 Found 149 hits with Last Name = 'fjellström' and Initial = 'o'
Found 149 hits with Last Name = 'fjellström' and Initial = 'o' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447515
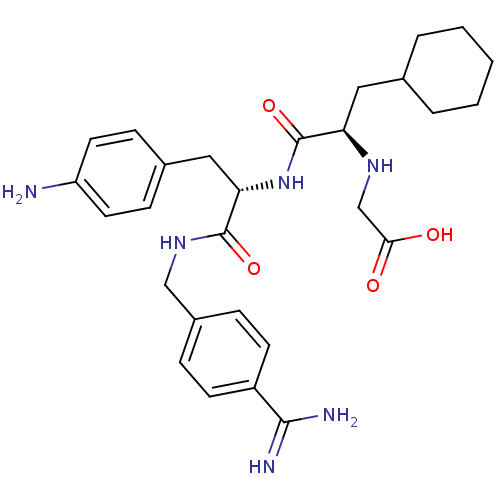
(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447528
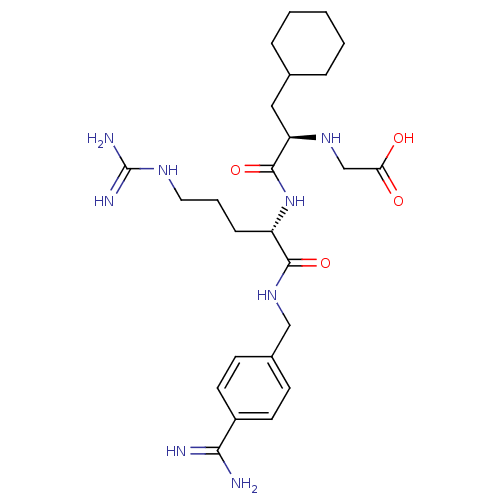
(CHEMBL3115904)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C25H40N8O4/c26-22(27)18-10-8-17(9-11-18)14-32-23(36)19(7-4-12-30-25(28)29)33-24(37)20(31-15-21(34)35)13-16-5-2-1-3-6-16/h8-11,16,19-20,31H,1-7,12-15H2,(H3,26,27)(H,32,36)(H,33,37)(H,34,35)(H4,28,29,30)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447516
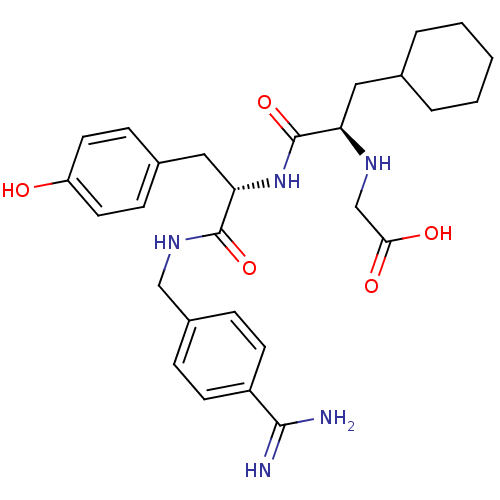
(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447523
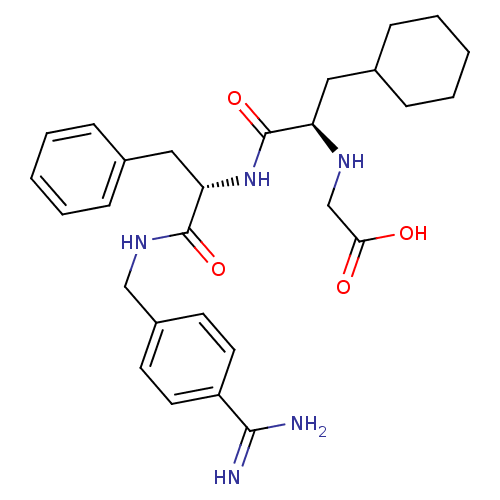
(CHEMBL3115897)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O4/c29-26(30)22-13-11-21(12-14-22)17-32-27(36)24(16-20-9-5-2-6-10-20)33-28(37)23(31-18-25(34)35)15-19-7-3-1-4-8-19/h2,5-6,9-14,19,23-24,31H,1,3-4,7-8,15-18H2,(H3,29,30)(H,32,36)(H,33,37)(H,34,35)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447515

