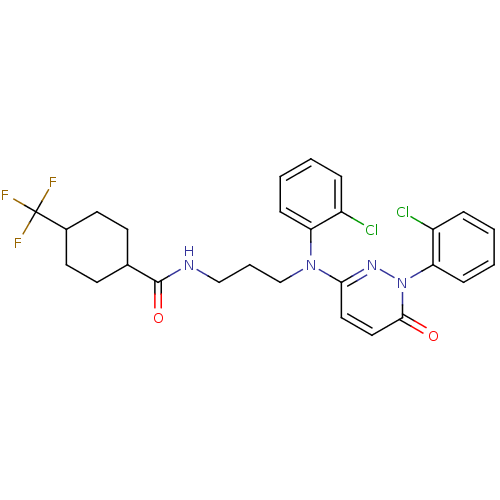
(2-arylpyridazin-3-one, 36 | N-{3-[(2-chlorophenyl)...)Show SMILES FC(F)(F)C1CCC(CC1)C(=O)NCCCN(c1ccc(=O)n(n1)-c1ccccc1Cl)c1ccccc1Cl |(13.52,-3.17,;12.75,-4.5,;14.08,-5.27,;11.98,-5.84,;11.41,-3.73,;11.41,-2.19,;10.08,-1.42,;8.74,-2.19,;8.74,-3.73,;10.08,-4.5,;7.41,-1.42,;7.41,.12,;6.08,-2.19,;4.74,-1.42,;3.41,-2.19,;2.08,-1.42,;.74,-2.19,;-.59,-1.42,;-1.92,-2.19,;-3.26,-1.42,;-3.26,.12,;-4.59,.89,;-1.92,.89,;-.59,.12,;-1.92,2.43,;-.59,3.2,;-.59,4.74,;-1.92,5.51,;-3.26,4.74,;-3.26,3.2,;-4.59,2.43,;.74,-3.73,;-.59,-4.5,;-.59,-6.04,;.74,-6.81,;2.08,-6.04,;2.08,-4.5,;3.41,-3.73,)| Show InChI InChI=1S/C27H27Cl2F3N4O2/c28-20-6-1-3-8-22(20)35(24-14-15-25(37)36(34-24)23-9-4-2-7-21(23)29)17-5-16-33-26(38)18-10-12-19(13-11-18)27(30,31)32/h1-4,6-9,14-15,18-19H,5,10-13,16-17H2,(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222101

(CHEMBL329635)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CCOCC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C23H18Cl2F2N4O2S/c24-14-2-1-3-15(25)21(14)31-18-11-19(30-6-8-33-9-7-30)22(29-17(18)12-28-23(31)32)34-20-5-4-13(26)10-16(20)27/h1-5,10-11H,6-9,12H2,(H,28,32) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222098

(CHEMBL93161)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CCN(CCN3CCCC3)CC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C29H30Cl2F2N6OS/c30-20-4-3-5-21(31)27(20)39-24-17-25(38-14-12-37(13-15-38)11-10-36-8-1-2-9-36)28(35-23(24)18-34-29(39)40)41-26-7-6-19(32)16-22(26)33/h3-7,16-17H,1-2,8-15,18H2,(H,34,40) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421133

