 Found 2040 hits with Last Name = 'fitzgerald' and Initial = 'm'
Found 2040 hits with Last Name = 'fitzgerald' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prothrombin
(Homo sapiens (Human)) | BDBM50123504
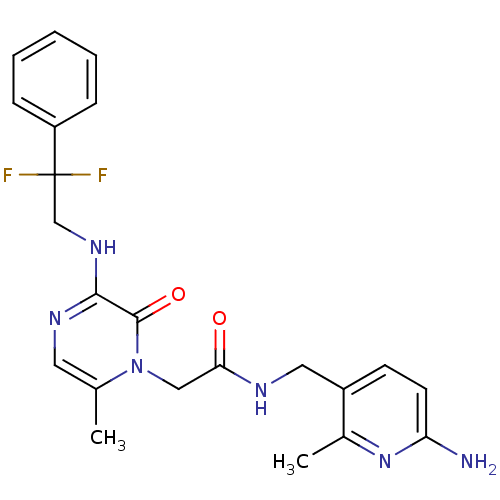
(CHEMBL142546 | N-((6-amino-2-methylpyridin-3-yl)me...)Show SMILES Cc1cnc(NCC(F)(F)c2ccccc2)c(=O)n1CC(=O)NCc1ccc(N)nc1C Show InChI InChI=1S/C22H24F2N6O2/c1-14-10-27-20(28-13-22(23,24)17-6-4-3-5-7-17)21(32)30(14)12-19(31)26-11-16-8-9-18(25)29-15(16)2/h3-10H,11-13H2,1-2H3,(H2,25,29)(H,26,31)(H,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377618
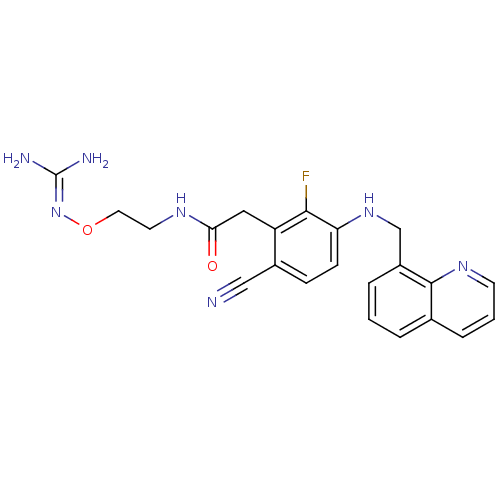
(CHEMBL254353)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]-c2cccc3cccnc23)ccc1C#N Show InChI InChI=1S/C22H22FN7O2/c23-20-17(11-19(31)27-9-10-32-30-22(25)26)15(12-24)6-7-18(20)29-13-16-4-1-3-14-5-2-8-28-21(14)16/h1-8,29H,9-11,13H2,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377625
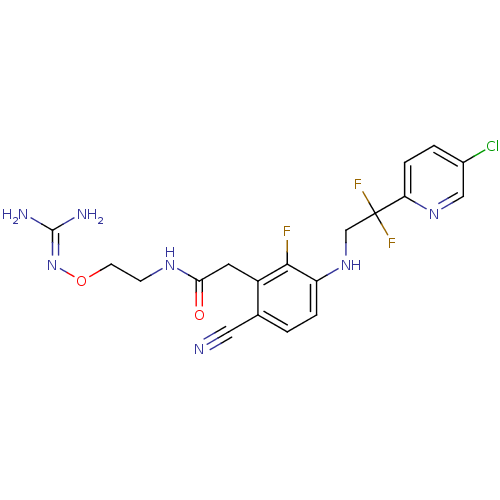
(CHEMBL254557)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccc(Cl)cn2)ccc1C#N Show InChI InChI=1S/C19H19ClF3N7O2/c20-12-2-4-15(28-9-12)19(22,23)10-29-14-3-1-11(8-24)13(17(14)21)7-16(31)27-5-6-32-30-18(25)26/h1-4,9,29H,5-7,10H2,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377615
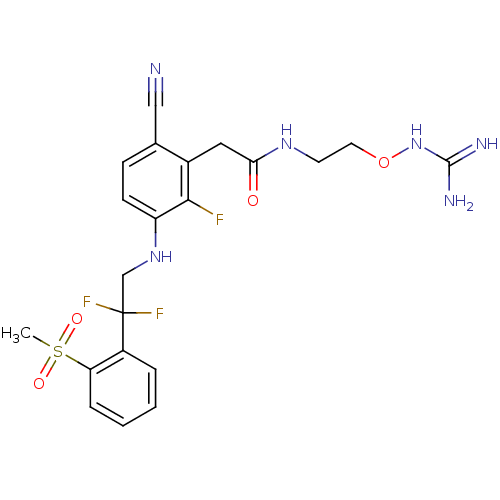
(CHEMBL254962)Show SMILES CS(=O)(=O)c1ccccc1C(F)(F)CNc1ccc(C#N)c(CC(=O)NCCONC(N)=N)c1F Show InChI InChI=1S/C21H23F3N6O4S/c1-35(32,33)17-5-3-2-4-15(17)21(23,24)12-29-16-7-6-13(11-25)14(19(16)22)10-18(31)28-8-9-34-30-20(26)27/h2-7,29H,8-10,12H2,1H3,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377623
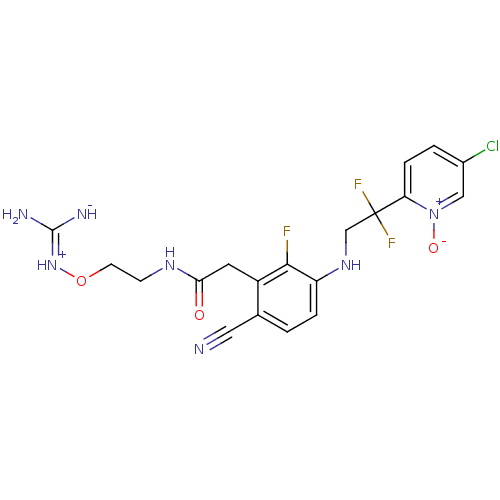
(CHEMBL254759)Show SMILES N\C([NH-])=[NH+]\OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccc(Cl)c[n+]2[O-])ccc1C#N Show InChI InChI=1S/C19H19ClF3N7O3/c20-12-2-4-15(30(32)9-12)19(22,23)10-28-14-3-1-11(8-24)13(17(14)21)7-16(31)27-5-6-33-29-18(25)26/h1-4,9,28H,5-7,10H2,(H5,25,26,27,29,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377611
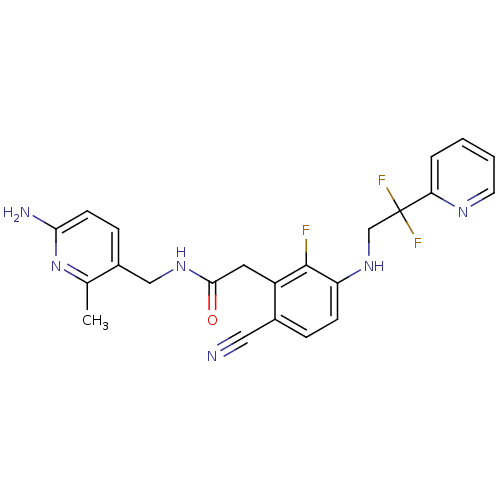
(CHEMBL258018)Show SMILES Cc1nc(N)ccc1CNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C23H21F3N6O/c1-14-16(6-8-20(28)32-14)12-30-21(33)10-17-15(11-27)5-7-18(22(17)24)31-13-23(25,26)19-4-2-3-9-29-19/h2-9,31H,10,12-13H2,1H3,(H2,28,32)(H,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377619
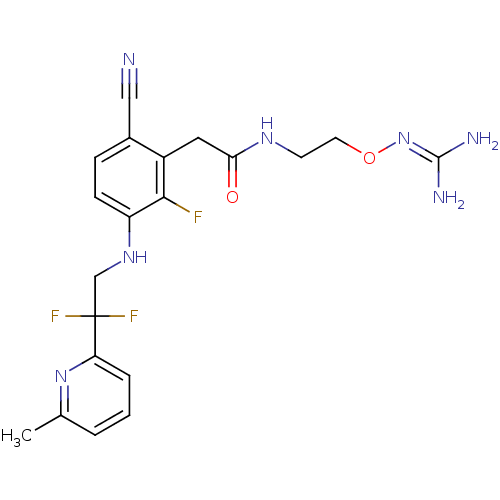
