

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM894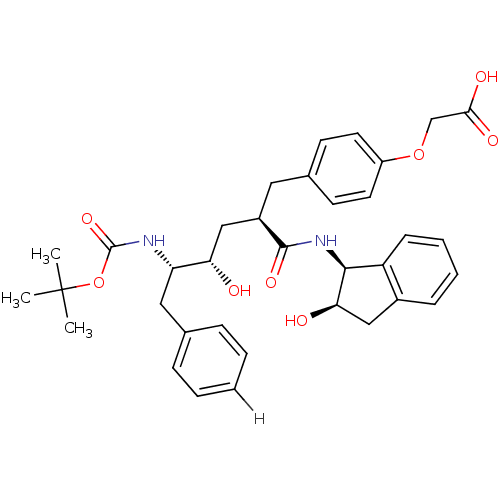 (2-{4-[(2R)-2-[(2S,3S)-3-{[(tert-butoxy)carbonyl]am...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM9291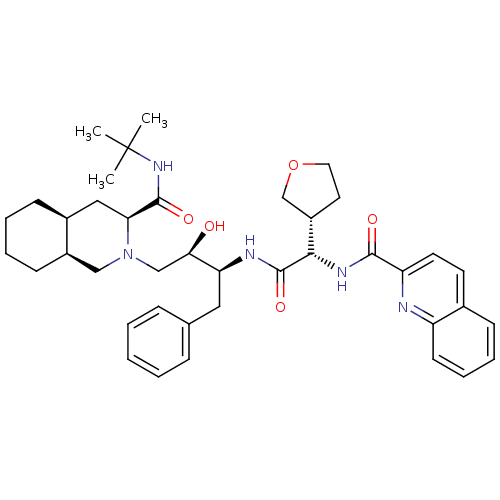 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3S)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383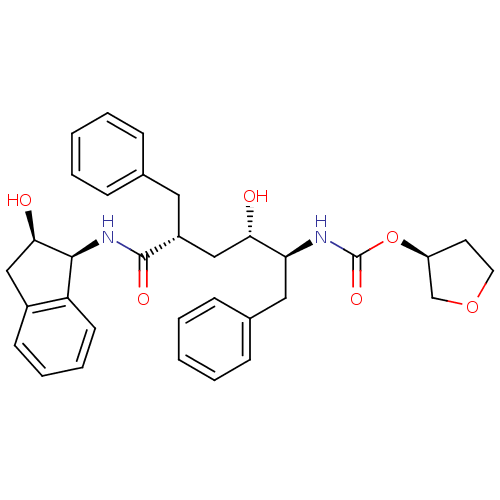 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM383 ((3S)-oxolan-3-yl N-[(2S,3S,5R)-5-benzyl-3-hydroxy-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibitory activity against Human immunodeficiency virus (HIV-1) protease | Bioorg Med Chem Lett 4: 499-504 (1994) Article DOI: 10.1016/0960-894X(94)80025-1 BindingDB Entry DOI: 10.7270/Q2GM8777 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM891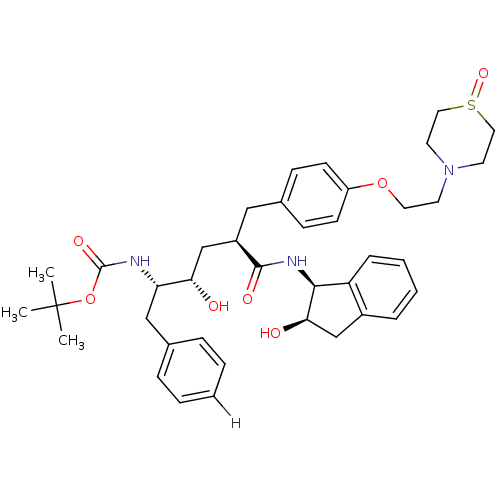 (L-685,434 deriv. 39 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM892 (L-685,434 deriv. 40 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM895 (L-685,434 deriv. 44 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM842 (Benzocycloalkyl Amines deriv. 12 | CHEMBL419923 | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM880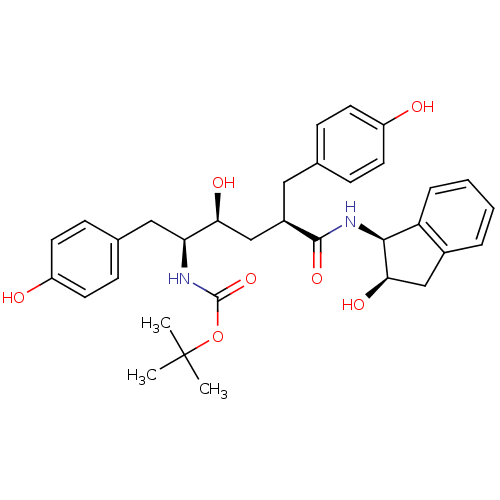 (L-685,434 deriv. 