(CHEMBL2088575)Show SMILES Fc1ccc(Oc2ccc3c(-c4cc(ccc4C=C)C(=O)NC4CC4)c(=O)ccn3n2)c(F)c1 |(13.19,-41.38,;13.18,-39.84,;11.85,-39.08,;11.84,-37.54,;13.17,-36.77,;13.17,-35.23,;11.83,-34.46,;11.82,-32.92,;10.5,-32.17,;9.18,-32.93,;7.85,-32.17,;7.85,-30.63,;9.18,-29.86,;9.18,-28.31,;7.84,-27.55,;6.52,-28.32,;6.51,-29.86,;5.18,-30.63,;3.85,-29.86,;10.51,-27.54,;10.5,-26,;11.85,-28.3,;13.18,-27.53,;14.72,-27.52,;13.94,-26.19,;6.51,-32.94,;5.18,-32.17,;6.51,-34.48,;7.85,-35.25,;9.18,-34.47,;10.51,-35.23,;14.51,-37.52,;15.84,-36.75,;14.52,-39.06,)| Show InChI InChI=1S/C26H19F2N3O3/c1-2-15-3-4-16(26(33)29-18-6-7-18)13-19(15)25-21-8-10-24(30-31(21)12-11-22(25)32)34-23-9-5-17(27)14-20(23)28/h2-5,8-14,18H,1,6-7H2,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421128
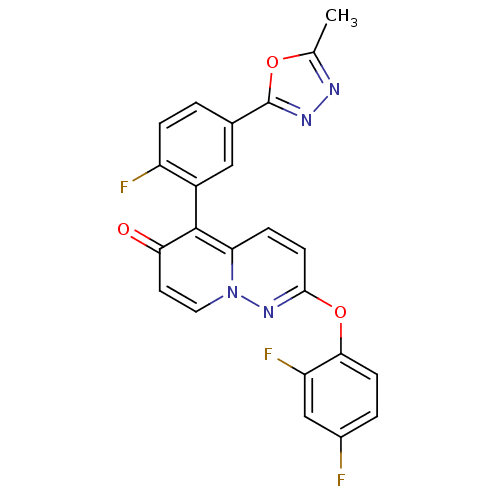
(CHEMBL2088583)Show SMILES Cc1nnc(o1)-c1ccc(F)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(-2.47,-10.49,;-2.95,-11.95,;-2.04,-13.2,;-2.96,-14.44,;-4.42,-13.96,;-4.41,-12.42,;-5.68,-14.86,;-7.01,-14.09,;-8.34,-14.86,;-8.34,-16.41,;-9.68,-17.18,;-7.01,-17.18,;-5.67,-16.41,;-7.01,-18.71,;-5.68,-19.48,;-4.36,-18.71,;-3.03,-19.47,;-3.02,-21.01,;-1.69,-21.77,;-1.68,-23.31,;-3.02,-24.08,;-3.01,-25.62,;-1.67,-26.39,;-1.66,-27.93,;-.34,-25.6,;-.35,-24.07,;.98,-23.29,;-4.35,-21.77,;-5.67,-21.01,;-7.01,-21.79,;-8.34,-21.02,;-8.34,-19.48,;-9.67,-18.71,)| Show InChI InChI=1S/C23H13F3N4O3/c1-12-27-28-23(32-12)13-2-4-16(25)15(10-13)22-18-5-7-21(29-30(18)9-8-19(22)31)33-20-6-3-14(24)11-17(20)26/h2-11H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15241

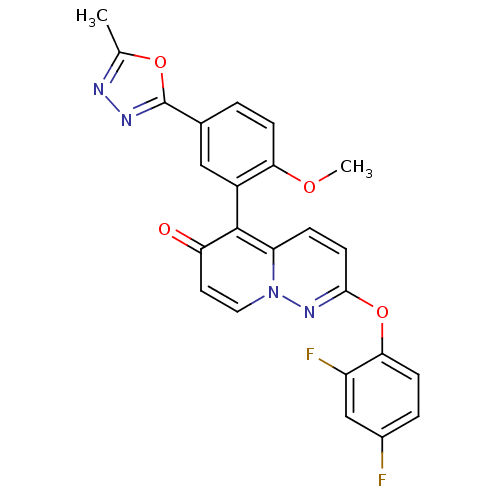
(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)N1CCNCC1 Show InChI InChI=1S/C24H20Cl2F2N4O/c25-19-2-1-3-20(26)23(19)32-22-12-15(31-8-6-29-7-9-31)11-17(18(22)13-30-24(32)33)16-5-4-14(27)10-21(16)28/h1-5,10-12,29H,6-9,13H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38alpha after 3 hrs by SPA method |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222097
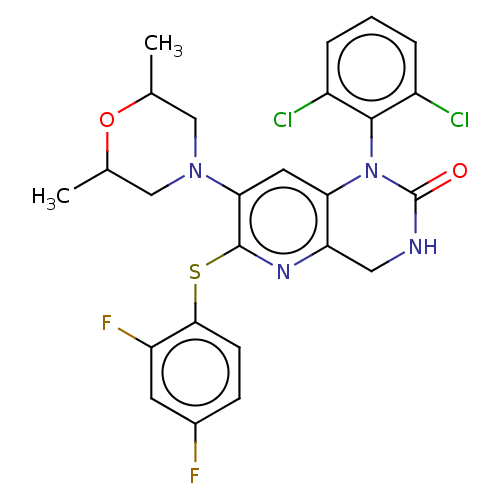
(CHEMBL92851)Show SMILES CC1CN(CC(C)O1)c1cc2N(C(=O)NCc2nc1Sc1ccc(F)cc1F)c1c(Cl)cccc1Cl Show InChI InChI=1S/C25H22Cl2F2N4O2S/c1-13-11-32(12-14(2)35-13)21-9-20-19(31-24(21)36-22-7-6-15(28)8-18(22)29)10-30-25(34)33(20)23-16(26)4-3-5-17(23)27/h3-9,13-14H,10-12H2,1-2H3,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222096