(CHEMBL402758)Show SMILES [#6]-c1cccc(n1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-3-2-4-16(29-12)20(22,23)11-28-15-6-5-13(10-24)14(18(15)21)9-17(31)27-7-8-32-30-19(25)26/h2-6,28H,7-9,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377622
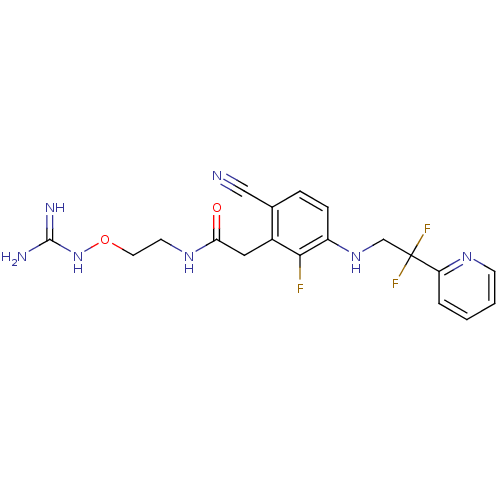
(CHEMBL257543)Show SMILES NC(=N)NOCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C19H20F3N7O2/c20-17-13(9-16(30)27-7-8-31-29-18(24)25)12(10-23)4-5-14(17)28-11-19(21,22)15-3-1-2-6-26-15/h1-6,28H,7-9,11H2,(H,27,30)(H4,24,25,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50377620
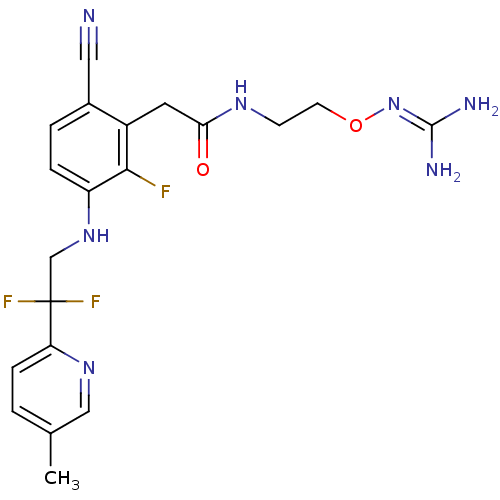
(CHEMBL254784)Show SMILES [#6]-c1ccc(nc1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-2-5-16(28-10-12)20(22,23)11-29-15-4-3-13(9-24)14(18(15)21)8-17(31)27-6-7-32-30-19(25)26/h2-5,10,29H,6-8,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377614
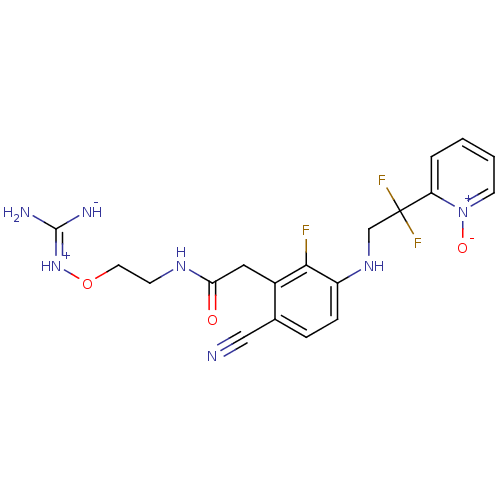
(CHEMBL401655)Show SMILES N\C([NH-])=[NH+]\OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2cccc[n+]2[O-])ccc1C#N Show InChI InChI=1S/C19H20F3N7O3/c20-17-13(9-16(30)26-6-8-32-28-18(24)25)12(10-23)4-5-14(17)27-11-19(21,22)15-3-1-2-7-29(15)31/h1-5,7,27H,6,8-9,11H2,(H5,24,25,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377617
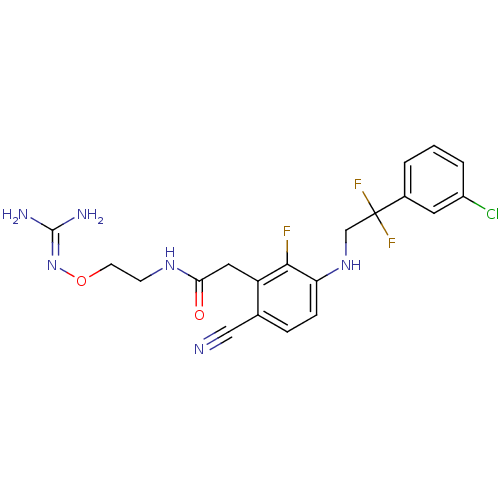
(CHEMBL403359)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2cccc(Cl)c2)ccc1C#N Show InChI InChI=1S/C20H20ClF3N6O2/c21-14-3-1-2-13(8-14)20(23,24)11-29-16-5-4-12(10-25)15(18(16)22)9-17(31)28-6-7-32-30-19(26)27/h1-5,8,29H,6-7,9,11H2,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377624
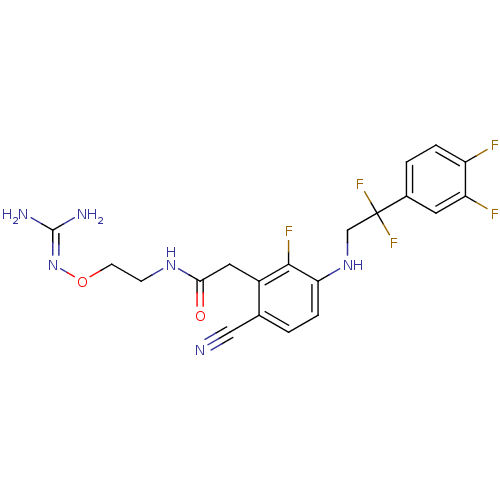
(CHEMBL403310)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccc(F)c(F)c2)ccc1C#N Show InChI InChI=1S/C20H19F5N6O2/c21-14-3-2-12(7-15(14)22)20(24,25)10-30-16-4-1-11(9-26)13(18(16)23)8-17(32)29-5-6-33-31-19(27)28/h1-4,7,30H,5-6,8,10H2,(H,29,32)(H4,27,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377607
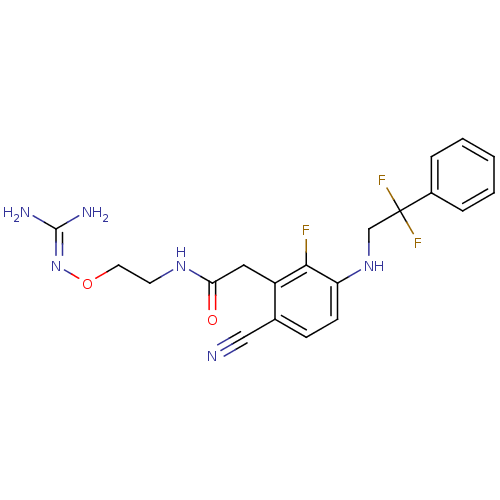
(CHEMBL404025)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2ccccc2)ccc1C#N Show InChI InChI=1S/C20H21F3N6O2/c21-18-15(10-17(30)27-8-9-31-29-19(25)26)13(11-24)6-7-16(18)28-12-20(22,23)14-4-2-1-3-5-14/h1-7,28H,8-10,12H2,(H,27,30)(H4,25,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377626
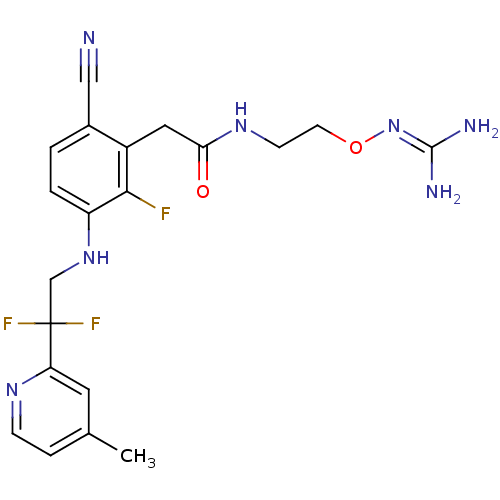
(CHEMBL254786)Show SMILES [#6]-c1ccnc(c1)C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-4-5-27-16(8-12)20(22,23)11-29-15-3-2-13(10-24)14(18(15)21)9-17(31)28-6-7-32-30-19(25)26/h2-5,8,29H,6-7,9,11H2,1H3,(H,28,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377616