(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447517
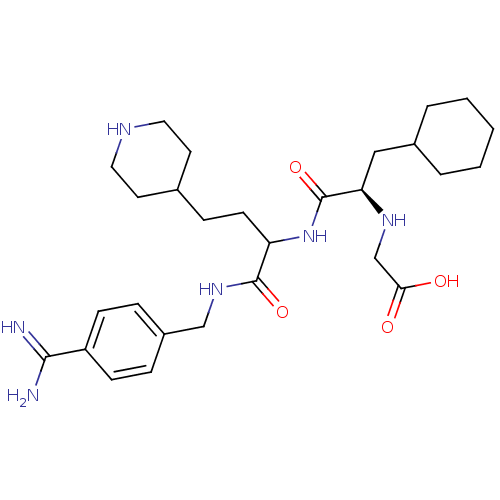
(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447521
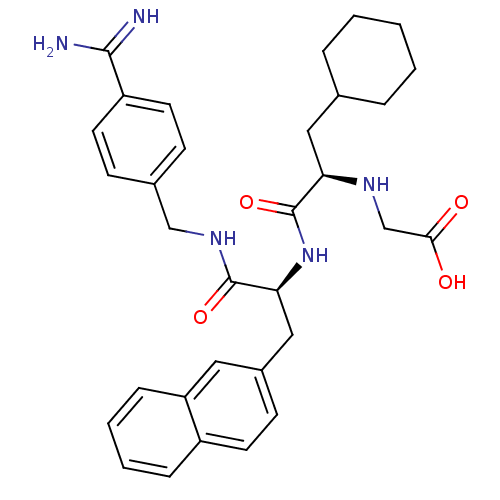
(CHEMBL3115899)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)25-14-10-22(11-15-25)19-36-31(40)28(18-23-12-13-24-8-4-5-9-26(24)16-23)37-32(41)27(35-20-29(38)39)17-21-6-2-1-3-7-21/h4-5,8-16,21,27-28,35H,1-3,6-7,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447514
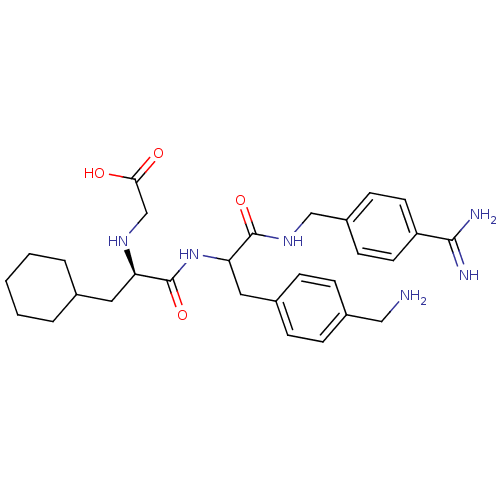
(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447522

(CHEMBL3115898)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)24-15-13-22(14-16-24)19-36-31(40)28(18-25-11-6-10-23-9-4-5-12-26(23)25)37-32(41)27(35-20-29(38)39)17-21-7-2-1-3-8-21/h4-6,9-16,21,27-28,35H,1-3,7-8,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447525
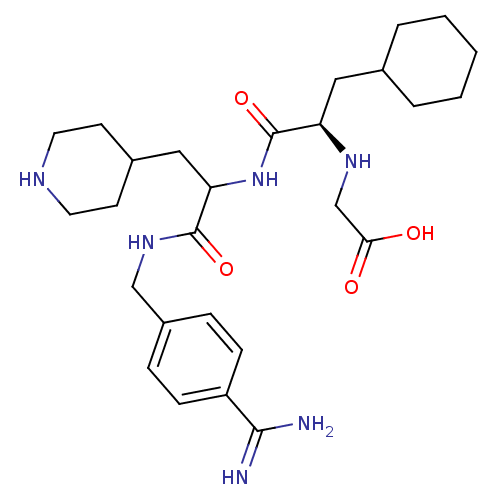
(CHEMBL3115893)Show SMILES NC(=N)c1ccc(CNC(=O)C(CC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C27H42N6O4/c28-25(29)21-8-6-20(7-9-21)16-32-26(36)23(15-19-10-12-30-13-11-19)33-27(37)22(31-17-24(34)35)14-18-4-2-1-3-5-18/h6-9,18-19,22-23,30-31H,1-5,10-17H2,(H3,28,29)(H,32,36)(H,33,37)(H,34,35)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447527
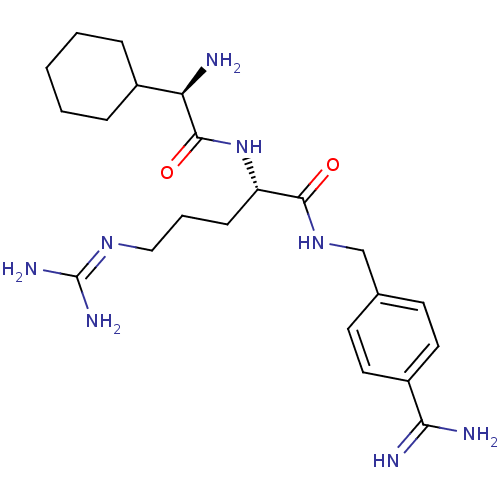
(CHEMBL3115905)Show SMILES [#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C22H36N8O2/c23-18(15-5-2-1-3-6-15)21(32)30-17(7-4-12-28-22(26)27)20(31)29-13-14-8-10-16(11-9-14)19(24)25/h8-11,15,17-18H,1-7,12-13,23H2,(H3,24,25)(H,29,31)(H,30,32)(H4,26,27,28)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50193243
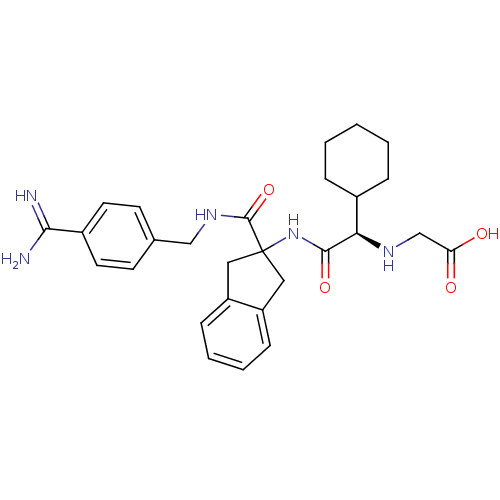
(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447516