24 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1046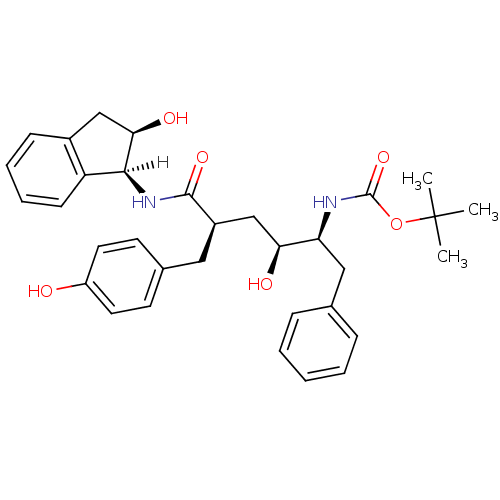 (CHEMBL298316 | L-685,927 | tert-butyl N-[(2S,3S,5R...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1046 (CHEMBL298316 | L-685,927 | tert-butyl N-[(2S,3S,5R...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1039 (CHEMBL297620 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM877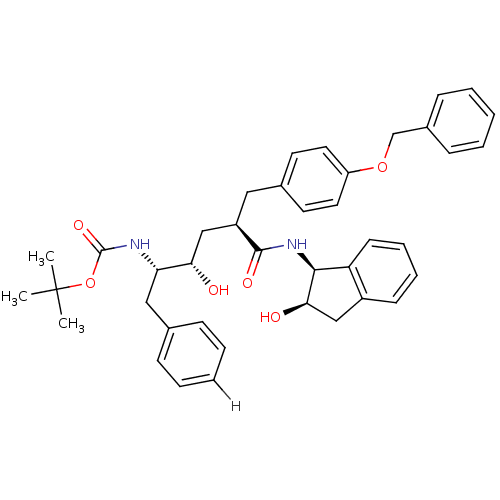 (L-685,434 deriv. 19 | N-(2(R)-Hydroxy-1(S)-indany1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035633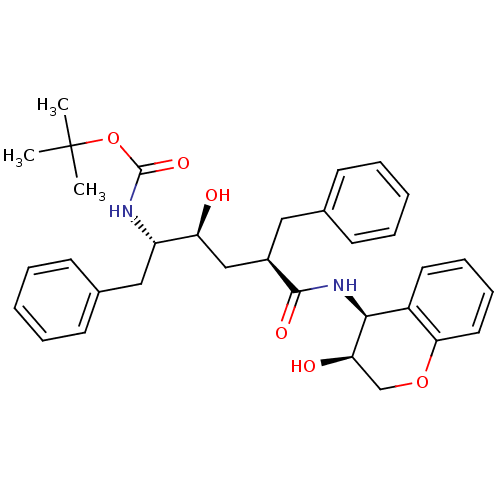 (CHEMBL111143 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035635 (CHEMBL322984 | [(1S,2S,4R)-1-Benzyl-4-((1S,2S,3R)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM888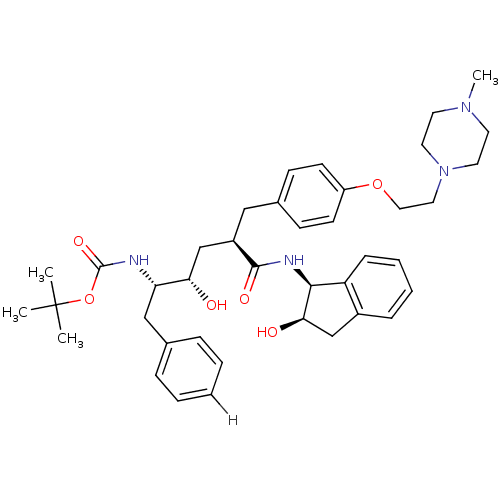 (L-685,434 deriv. 36 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM896 (L-685,434 deriv. 45 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM893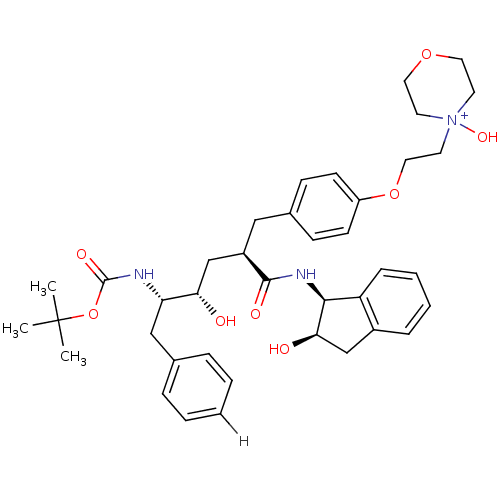 (4-(2-{4-[(2R)-2-[(2S,3S)-3-{[(tert-butoxy)carbonyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1031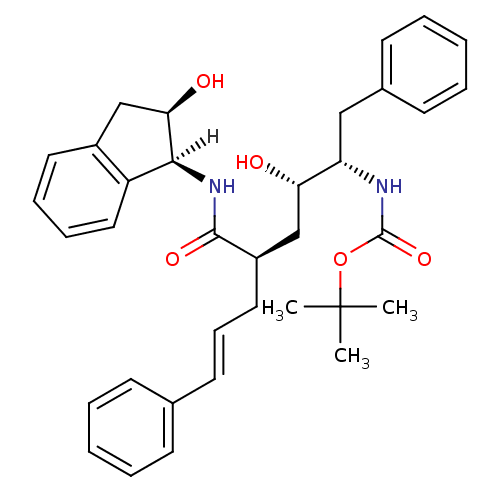 (CHEMBL265514 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50039563 ((R)-N*1*-[(1S,2R)-1-Benzyl-3-((3S,4aS,8aS)-3-tert-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Tested for inhibitory activity against HIV protease enzyme | J Med Chem 37: 2506-8 (1994) BindingDB Entry DOI: 10.7270/Q2WQ02VZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM519 ((2S)-N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarba...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1030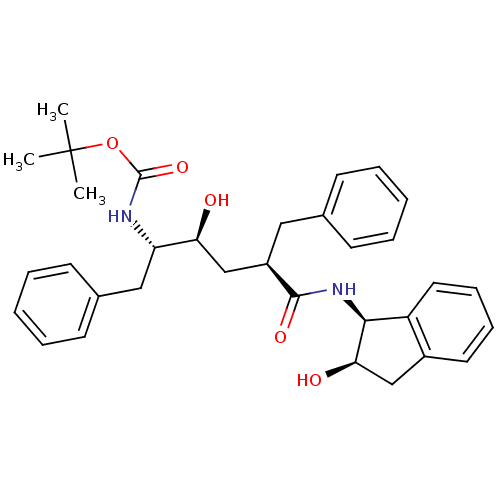 (CHEMBL296115 | L-685,434 | Urethane deriv. 1 | ter...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035630 (CHEMBL108918 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1040 (CHEMBL296484 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1036 (CHEMBL295904 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1038 (CHEMBL265516 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50162799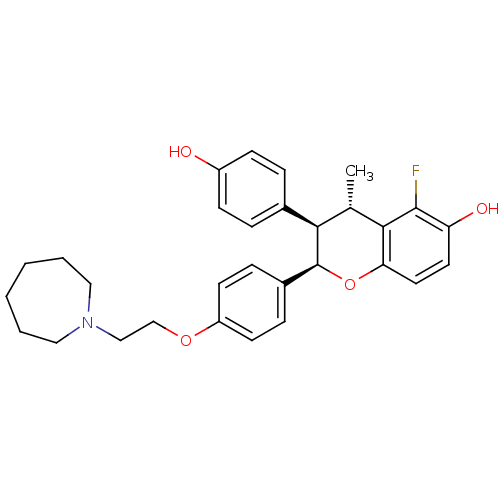 ((2R,3R,4S)-2-[4-(2-Azepan-1-yl-ethoxy)-phenyl]-5-f...