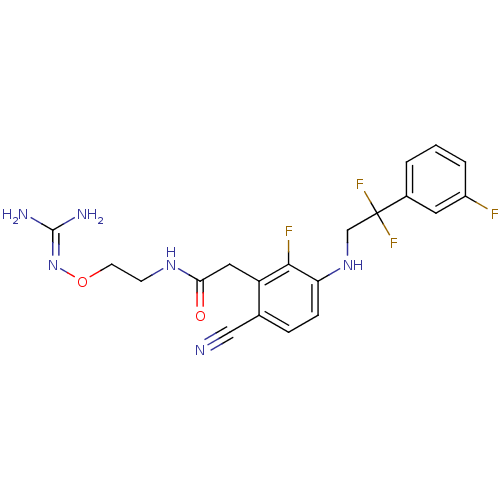
(CHEMBL258198)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(F)c(-[#7]-[#6]C(F)(F)c2cccc(F)c2)ccc1C#N Show InChI InChI=1S/C20H20F4N6O2/c21-14-3-1-2-13(8-14)20(23,24)11-29-16-5-4-12(10-25)15(18(16)22)9-17(31)28-6-7-32-30-19(26)27/h1-5,8,29H,6-7,9,11H2,(H,28,31)(H4,26,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023
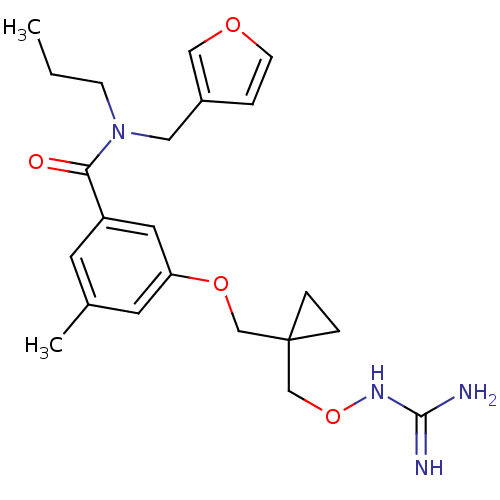
(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377608
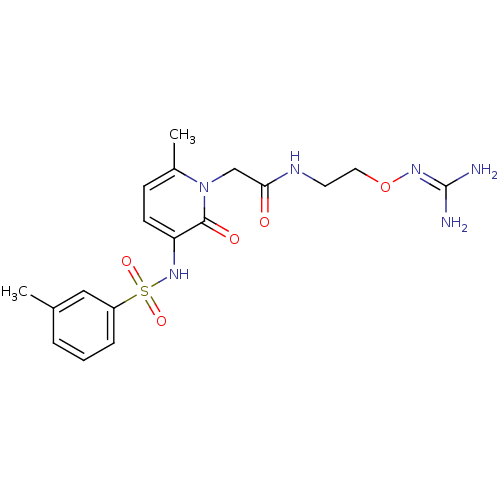
(CHEMBL256941)Show SMILES [#6]-c1cccc(c1)S(=O)(=O)[#7]-c1ccc(-[#6])n(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1=O Show InChI InChI=1S/C18H24N6O5S/c1-12-4-3-5-14(10-12)30(27,28)23-15-7-6-13(2)24(17(15)26)11-16(25)21-8-9-29-22-18(19)20/h3-7,10,23H,8-9,11H2,1-2H3,(H,21,25)(H4,19,20,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50149023

(3-{[1-({[(diaminomethylidene)amino]oxy}methyl)cycl...)Show SMILES CCCN(Cc1ccoc1)C(=O)c1cc(C)cc(OCC2(CONC(N)=N)CC2)c1 Show InChI InChI=1S/C22H30N4O4/c1-3-7-26(12-17-4-8-28-13-17)20(27)18-9-16(2)10-19(11-18)29-14-22(5-6-22)15-30-25-21(23)24/h4,8-11,13H,3,5-7,12,14-15H2,1-2H3,(H4,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377613
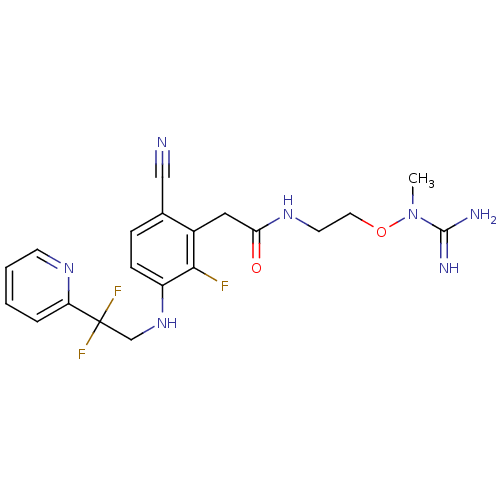
(CHEMBL258225)Show SMILES CN(OCCNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N)C(N)=N Show InChI InChI=1S/C20H22F3N7O2/c1-30(19(25)26)32-9-8-28-17(31)10-14-13(11-24)5-6-15(18(14)21)29-12-20(22,23)16-4-2-3-7-27-16/h2-7,29H,8-10,12H2,1H3,(H3,25,26)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377621
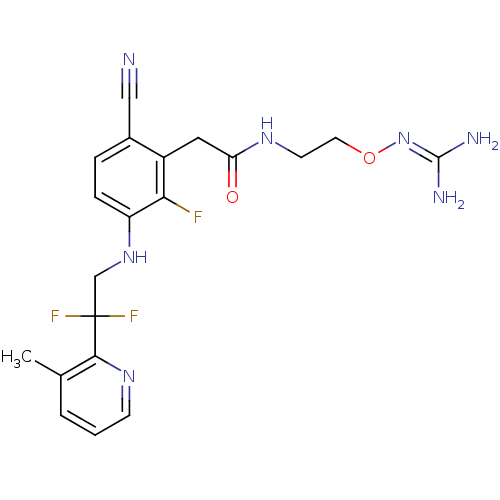
(CHEMBL254785)Show SMILES [#6]-c1cccnc1C(F)(F)[#6]-[#7]-c1ccc(C#N)c(-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#8]\[#7]=[#6](\[#7])-[#7])c1F Show InChI InChI=1S/C20H22F3N7O2/c1-12-3-2-6-28-18(12)20(22,23)11-29-15-5-4-13(10-24)14(17(15)21)9-16(31)27-7-8-32-30-19(25)26/h2-6,29H,7-9,11H2,1H3,(H,27,31)(H4,25,26,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50383265
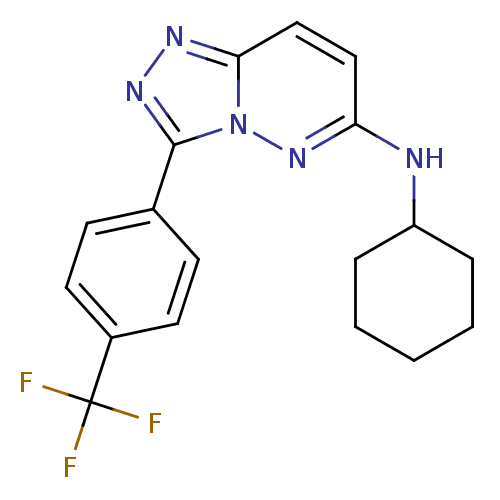
(CHEMBL2032368)Show SMILES FC(F)(F)c1ccc(cc1)-c1nnc2ccc(NC3CCCCC3)nn12 Show InChI InChI=1S/C18H18F3N5/c19-18(20,21)13-8-6-12(7-9-13)17-24-23-16-11-10-15(25-26(16)17)22-14-4-2-1-3-5-14/h6-11,14H,1-5H2,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genzyme Corp.
Curated by ChEMBL
| Assay Description
Inhibition of human Pim-1 using 5-FAM-RSRHSSYPAGT-CONH2 as substrate preincubated for 15 mins prior substrate addition measured after 45 mins by fluo... |