(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447519
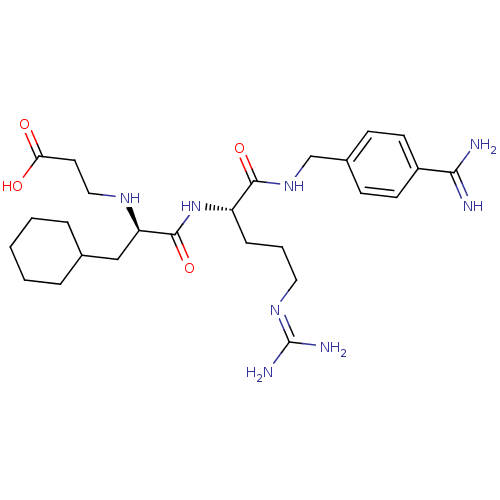
(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447518
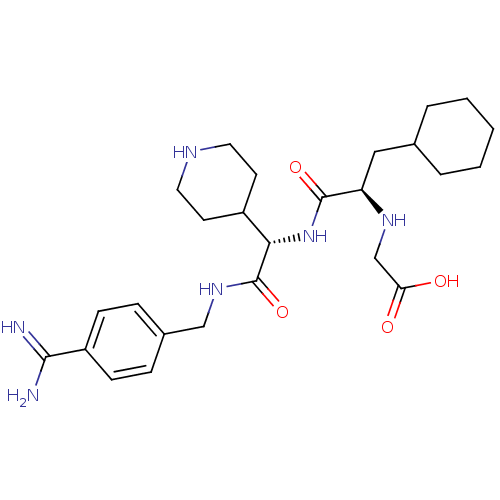
(CHEMBL3115892)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H](NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)20-8-6-18(7-9-20)15-31-26(36)23(19-10-12-29-13-11-19)32-25(35)21(30-16-22(33)34)14-17-4-2-1-3-5-17/h6-9,17,19,21,23,29-30H,1-5,10-16H2,(H3,27,28)(H,31,36)(H,32,35)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447524
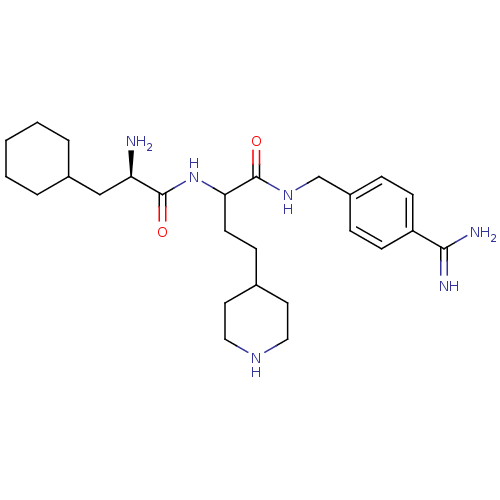
(CHEMBL3115896)Show SMILES N[C@H](CC1CCCCC1)C(=O)NC(CCC1CCNCC1)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H42N6O2/c27-22(16-19-4-2-1-3-5-19)25(33)32-23(11-8-18-12-14-30-15-13-18)26(34)31-17-20-6-9-21(10-7-20)24(28)29/h6-7,9-10,18-19,22-23,30H,1-5,8,11-17,27H2,(H3,28,29)(H,31,34)(H,32,33)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447520
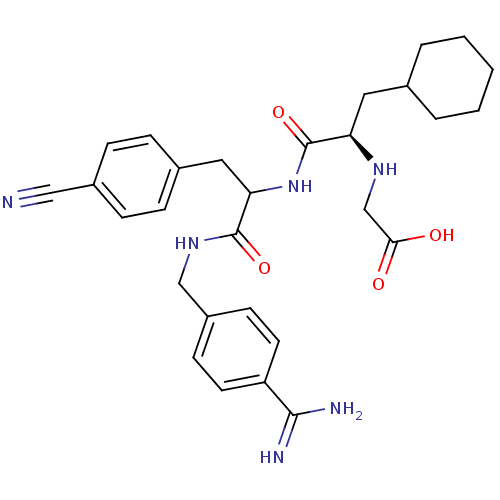
(CHEMBL3115902)Show SMILES NC(=N)c1ccc(CNC(=O)C(Cc2ccc(cc2)C#N)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C29H36N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-15,17-18H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447526
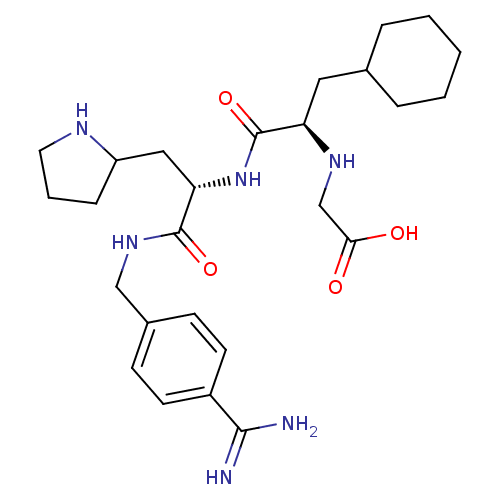
(CHEMBL3115907)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CC2CCCN2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)19-10-8-18(9-11-19)15-31-25(35)22(14-20-7-4-12-29-20)32-26(36)21(30-16-23(33)34)13-17-5-2-1-3-6-17/h8-11,17,20-22,29-30H,1-7,12-16H2,(H3,27,28)(H,31,35)(H,32,36)(H,33,34)/t20?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447516