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha | Bioorg Med Chem Lett 15: 1675-81 (2005) Article DOI: 10.1016/j.bmcl.2005.01.046 BindingDB Entry DOI: 10.7270/Q2S46RFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157151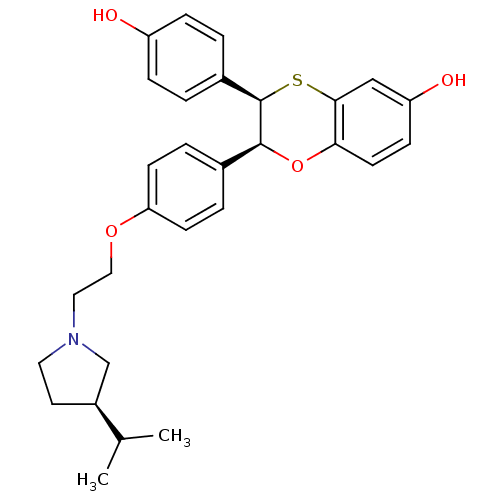 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-(4-{2-[3-((R)-isopr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1037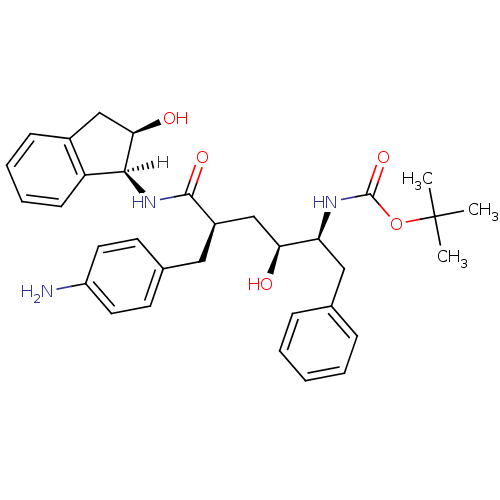 (CHEMBL296493 | Hydroxyethylene dipeptide isostere ...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM879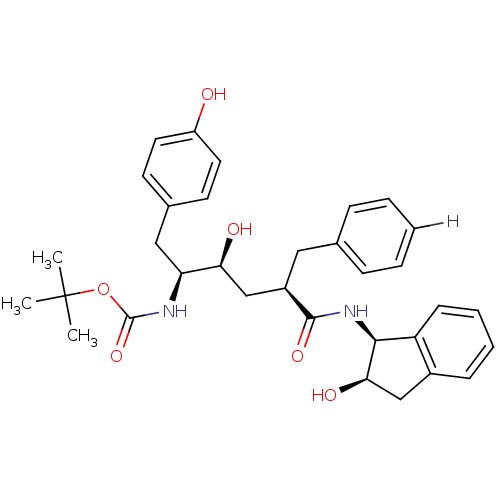 (L-685,434 deriv. 23 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1030 (CHEMBL296115 | L-685,434 | Urethane deriv. 1 | ter...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157160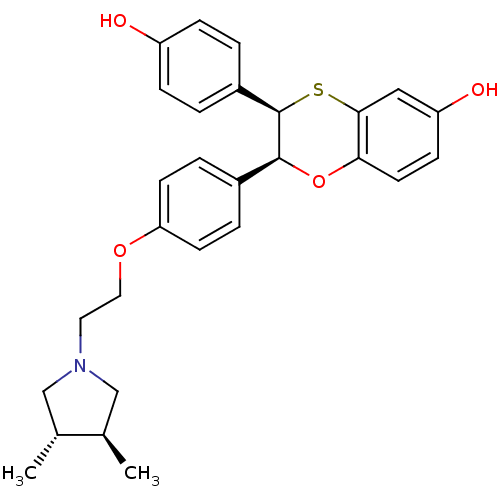 ((2S,3R)-2-(4-{2-[(3S,4S)-3,4-DIMETHYLPYRROLIDIN-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157161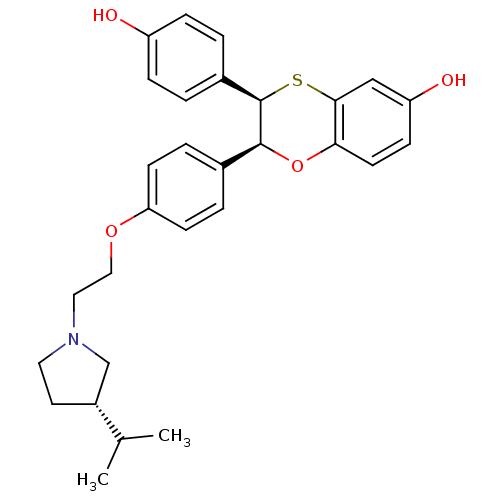 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-(4-{2-[3-((S)-isopr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM881 (L-685,434 derivative | L-689,502 | N-(2(R)-Hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM890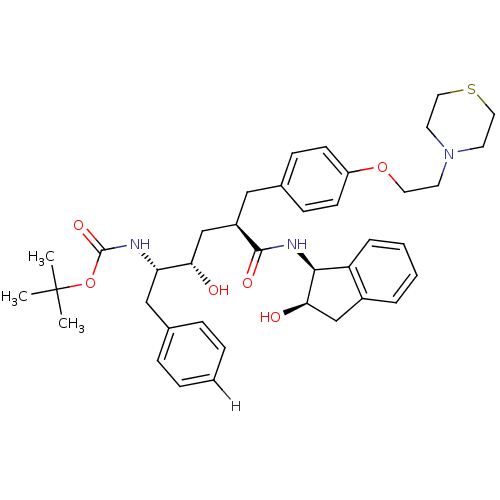 (L-685,434 deriv. 