(CHEMBL92648)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CC3CC2CN3CC2CC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C28H25Cl2F2N5OS/c29-19-2-1-3-20(30)26(19)37-23-10-24(36-14-17-9-18(36)13-35(17)12-15-4-5-15)27(34-22(23)11-33-28(37)38)39-25-7-6-16(31)8-21(25)32/h1-3,6-8,10,15,17-18H,4-5,9,11-14H2,(H,33,38) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222084

(CHEMBL327632)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CC3CC2CN3C2CC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C27H23Cl2F2N5OS/c28-18-2-1-3-19(29)25(18)36-22-10-23(35-13-16-9-17(35)12-34(16)15-5-6-15)26(33-21(22)11-32-27(36)37)38-24-7-4-14(30)8-20(24)31/h1-4,7-8,10,15-17H,5-6,9,11-13H2,(H,32,37) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15241

(1-(2,6-dichlorophenyl)-5-(2,4-difluorophenyl)-7-(p...)Show SMILES Fc1ccc(c(F)c1)-c1cc(cc2N(C(=O)NCc12)c1c(Cl)cccc1Cl)N1CCNCC1 Show InChI InChI=1S/C24H20Cl2F2N4O/c25-19-2-1-3-20(26)23(19)32-22-12-15(31-8-6-29-7-9-31)11-17(18(22)13-30-24(32)33)16-5-4-14(27)10-21(16)28/h1-5,10-12,29H,6-9,13H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421132
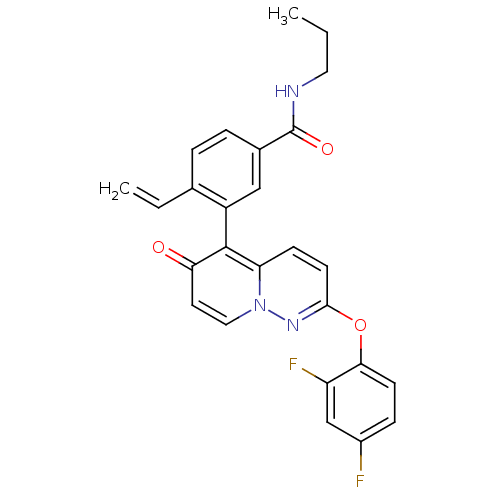
(CHEMBL2088579)Show SMILES CCCNC(=O)c1ccc(C=C)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(1.15,-29.44,;-.18,-30.21,;-1.51,-29.45,;-2.85,-30.22,;-4.18,-29.46,;-4.19,-27.92,;-5.51,-30.23,;-6.85,-29.47,;-8.18,-30.24,;-8.18,-31.78,;-9.51,-32.55,;-10.85,-31.78,;-6.84,-32.56,;-5.51,-31.78,;-6.84,-34.09,;-5.51,-34.86,;-4.2,-34.09,;-2.87,-34.84,;-2.86,-36.38,;-1.52,-37.15,;-1.52,-38.69,;-2.85,-39.46,;-2.85,-41,;-1.51,-41.76,;-1.5,-43.3,;-.17,-40.98,;-.19,-39.45,;1.14,-38.67,;-4.19,-37.15,;-5.51,-36.39,;-6.85,-37.17,;-8.18,-36.4,;-8.18,-34.86,;-9.51,-34.09,)| Show InChI InChI=1S/C26H21F2N3O3/c1-3-12-29-26(33)17-6-5-16(4-2)19(14-17)25-21-8-10-24(30-31(21)13-11-22(25)32)34-23-9-7-18(27)15-20(23)28/h4-11,13-15H,2-3,12H2,1H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222110
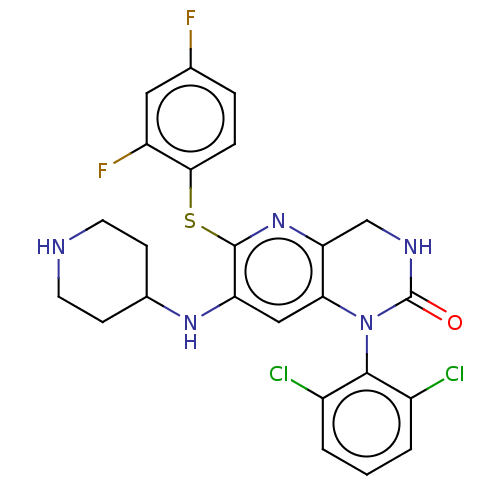
(CHEMBL96629)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2NC2CCNCC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C24H21Cl2F2N5OS/c25-15-2-1-3-16(26)22(15)33-20-11-18(31-14-6-8-29-9-7-14)23(32-19(20)12-30-24(33)34)35-21-5-4-13(27)10-17(21)28/h1-5,10-11,14,29,31H,6-9,12H2,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222100