J Med Chem 55: 2641-8 (2012)
Article DOI: 10.1021/jm2014698
BindingDB Entry DOI: 10.7270/Q2Q2418B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377612
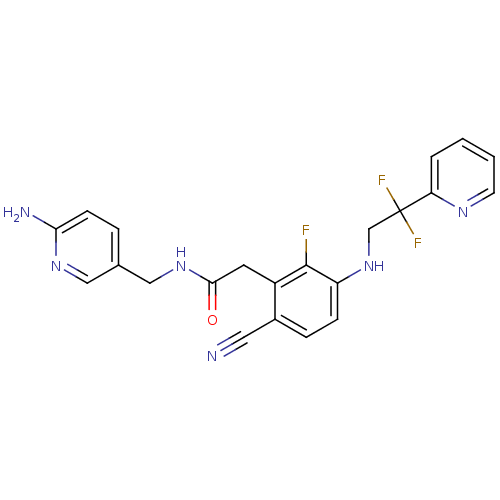
(CHEMBL255916)Show SMILES Nc1ccc(CNC(=O)Cc2c(F)c(NCC(F)(F)c3ccccn3)ccc2C#N)cn1 Show InChI InChI=1S/C22H19F3N6O/c23-21-16(9-20(32)30-12-14-4-7-19(27)29-11-14)15(10-26)5-6-17(21)31-13-22(24,25)18-3-1-2-8-28-18/h1-8,11,31H,9,12-13H2,(H2,27,29)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377609
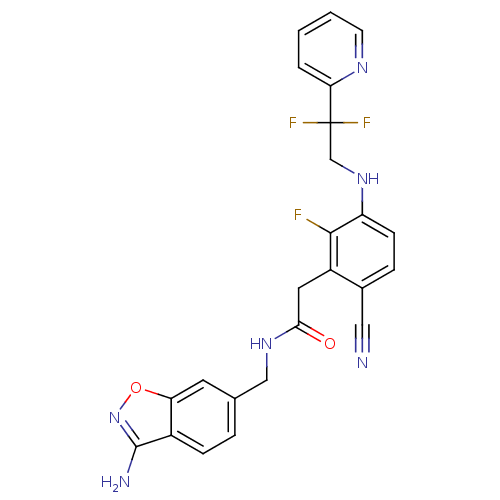
(CHEMBL256091)Show SMILES Nc1noc2cc(CNC(=O)Cc3c(F)c(NCC(F)(F)c4ccccn4)ccc3C#N)ccc12 Show InChI InChI=1S/C24H19F3N6O2/c25-22-17(10-21(34)31-12-14-4-6-16-19(9-14)35-33-23(16)29)15(11-28)5-7-18(22)32-13-24(26,27)20-3-1-2-8-30-20/h1-9,32H,10,12-13H2,(H2,29,33)(H,31,34) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50377610
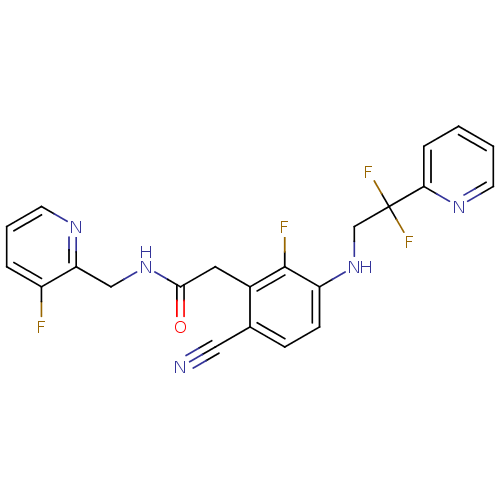
(CHEMBL250551)Show SMILES Fc1cccnc1CNC(=O)Cc1c(F)c(NCC(F)(F)c2ccccn2)ccc1C#N Show InChI InChI=1S/C22H17F4N5O/c23-16-4-3-9-28-18(16)12-30-20(32)10-15-14(11-27)6-7-17(21(15)24)31-13-22(25,26)19-5-1-2-8-29-19/h1-9,31H,10,12-13H2,(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50223072
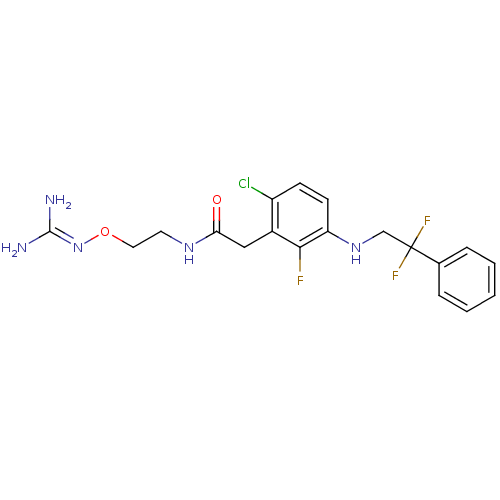
(CHEMBL401842 | N-[2-(carbamimidamidooxy)ethyl]-2-{...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#8]-[#6]-[#6]-[#7]-[#6](=O)-[#6]-c1c(Cl)ccc(-[#7]-[#6]C(F)(F)c2ccccc2)c1F Show InChI InChI=1S/C19H21ClF3N5O2/c20-14-6-7-15(27-11-19(22,23)12-4-2-1-3-5-12)17(21)13(14)10-16(29)26-8-9-30-28-18(24)25/h1-7,27H,8-11H2,(H,26,29)(H4,24,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to human thrombin |
Bioorg Med Chem Lett 18: 2865-70 (2008)
Article DOI: 10.1016/j.bmcl.2008.03.087
BindingDB Entry DOI: 10.7270/Q2FJ2HP3 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50005480
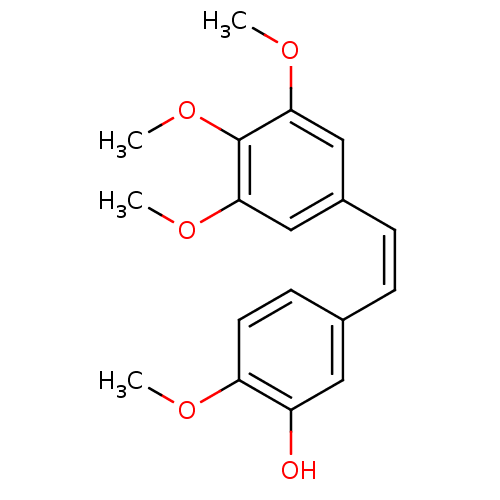
((-)-combretastatin | (Z)-3'-hydroxy-3,4,4',5-tetra...)Show InChI InChI=1S/C18H20O5/c1-20-15-8-7-12(9-14(15)19)5-6-13-10-16(21-2)18(23-4)17(11-13)22-3/h5-11,19H,1-4H3/b6-5- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109343
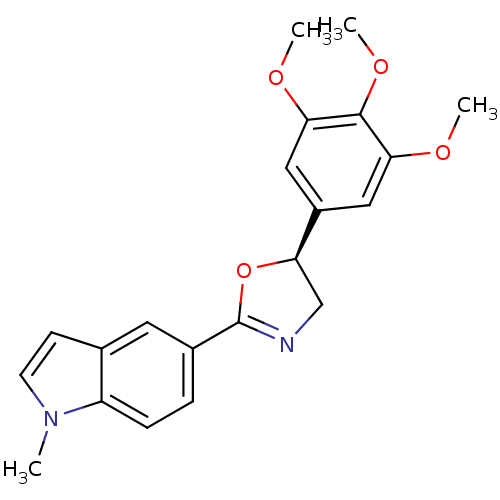
(1-Methyl-5-[(S)-5-(3,4,5-trimethoxy-phenyl)-4,5-di...)Show SMILES COc1cc(cc(OC)c1OC)[C@H]1CN=C(O1)c1ccc2n(C)ccc2c1 |c:15| Show InChI InChI=1S/C21H22N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)21-22-12-19(27-21)15-10-17(24-2)20(26-4)18(11-15)25-3/h5-11,19H,12H2,1-4H3/t19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50014846
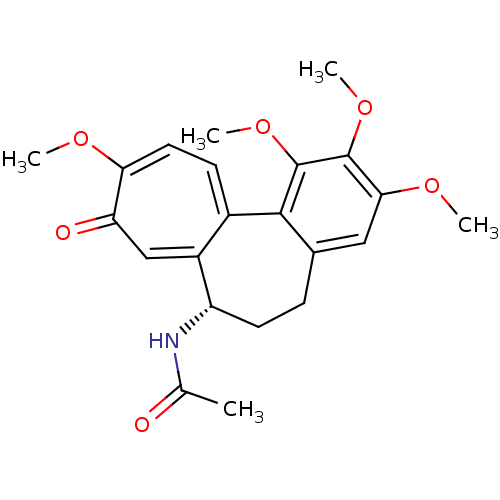