38 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50162804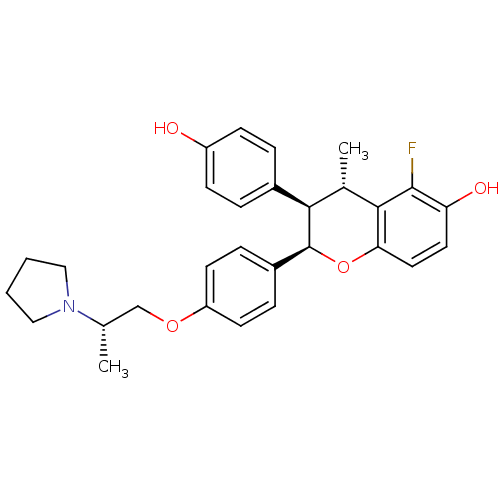 ((2R,3R,4S)-5-Fluoro-3-(4-hydroxy-phenyl)-4-methyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha | Bioorg Med Chem Lett 15: 1675-81 (2005) Article DOI: 10.1016/j.bmcl.2005.01.046 BindingDB Entry DOI: 10.7270/Q2S46RFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157155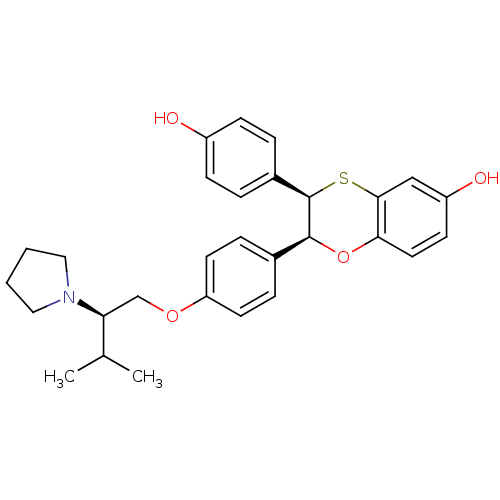 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-[4-((R)-3-methyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157166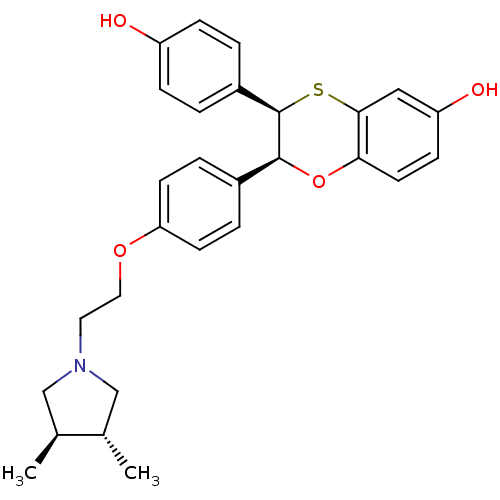 ((2S,3R)-2-(4-{2-[(3R,4R)-3,4-DIMETHYLPYRROLIDIN-1-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157143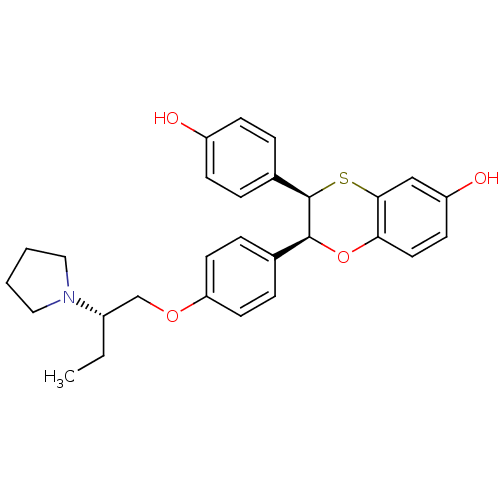 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-[4-((S)-2-pyrrolidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035628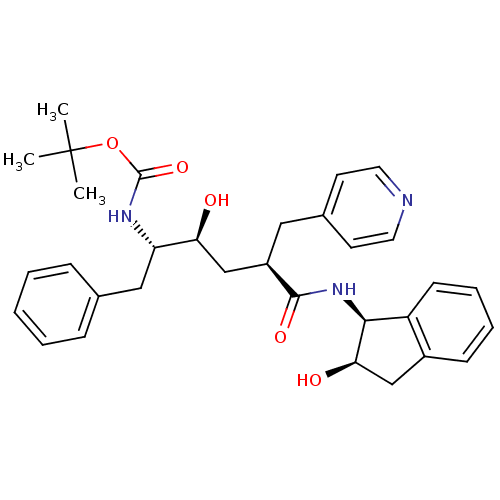 (CHEMBL430692 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM882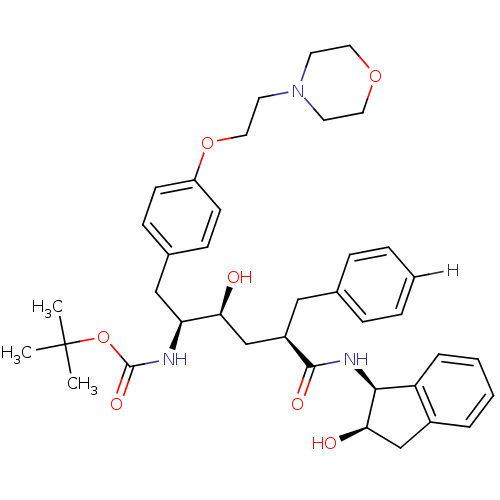 (L-685,434 deriv. 30 | N-(2(R)-Hydroxy-1(S)-indanyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | 5.5 | 30 |