(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447514

(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447515

(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193243

(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447519

(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447518

(CHEMBL3115892)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H](NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)20-8-6-18(7-9-20)15-31-26(36)23(19-10-12-29-13-11-19)32-25(35)21(30-16-22(33)34)14-17-4-2-1-3-5-17/h6-9,17,19,21,23,29-30H,1-5,10-16H2,(H3,27,28)(H,31,36)(H,32,35)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447517

(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447514

(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50193243

(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447517

(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50210424
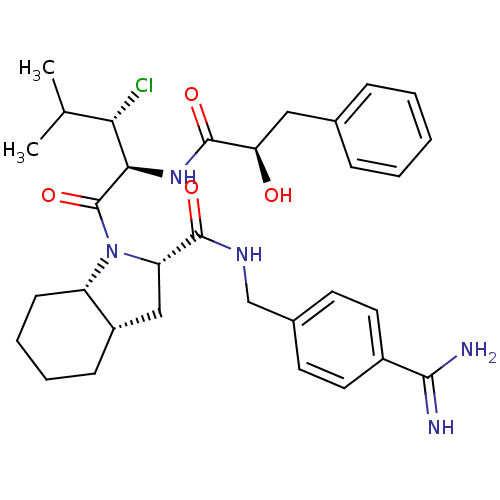
((2S,3aS,7aS)-1-[3-chloro-2-((S)-(2S,3R)-2-hydroxy-...)Show SMILES CC(C)[C@H](Cl)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1[C@@H](C[C@@H]2CCCC[C@H]12)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C32H42ClN5O4/c1-19(2)27(33)28(37-31(41)26(39)16-20-8-4-3-5-9-20)32(42)38-24-11-7-6-10-23(24)17-25(38)30(40)36-18-21-12-14-22(15-13-21)29(34)35/h3-5,8-9,12-15,19,23-28,39H,6-7,10-11,16-18H2,1-2H3,(H3,34,35)(H,36,40)(H,37,41)/t23-,24-,25-,26+,27-,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3480-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.075
BindingDB Entry DOI: 10.7270/Q25M65FJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301573
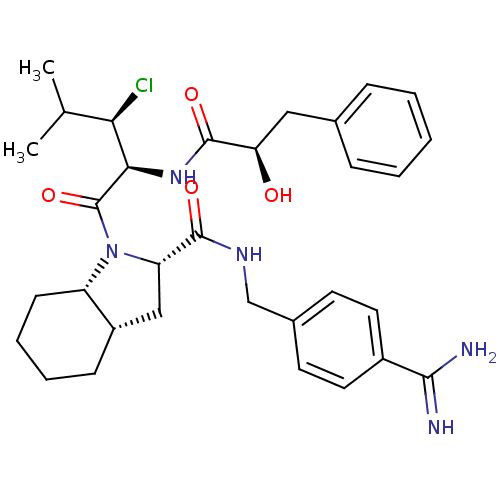