((S)-N-(5,6,7,9-tetrahydro-1,2,3,10-tetramethoxy-9-...)Show SMILES COc1cc2CC[C@H](NC(C)=O)c3cc(=O)c(OC)ccc3-c2c(OC)c1OC |r| Show InChI InChI=1S/C22H25NO6/c1-12(24)23-16-8-6-13-10-19(27-3)21(28-4)22(29-5)20(13)14-7-9-18(26-2)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| 780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tubulin beta-2B chain
(Bos taurus) | BDBM50109342
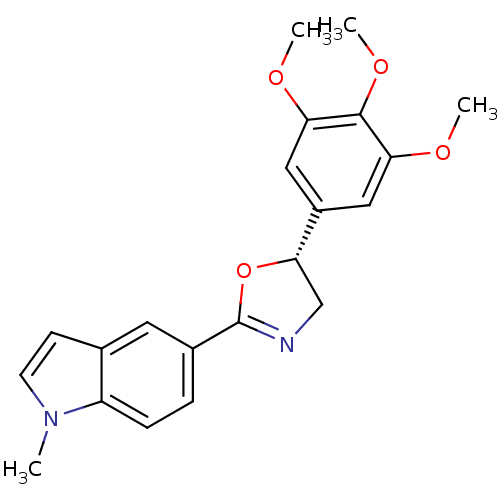
(1-Methyl-5-[(R)-5-(3,4,5-trimethoxy-phenyl)-4,5-di...)Show SMILES COc1cc(cc(OC)c1OC)[C@@H]1CN=C(O1)c1ccc2n(C)ccc2c1 |c:15| Show InChI InChI=1S/C21H22N2O4/c1-23-8-7-13-9-14(5-6-16(13)23)21-22-12-19(27-21)15-10-17(24-2)20(26-4)18(11-15)25-3/h5-11,19H,12H2,1-4H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | |
Tubulin beta-2B chain
(Bos taurus) | BDBM50012278
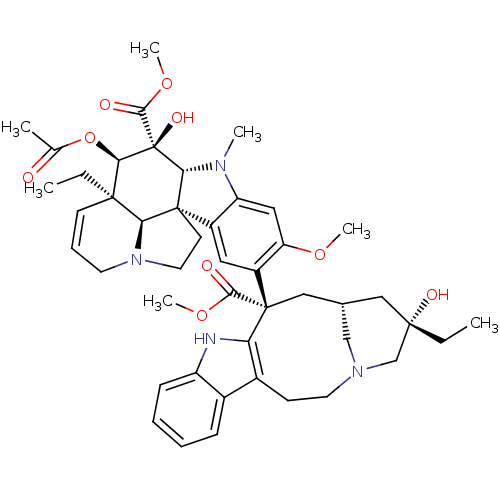
((2ALPHA,2''BETA,3BETA,4ALPHA,5BETA)-VINCALEUKOBLAS...)Show SMILES CC[C@]1(O)C[C@@H]2CN(C1)CCc1c([nH]c3ccccc13)[C@@](C2)(C(=O)OC)c1cc2c(cc1OC)N(C)[C@@H]1[C@]22CCN3CC=C[C@](CC)([C@@H]23)[C@@H](OC(C)=O)[C@]1(O)C(=O)OC |r,c:48| Show InChI InChI=1S/C46H58N4O9/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3/t28-,37-,38+,39+,42-,43+,44+,45-,46-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories
Curated by ChEMBL
| Assay Description
Binding constant at colchicine site of bovine brain tubulin |
Bioorg Med Chem Lett 12: 465-9 (2002)
BindingDB Entry DOI: 10.7270/Q2PC32W7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605778
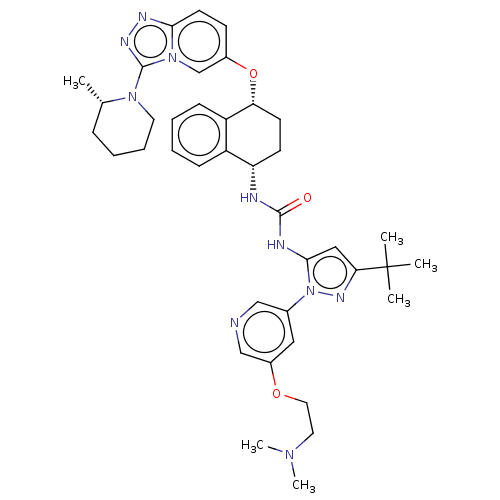
(CHEMBL5186216)Show SMILES C[C@H]1CCCCN1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cncc(OCCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM894
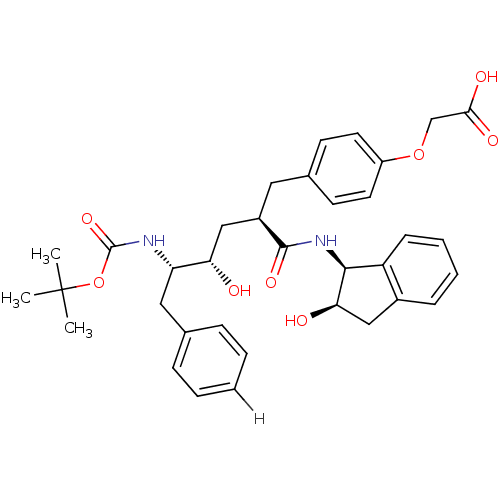
(2-{4-[(2R)-2-[(2S,3S)-3-{[(tert-butoxy)carbonyl]am...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(OCC(O)=O)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H42N2O8/c1-35(2,3)45-34(43)36-28(18-22-9-5-4-6-10-22)29(38)20-25(17-23-13-15-26(16-14-23)44-21-31(40)41)33(42)37-32-27-12-8-7-11-24(27)19-30(32)39/h4-16,25,28-30,32,38-39H,17-21H2,1-3H3,(H,36,43)(H,37,42)(H,40,41)/t25-,28+,29+,30-,32+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 35: 1685-701 (1992)
Article DOI: 10.1021/jm00088a003
BindingDB Entry DOI: 10.7270/Q2JH3JCF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM9291
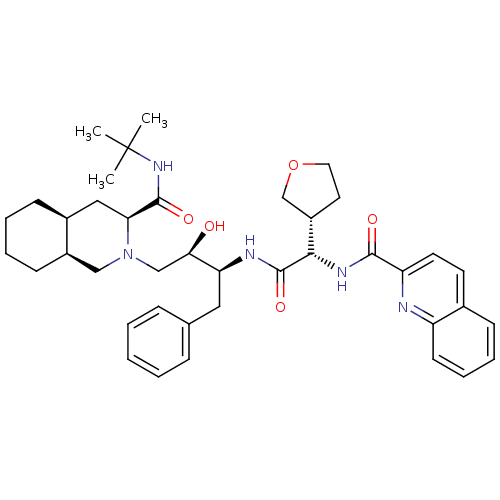