(CHEMBL329005)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CCCCC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C24H20Cl2F2N4OS/c25-15-5-4-6-16(26)22(15)32-19-12-20(31-9-2-1-3-10-31)23(30-18(19)13-29-24(32)33)34-21-8-7-14(27)11-17(21)28/h4-8,11-12H,1-3,9-10,13H2,(H,29,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM15243

(1-(2,6-dichlorophenyl)-6-[(2,4-difluorophenyl)sulf...)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2C2=CCNCC2)c2c(Cl)cccc2Cl)c(F)c1 |t:19| Show InChI InChI=1S/C24H18Cl2F2N4OS/c25-16-2-1-3-17(26)22(16)32-20-11-15(13-6-8-29-9-7-13)23(31-19(20)12-30-24(32)33)34-21-5-4-14(27)10-18(21)28/h1-6,10-11,29H,7-9,12H2,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with wild-type or mutant p38alpha enzymes, and substrates in the presence ATP/[gam... |
Nat Struct Biol 10: 764-9 (2003)
Article DOI: 10.1038/nsb949
BindingDB Entry DOI: 10.7270/Q2SX6BF9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421125
(CHEMBL2088586)Show SMILES Fc1ccc(Oc2ccc3c(-c4c(F)cccc4F)c(=O)ccn3n2)c(F)c1 |(-3.31,-9.21,;-3.32,-7.66,;-4.65,-6.9,;-4.66,-5.36,;-3.32,-4.59,;-3.33,-3.04,;-4.67,-2.28,;-4.68,-.74,;-6,.02,;-7.32,-.75,;-8.65,.01,;-8.66,1.55,;-9.99,2.32,;-11.34,1.55,;-9.99,3.87,;-8.66,4.64,;-7.32,3.88,;-7.32,2.32,;-5.98,1.56,;-9.99,-.75,;-11.33,.02,;-9.99,-2.29,;-8.66,-3.07,;-7.32,-2.28,;-5.99,-3.05,;-1.99,-5.34,;-.66,-4.56,;-1.98,-6.88,)| Show InChI InChI=1S/C20H10F4N2O2/c21-11-4-6-17(14(24)10-11)28-18-7-5-15-20(16(27)8-9-26(15)25-18)19-12(22)2-1-3-13(19)23/h1-10H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50222109

(CHEMBL93059)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CCNCC2)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C23H19Cl2F2N5OS/c24-14-2-1-3-15(25)21(14)32-18-11-19(31-8-6-28-7-9-31)22(30-17(18)12-29-23(32)33)34-20-5-4-13(26)10-16(20)27/h1-5,10-11,28H,6-9,12H2,(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Anti human TNF-alpha activity determined through TNF-alpha release was measured in the supernatants by ELISA |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM16524
(2-arylpyridazin-3-one, 30 | 2-{3-[(2-chlorophenyl)...)Show SMILES Clc1ccccc1N(CCCN1C(=O)c2ccccc2C1=O)c1ccc(=O)n(n1)-c1ccccc1Cl Show InChI InChI=1S/C27H20Cl2N4O3/c28-20-10-3-5-12-22(20)31(16-7-17-32-26(35)18-8-1-2-9-19(18)27(32)36)24-14-15-25(34)33(30-24)23-13-6-4-11-21(23)29/h1-6,8-15H,7,16-17H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.0 | 30 |
Merck Research Laboratories
| Assay Description
The biochemical activity of compounds was determined by incubation with p38 enzyme, and substrates in the presence ATP/[gamma-33P] ATP. After the rea... |
Bioorg Med Chem Lett 16: 5809-13 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.074
BindingDB Entry DOI: 10.7270/Q28P5XSC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 13
(Mus musculus) | BDBM50222093