((2S,3aS,7aS)-N-(4-carbamimidoylbenzyl)-1-((2S,3R)-...)Show SMILES CC(C)[C@@H](Cl)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1[C@@H](C[C@@H]2CCCC[C@H]12)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C32H42ClN5O4/c1-19(2)27(33)28(37-31(41)26(39)16-20-8-4-3-5-9-20)32(42)38-24-11-7-6-10-23(24)17-25(38)30(40)36-18-21-12-14-22(15-13-21)29(34)35/h3-5,8-9,12-15,19,23-28,39H,6-7,10-11,16-18H2,1-2H3,(H3,34,35)(H,36,40)(H,37,41)/t23-,24-,25-,26+,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301562
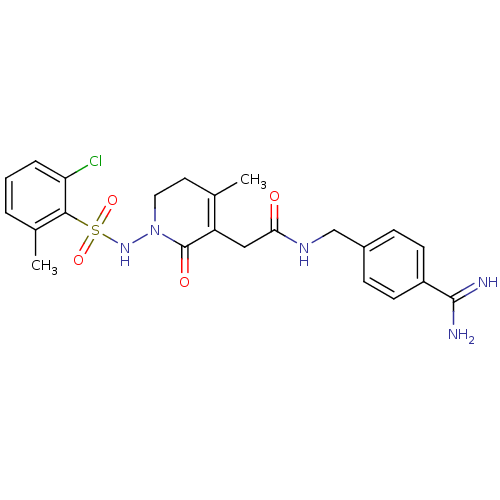
(CHEMBL584260 | N-(4-carbamimidoylbenzyl)-2-(1-(2-c...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1c(C)cccc1Cl |c:1| Show InChI InChI=1S/C23H26ClN5O4S/c1-14-10-11-29(28-34(32,33)21-15(2)4-3-5-19(21)24)23(31)18(14)12-20(30)27-13-16-6-8-17(9-7-16)22(25)26/h3-9,28H,10-13H2,1-2H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50210431
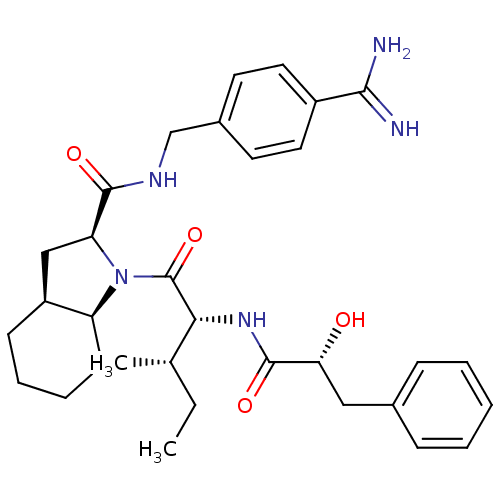
((2S,3aS,7aS)-1-[2-((R)-(2S,3R)-2-hydroxy-3-phenyl-...)Show SMILES CC[C@H](C)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C32H43N5O4/c1-3-20(2)28(36-31(40)27(38)17-21-9-5-4-6-10-21)32(41)37-25-12-8-7-11-24(25)18-26(37)30(39)35-19-22-13-15-23(16-14-22)29(33)34/h4-6,9-10,13-16,20,24-28,38H,3,7-8,11-12,17-19H2,1-2H3,(H3,33,34)(H,35,39)(H,36,40)/t20-,24-,25-,26-,27+,28+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3480-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.075
BindingDB Entry DOI: 10.7270/Q25M65FJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301571
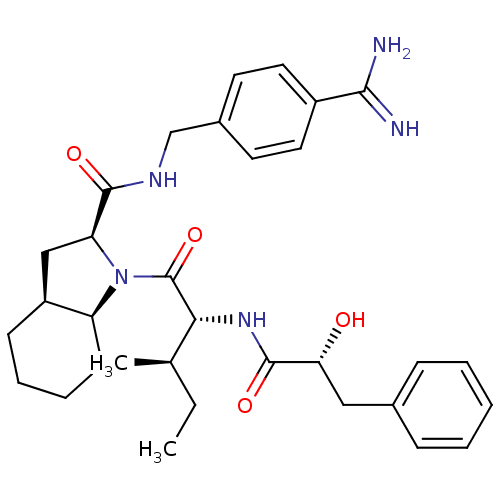
((2S,3aS,7aS)-N-(4-carbamimidoylbenzyl)-1-((2R,3R)-...)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1[C@H]2CCCC[C@H]2C[C@H]1C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C32H43N5O4/c1-3-20(2)28(36-31(40)27(38)17-21-9-5-4-6-10-21)32(41)37-25-12-8-7-11-24(25)18-26(37)30(39)35-19-22-13-15-23(16-14-22)29(33)34/h4-6,9-10,13-16,20,24-28,38H,3,7-8,11-12,17-19H2,1-2H3,(H3,33,34)(H,35,39)(H,36,40)/t20-,24+,25+,26+,27-,28-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301572