((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...)Show SMILES CC(C)(C)NC(=O)[C@@H]1C[C@@H]2CCCC[C@@H]2CN1C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)c1ccc2ccccc2n1)[C@H]1CCOC1 |r| Show InChI InChI=1S/C40H53N5O5/c1-40(2,3)44-38(48)34-22-28-14-7-8-15-29(28)23-45(34)24-35(46)33(21-26-11-5-4-6-12-26)42-39(49)36(30-19-20-50-25-30)43-37(47)32-18-17-27-13-9-10-16-31(27)41-32/h4-6,9-13,16-18,28-30,33-36,46H,7-8,14-15,19-25H2,1-3H3,(H,42,49)(H,43,47)(H,44,48)/t28-,29+,30-,33-,34-,35+,36-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605782

(CHEMBL5174953)Show SMILES C[C@H]1CCC[C@@H](C)N1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cnn(CCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605750
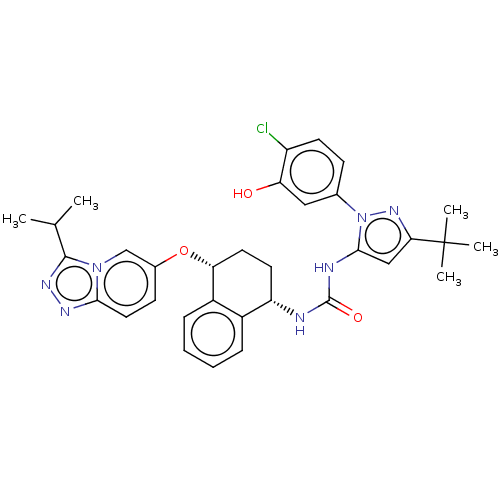
(CHEMBL5172781)Show SMILES CC(C)c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4ccc(Cl)c(O)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM383
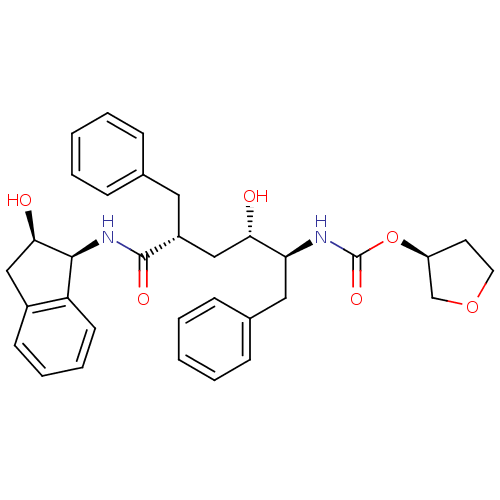
((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 |r| Show InChI InChI=1S/C33H38N2O6/c36-29(28(18-23-11-5-2-6-12-23)34-33(39)41-26-15-16-40-21-26)20-25(17-22-9-3-1-4-10-22)32(38)35-31-27-14-8-7-13-24(27)19-30(31)37/h1-14,25-26,28-31,36-37H,15-21H2,(H,34,39)(H,35,38)/t25-,26+,28+,29+,30-,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of HIV-1 protease was determined in vitro |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605751

(CHEMBL5180443)Show SMILES CC(C)c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4ccccc4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM383

((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...)Show SMILES O[C@@H](C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1 |r| Show InChI InChI=1S/C33H38N2O6/c36-29(28(18-23-11-5-2-6-12-23)34-33(39)41-26-15-16-40-21-26)20-25(17-22-9-3-1-4-10-22)32(38)35-31-27-14-8-7-13-24(27)19-30(31)37/h1-14,25-26,28-31,36-37H,15-21H2,(H,34,39)(H,35,38)/t25-,26+,28+,29+,30-,31+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory activity against Human immunodeficiency virus (HIV-1) protease |
Bioorg Med Chem Lett 4: 499-504 (1994)
Article DOI: 10.1016/0960-894X(94)80025-1
BindingDB Entry DOI: 10.7270/Q2GM8777 |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM891
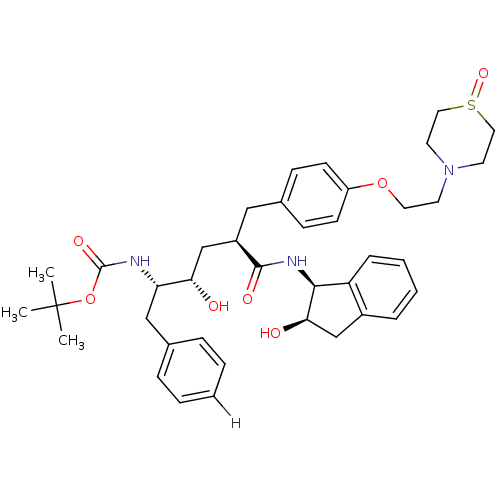
(L-685,434 deriv. 39 | N-(2(R)-Hydroxy-1(S)-indanyl...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(OCCN2CCS(=O)CC2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C39H51N3O7S/c1-39(2,3)49-38(46)40-33(24-27-9-5-4-6-10-27)34(43)26-30(37(45)41-36-32-12-8-7-11-29(32)25-35(36)44)23-28-13-15-31(16-14-28)48-20-17-42-18-21-50(47)22-19-42/h4-16,30,33-36,43-44H,17-26H2,1-3H3,(H,40,46)(H,41,45)/t30-,33+,34+,35-,36+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 35: 1685-701 (1992)