Merck Research Laboratories | Assay Description Sensitivity of HIV-1 protease activity to protease inhibitors was determined by a peptide substrate cleavage assay. Protease products were analyzed o... | J Med Chem 35: 1685-701 (1992) Article DOI: 10.1021/jm00088a003 BindingDB Entry DOI: 10.7270/Q2JH3JCF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM1041 (CHEMBL47514 | Hydroxyethylene dipeptide isostere 3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50162794 ((2R,3R,4S)-3-(4-Hydroxy-phenyl)-4-methyl-2-[4-(2-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha | Bioorg Med Chem Lett 15: 1675-81 (2005) Article DOI: 10.1016/j.bmcl.2005.01.046 BindingDB Entry DOI: 10.7270/Q2S46RFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50422052 (CHEMBL109952 | L-700417) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HIV-1 protease was determined in vitro | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157159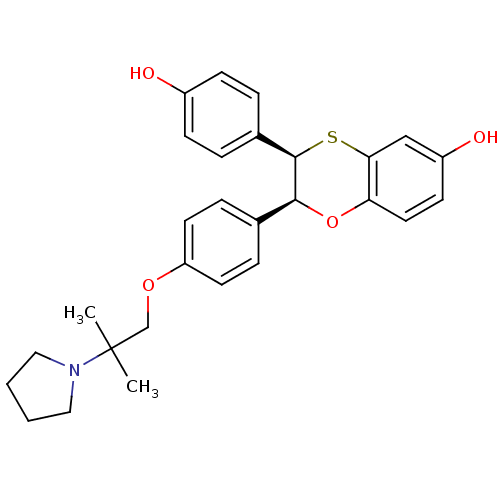 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-[4-(2-methyl-2-pyrr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50035631 (CHEMBL325551 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-4-((...