((2S,3aS,7aS)-N-(4-carbamimidoylbenzyl)-1-((R)-3,3-...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2C[C@@H]3CCCC[C@@H]3N2C(=O)[C@H](NC(=O)[C@H](O)Cc2ccccc2)C(C2CCCCC2)C2CCCCC2)cc1 |r| Show InChI InChI=1S/C41H57N5O4/c42-38(43)31-22-20-28(21-23-31)26-44-39(48)34-25-32-18-10-11-19-33(32)46(34)41(50)37(45-40(49)35(47)24-27-12-4-1-5-13-27)36(29-14-6-2-7-15-29)30-16-8-3-9-17-30/h1,4-5,12-13,20-23,29-30,32-37,47H,2-3,6-11,14-19,24-26H2,(H3,42,43)(H,44,48)(H,45,49)/t32-,33-,34-,35+,37+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50210427
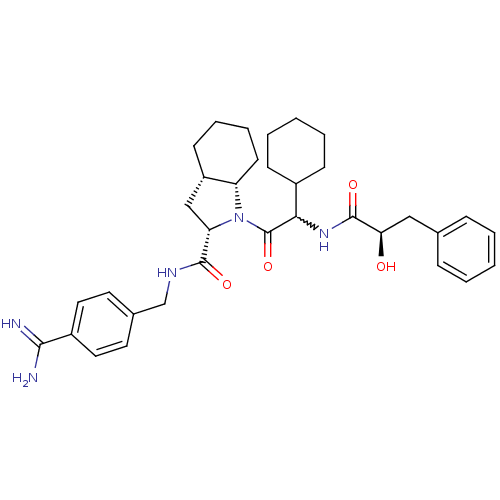
((2S,3aS,7aS)-1-[2-cyclohexyl-2-((R)-2-hydroxy-3-ph...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H]2C[C@@H]3CCCC[C@@H]3N2C(=O)C(NC(=O)[C@H](O)Cc2ccccc2)C2CCCCC2)cc1 |w:22.24| Show InChI InChI=1S/C34H45N5O4/c35-31(36)25-17-15-23(16-18-25)21-37-32(41)28-20-26-13-7-8-14-27(26)39(28)34(43)30(24-11-5-2-6-12-24)38-33(42)29(40)19-22-9-3-1-4-10-22/h1,3-4,9-10,15-18,24,26-30,40H,2,5-8,11-14,19-21H2,(H3,35,36)(H,37,41)(H,38,42)/t26-,27-,28-,29+,30?/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3480-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.075
BindingDB Entry DOI: 10.7270/Q25M65FJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301574

(CHEMBL568990 | Chlorodysinosin A)Show SMILES [#6]-[#8]-[#6@H](-[#6]-[#8]S([#8-])(=O)=O)-[#6](=O)-[#7]-[#6@H](-[#6@H](Cl)-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6@H](-[#8])-[#6@@H](-[#8])-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6]-[#6]-1=[#6]-[#6]\[#7+](-[#6]-1)=[#6](\[#7])-[#7] |r,t:38| Show InChI InChI=1S/C26H43ClN6O10S/c1-13(2)21(27)22(31-24(37)20(42-3)12-43-44(39,40)41)25(38)33-16-10-19(35)18(34)9-15(16)8-17(33)23(36)30-6-4-14-5-7-32(11-14)26(28)29/h5,13,15-22,34-35H,4,6-12H2,1-3H3,(H6,28,29,30,31,36,37,39,40,41)/t15-,16+,17+,18+,19+,20-,21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301559

(CHEMBL569249 | N-(4-carbamimidoylbenzyl)-2-(1-(2,5...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1cc(C)ccc1C |c:1| Show InChI InChI=1S/C24H29N5O4S/c1-15-4-5-17(3)21(12-15)34(32,33)28-29-11-10-16(2)20(24(29)31)13-22(30)27-14-18-6-8-19(9-7-18)23(25)26/h4-9,12,28H,10-11,13-14H2,1-3H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50210435
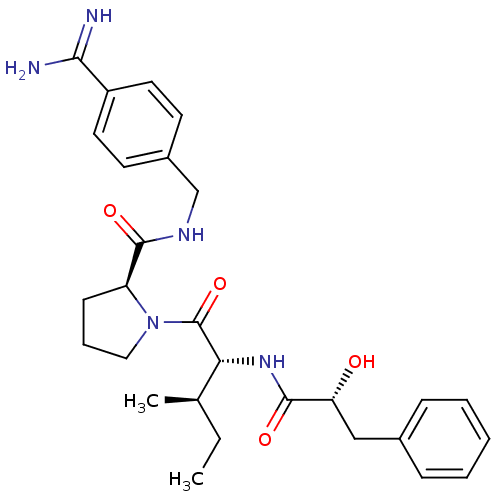
((S)-1-[(2R,3R)-2-((R)-2-hydroxy-3-phenyl-propionyl...)Show SMILES CC[C@@H](C)[C@@H](NC(=O)[C@H](O)Cc1ccccc1)C(=O)N1CCC[C@H]1C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C28H37N5O4/c1-3-18(2)24(32-27(36)23(34)16-19-8-5-4-6-9-19)28(37)33-15-7-10-22(33)26(35)31-17-20-11-13-21(14-12-20)25(29)30/h4-6,8-9,11-14,18,22-24,34H,3,7,10,15-17H2,1-2H3,(H3,29,30)(H,31,35)(H,32,36)/t18-,22+,23-,24-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3480-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.075
BindingDB Entry DOI: 10.7270/Q25M65FJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301561