Article DOI: 10.1021/jm00088a003
BindingDB Entry DOI: 10.7270/Q2JH3JCF |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605749

(CHEMBL5206397)Show SMILES CC(C)c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4ccc(O)c(Cl)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM892

(L-685,434 deriv. 40 | N-(2(R)-Hydroxy-1(S)-indanyl...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(OCCN2CCS(=O)(=O)CC2)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C39H51N3O8S/c1-39(2,3)50-38(46)40-33(24-27-9-5-4-6-10-27)34(43)26-30(37(45)41-36-32-12-8-7-11-29(32)25-35(36)44)23-28-13-15-31(16-14-28)49-20-17-42-18-21-51(47,48)22-19-42/h4-16,30,33-36,43-44H,17-26H2,1-3H3,(H,40,46)(H,41,45)/t30-,33+,34+,35-,36+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 35: 1685-701 (1992)
Article DOI: 10.1021/jm00088a003
BindingDB Entry DOI: 10.7270/Q2JH3JCF |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM895

(L-685,434 deriv. 44 | N-(2(R)-Hydroxy-1(S)-indanyl...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccc(OCCO)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C35H44N2O7/c1-35(2,3)44-34(42)36-29(20-23-9-5-4-6-10-23)30(39)22-26(19-24-13-15-27(16-14-24)43-18-17-38)33(41)37-32-28-12-8-7-11-25(28)21-31(32)40/h4-16,26,29-32,38-40H,17-22H2,1-3H3,(H,36,42)(H,37,41)/t26-,29+,30+,31-,32+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 35: 1685-701 (1992)
Article DOI: 10.1021/jm00088a003
BindingDB Entry DOI: 10.7270/Q2JH3JCF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM842

(Benzocycloalkyl Amines deriv. 12 | CHEMBL419923 | ...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H]1[C@H](O)[C@H](O)c2ccccc12 |r| Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-26(19-22-14-8-5-9-15-22)27(36)20-23(18-21-12-6-4-7-13-21)31(39)35-28-24-16-10-11-17-25(24)29(37)30(28)38/h4-17,23,26-30,36-38H,18-20H2,1-3H3,(H,34,40)(H,35,39)/t23-,26+,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 11
(Homo sapiens (Human)) | BDBM50605782

(CHEMBL5174953)Show SMILES C[C@H]1CCC[C@@H](C)N1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cnn(CCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605781

(CHEMBL5202475)Show SMILES C[C@H]1CCCCN1c1nnc2ccc(O[C@@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4cnn(CCN(C)C)c4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605753
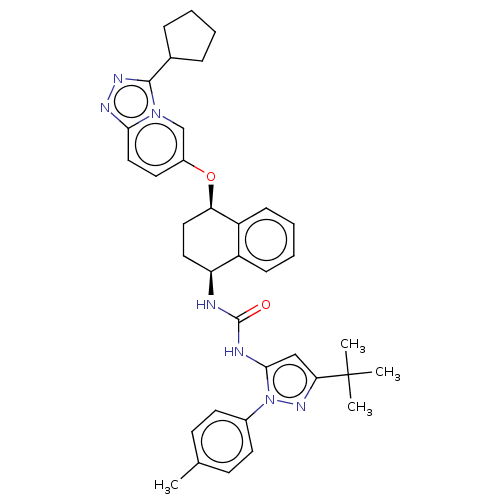