(CHEMBL178031)Show SMILES Fc1ccc(Sc2nc3CNC(=O)N(c3cc2N2CC3CCC2C3)c2c(Cl)cccc2Cl)c(F)c1 Show InChI InChI=1S/C25H20Cl2F2N4OS/c26-16-2-1-3-17(27)23(16)33-20-10-21(32-12-13-4-6-15(32)8-13)24(31-19(20)11-30-25(33)34)35-22-7-5-14(28)9-18(22)29/h1-3,5,7,9-10,13,15H,4,6,8,11-12H2,(H,30,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory activity against mouse Mitogen-activated protein kinase p38 |
Bioorg Med Chem Lett 13: 273-6 (2003)
BindingDB Entry DOI: 10.7270/Q2BZ688J |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50317584

(1-(2,6-difluoro-4-(1,3,4-oxadiazol-2-yl)phenyl)-6-...)Show SMILES Fc1ccc(c(F)c1)-c1cccc2c(-c3c(F)cc(cc3F)-c3nnco3)c(=O)ccn12 |(13.24,-49.18,;13.24,-47.64,;11.91,-46.86,;11.91,-45.32,;13.25,-44.56,;14.58,-45.32,;15.91,-44.55,;14.58,-46.86,;13.25,-43.02,;14.58,-42.25,;14.57,-40.71,;13.25,-39.95,;11.92,-40.71,;10.59,-39.94,;10.59,-38.4,;11.93,-37.63,;13.26,-38.4,;11.93,-36.09,;10.6,-35.32,;9.26,-36.1,;9.26,-37.63,;7.93,-38.41,;10.6,-33.77,;11.84,-32.86,;11.37,-31.4,;9.83,-31.4,;9.35,-32.87,;9.26,-40.71,;7.93,-39.95,;9.26,-42.25,;10.59,-43.02,;11.92,-42.25,)| Show InChI InChI=1S/C23H11F4N3O2/c24-13-4-5-14(15(25)10-13)18-2-1-3-19-22(20(31)6-7-30(18)19)21-16(26)8-12(9-17(21)27)23-29-28-11-32-23/h1-11H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38alpha after 3 hrs by SPA method |
Bioorg Med Chem Lett 20: 2765-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.03.069
BindingDB Entry DOI: 10.7270/Q23T9HCC |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Mus musculus (mouse)) | BDBM50421134
(CHEMBL2088576)Show SMILES CCCNC(=O)c1ccc(CC)c(c1)-c1c2ccc(Oc3ccc(F)cc3F)nn2ccc1=O |(28.02,-27.74,;26.69,-28.52,;25.36,-27.75,;24.03,-28.53,;22.69,-27.76,;22.68,-26.22,;21.36,-28.54,;20.02,-27.77,;18.7,-28.54,;18.69,-30.09,;17.36,-30.86,;16.03,-30.09,;20.03,-30.86,;21.36,-30.09,;20.03,-32.4,;21.36,-33.16,;22.68,-32.39,;24,-33.15,;24.01,-34.69,;25.35,-35.45,;25.35,-36.99,;24.02,-37.76,;24.03,-39.3,;25.36,-40.07,;25.37,-41.61,;26.7,-39.29,;26.69,-37.75,;28.02,-36.97,;22.69,-35.46,;21.36,-34.69,;20.03,-35.47,;18.69,-34.7,;18.69,-33.16,;17.36,-32.39,)| Show InChI InChI=1S/C26H23F2N3O3/c1-3-12-29-26(33)17-6-5-16(4-2)19(14-17)25-21-8-10-24(30-31(21)13-11-22(25)32)34-23-9-7-18(27)15-20(23)28/h5-11,13-15H,3-4,12H2,1-2H3,(H,29,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of mouse p38apha assessed as reduction in GST-ATF2 substrate phosphorylation by SPA assay |
Bioorg Med Chem Lett 22: 5979-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.035
BindingDB Entry DOI: 10.7270/Q28P61S0 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11/12/13/14
(Homo sapiens (Human)) | BDBM50122920
(6-[2-(2,3-Dichloro-phenyl)-imidazo[1,2-a]pyridin-3...)Show SMILES Cc1ccccc1-n1nc(ccc1=O)-c1c(nc2ccccn12)-c1cccc(Cl)c1Cl Show InChI InChI=1S/C24H16Cl2N4O/c1-15-7-2-3-10-19(15)30-21(31)13-12-18(28-30)24-23(16-8-6-9-17(25)22(16)26)27-20-11-4-5-14-29(20)24/h2-14H,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of Tumor necrosis factor alpha release from LPS-induced THP-1 cells by ELISA |
J Med Chem 46: 349-52 (2003)
Article DOI: 10.1021/jm025585h
BindingDB Entry DOI: 10.7270/Q27P8XRR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data