(CHEMBL565328 | N-(4-carbamimidoylbenzyl)-2-(1-(2,5...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1cc(Cl)ccc1Cl |c:1| Show InChI InChI=1S/C22H23Cl2N5O4S/c1-13-8-9-29(28-34(32,33)19-10-16(23)6-7-18(19)24)22(31)17(13)11-20(30)27-12-14-2-4-15(5-3-14)21(25)26/h2-7,10,28H,8-9,11-12H2,1H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50210428
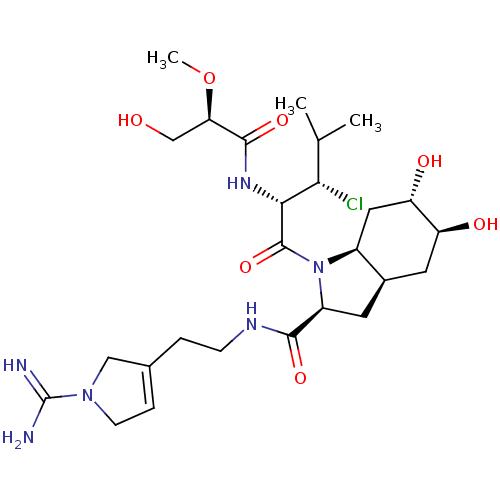
((2S,3aR,5S,6S,7aS)-1-{(S)-3-chloro-2-[(S)-3-hydrox...)Show SMILES CO[C@H](CO)C(=O)N[C@H]([C@@H](Cl)C(C)C)C(=O)N1[C@H]2C[C@H](O)[C@@H](O)C[C@H]2C[C@H]1C(=O)NCCC1=CCN(C1)C(N)=N |t:34| Show InChI InChI=1S/C26H43ClN6O7/c1-13(2)21(27)22(31-24(38)20(12-34)40-3)25(39)33-16-10-19(36)18(35)9-15(16)8-17(33)23(37)30-6-4-14-5-7-32(11-14)26(28)29/h5,13,15-22,34-36H,4,6-12H2,1-3H3,(H3,28,29)(H,30,37)(H,31,38)/t15-,16+,17+,18+,19+,20-,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Montréal
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 17: 3480-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.03.075
BindingDB Entry DOI: 10.7270/Q25M65FJ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301558

(CHEMBL565292 | N-(4-carbamimidoylbenzyl)-2-(1-(3-m...)Show SMILES COc1cccc(c1)S(=O)(=O)NN1CCC(C)=C(CC(=O)NCc2ccc(cc2)C(N)=N)C1=O |t:17| Show InChI InChI=1S/C23H27N5O5S/c1-15-10-11-28(27-34(31,32)19-5-3-4-18(12-19)33-2)23(30)20(15)13-21(29)26-14-16-6-8-17(9-7-16)22(24)25/h3-9,12,27H,10-11,13-14H2,1-2H3,(H3,24,25)(H,26,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177433
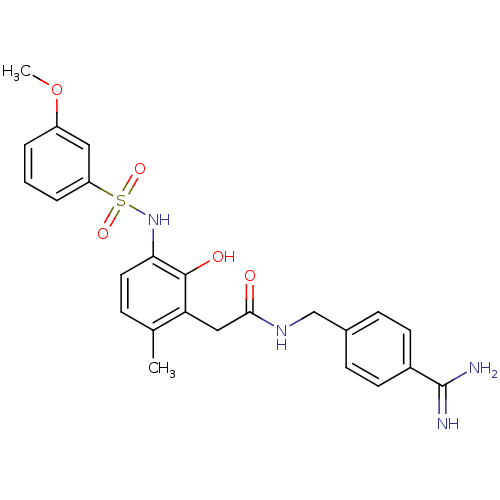
(CHEMBL535160 | N-(4-carbamimidoylbenzyl)-2-(2-hydr...)Show SMILES COc1cccc(c1)S(=O)(=O)Nc1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1O Show InChI InChI=1S/C24H26N4O5S/c1-15-6-11-21(28-34(31,32)19-5-3-4-18(12-19)33-2)23(30)20(15)13-22(29)27-14-16-7-9-17(10-8-16)24(25)26/h3-12,28,30H,13-14H2,1-2H3,(H3,25,26)(H,27,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177436

(CHEMBL557787 | N-(4-carbamimidoyl-benzyl)-2-[2-hyd...)Show SMILES Cc1ccc(NS(=O)(=O)c2cccc3ccccc23)c(O)c1CC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C27H26N4O4S/c1-17-9-14-23(31-36(34,35)24-8-4-6-19-5-2-3-7-21(19)24)26(33)22(17)15-25(32)30-16-18-10-12-20(13-11-18)27(28)29/h2-14,31,33H,15-16H2,1H3,(H3,28,29)(H,30,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50235535