(CHEMBL5200224)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)N[C@H]1CC[C@@H](Oc2ccc3nnc(C4CCCC4)n3c2)c2ccccc12)C(C)(C)C |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50605747
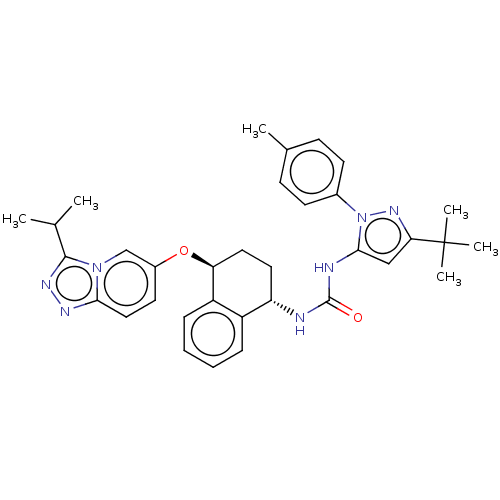
(CHEMBL5173417)Show SMILES CC(C)c1nnc2ccc(O[C@H]3CC[C@H](NC(=O)Nc4cc(nn4-c4ccc(C)cc4)C(C)(C)C)c4ccccc34)cn12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00115
BindingDB Entry DOI: 10.7270/Q2B280DT |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM880
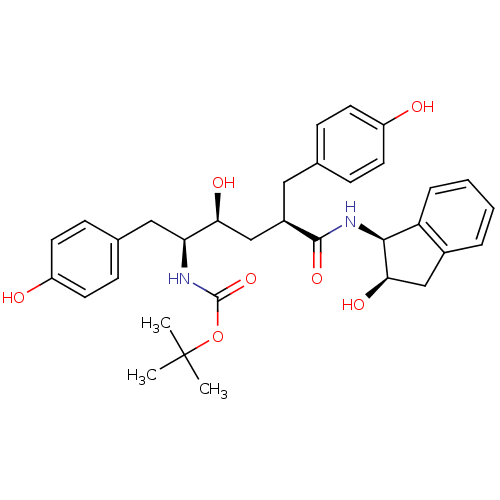
(L-685,434 deriv. 24 | N-(2(R)-Hydroxy-1(S)-indanyl...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccc(O)cc1)[C@@H](O)C[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H]1[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H40N2O7/c1-33(2,3)42-32(41)34-27(17-21-10-14-25(37)15-11-21)28(38)19-23(16-20-8-12-24(36)13-9-20)31(40)35-30-26-7-5-4-6-22(26)18-29(30)39/h4-15,23,27-30,36-39H,16-19H2,1-3H3,(H,34,41)(H,35,40)/t23-,27+,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 35: 1685-701 (1992)
Article DOI: 10.1021/jm00088a003
BindingDB Entry DOI: 10.7270/Q2JH3JCF |
More data for this
Ligand-Target Pair | |
Dimer of Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1046
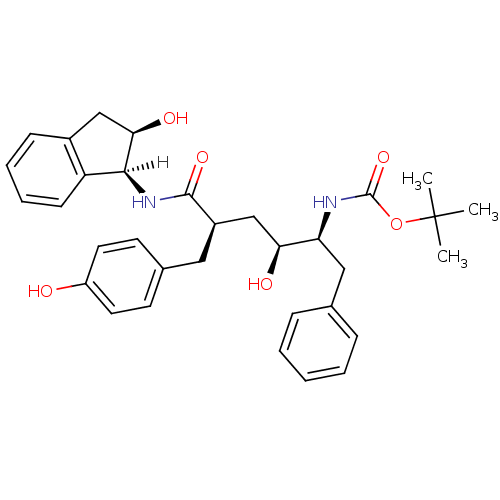
(CHEMBL298316 | L-685,927 | tert-butyl N-[(2S,3S,5R...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(O)cc2)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(36)16-14-22)31(39)35-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,36-38H,17-20H2,1-3H3,(H,34,40)(H,35,39)/t24-,27+,28+,29-,30+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories
| Assay Description
Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... |
J Med Chem 35: 1685-701 (1992)
Article DOI: 10.1021/jm00088a003
BindingDB Entry DOI: 10.7270/Q2JH3JCF |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM1046

(CHEMBL298316 | L-685,927 | tert-butyl N-[(2S,3S,5R...)Show SMILES [H][C@@]1(NC(=O)[C@@H](C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OC(C)(C)C)Cc2ccc(O)cc2)[C@H](O)Cc2ccccc12 |r| Show InChI InChI=1S/C33H40N2O6/c1-33(2,3)41-32(40)34-27(18-21-9-5-4-6-10-21)28(37)20-24(17-22-13-15-25(36)16-14-22)31(39)35-30-26-12-8-7-11-23(26)19-29(30)38/h4-16,24,27-30,36-38H,17-20H2,1-3H3,(H,34,40)(H,35,39)/t24-,27+,28+,29-,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of HIV-1 protease |
J Med Chem 38: 305-17 (1995)
Checked by Author
BindingDB Entry DOI: 10.7270/Q23T9G8M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data