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of HIV-1 protease | J Med Chem 38: 305-17 (1995) Checked by Author BindingDB Entry DOI: 10.7270/Q23T9G8M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157162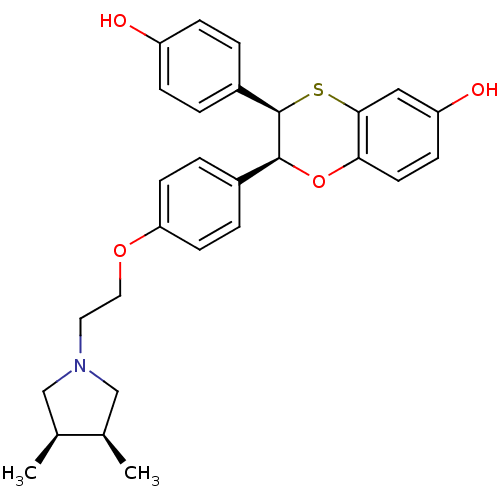 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-{4-[2-((R)-methyl-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50162807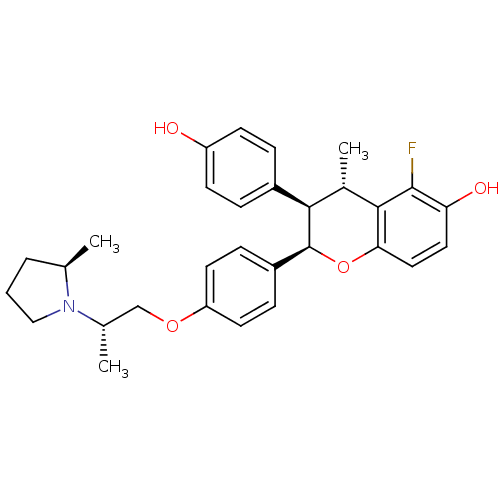 ((2R,3R,4S)-5-Fluoro-3-(4-hydroxy-phenyl)-4-methyl-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of estrogen receptor alpha | Bioorg Med Chem Lett 15: 1675-81 (2005) Article DOI: 10.1016/j.bmcl.2005.01.046 BindingDB Entry DOI: 10.7270/Q2S46RFB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157142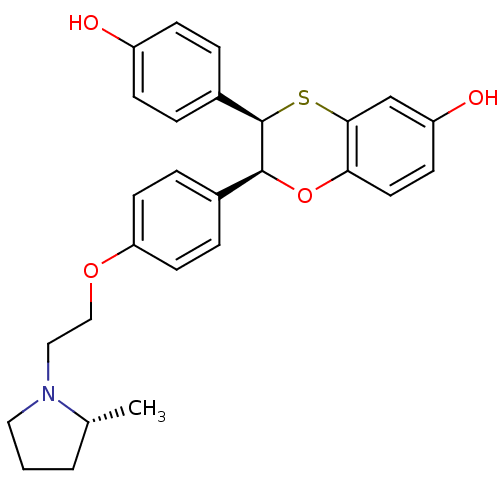 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-{4-[2-((R)-2-methyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50157149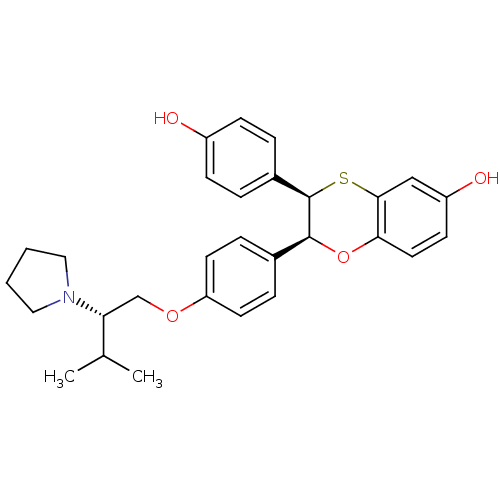 ((2S,3R)-3-(4-Hydroxy-phenyl)-2-[4-((S)-3-methyl-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of bindign to recombinant human estrogen receptor alpha | Bioorg Med Chem Lett 15: 107-13 (2004) Article DOI: 10.1016/j.bmcl.2004.10.036 BindingDB Entry DOI: 10.7270/Q2GM86S0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 530 total ) | Next | Last >> |