(CHEMBL559192 | N-(4-carbamimidoyl-benzyl)-2-[4-met...)Show SMILES Cc1ccccc1CCNn1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1=O Show InChI InChI=1S/C25H29N5O2/c1-17-5-3-4-6-20(17)11-13-29-30-14-12-18(2)22(25(30)32)15-23(31)28-16-19-7-9-21(10-8-19)24(26)27/h3-10,12,14,29H,11,13,15-16H2,1-2H3,(H3,26,27)(H,28,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of thrombin |
Bioorg Med Chem Lett 18: 1972-6 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.122
BindingDB Entry DOI: 10.7270/Q2833SV0 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50177425

(CHEMBL537420 | N-(4-carbamimidoylbenzyl)-2-(3-(2,5...)Show SMILES Cc1ccc(C)c(c1)S(=O)(=O)Nc1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1O Show InChI InChI=1S/C25H28N4O4S/c1-15-4-5-17(3)22(12-15)34(32,33)29-21-11-6-16(2)20(24(21)31)13-23(30)28-14-18-7-9-19(10-8-18)25(26)27/h4-12,29,31H,13-14H2,1-3H3,(H3,26,27)(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50294877
((2R,3AS,6R,7AS)-N-(2-{1-[AMINO(IMINO)METHYL]-2,5-D...)Show SMILES NC(=N)N1CC=C(CCNC(=O)[C@@H]2C[C@@H]3CC[C@@H](O)C[C@@H]3N2C(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](O)Cc2ccccc2)C1 |r,t:5| Show InChI InChI=1S/C34H44N6O5/c35-34(36)39-16-14-24(21-39)13-15-37-31(43)29-19-25-11-12-26(41)20-28(25)40(29)33(45)27(17-22-7-3-1-4-8-22)38-32(44)30(42)18-23-9-5-2-6-10-23/h1-10,14,25-30,41-42H,11-13,15-21H2,(H3,35,36)(H,37,43)(H,38,44)/t25-,26+,27+,28-,29-,30+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prothrombin
(Homo sapiens (Human)) | BDBM50177421

(CHEMBL558235 | N-(4-carbamimidoylbenzyl)-2-(3-(3,4...)Show SMILES COc1ccc(cc1OC)S(=O)(=O)Nc1ccc(C)c(CC(=O)NCc2ccc(cc2)C(N)=N)c1O Show InChI InChI=1S/C25H28N4O6S/c1-15-4-10-20(29-36(32,33)18-9-11-21(34-2)22(12-18)35-3)24(31)19(15)13-23(30)28-14-16-5-7-17(8-6-16)25(26)27/h4-12,29,31H,13-14H2,1-3H3,(H3,26,27)(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin |
Bioorg Med Chem Lett 16: 1032-6 (2006)
Article DOI: 10.1016/j.bmcl.2005.10.082
BindingDB Entry DOI: 10.7270/Q2V125K5 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301557

(CHEMBL565291 | N-(4-carbamimidoylbenzyl)-2-(4-meth...)Show SMILES CC1=C(CC(=O)NCc2ccc(cc2)C(N)=N)C(=O)N(CC1)NS(=O)(=O)c1ccccc1 |c:1| Show InChI InChI=1S/C22H25N5O4S/c1-15-11-12-27(26-32(30,31)18-5-3-2-4-6-18)22(29)19(15)13-20(28)25-14-16-7-9-17(10-8-16)21(23)24/h2-10,26H,11-14H2,1H3,(H3,23,24)(H,25,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50301563

(CHEMBL568099 | N-(4-carbamimidoylbenzyl)-2-(1-(2-m...)Show SMILES COc1cc(C)ccc1S(=O)(=O)NN1CCC(C)=C(CC(=O)NCc2ccc(cc2)C(N)=N)C1=O |t:18| Show InChI InChI=1S/C24H29N5O5S/c1-15-4-9-21(20(12-15)34-3)35(32,33)28-29-11-10-16(2)19(24(29)31)13-22(30)27-14-17-5-7-18(8-6-17)23(25)26/h4-9,12,28H,10-11,13-14H2,1-3H3,(H3,25,26)(H,27,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50269776

(CHEMBL502639 | Dysinosin A)Show SMILES [#6]-[#8]-[#6@H](-[#6]-[#8]S([#8-])(=O)=O)-[#6](=O)-[#7]-[#6@H](-[#6]-[#6](-[#6])-[#6])-[#6](=O)-[#7]-1-[#6@H]-2-[#6]-[#6@H](-[#8])-[#6@@H](-[#8])-[#6]-[#6@H]-2-[#6]-[#6@H]-1-[#6](=O)-[#7]-[#6]-[#6]-[#6]-1=[#6]-[#6]\[#7+](-[#6]-1)=[#6](\[#7])-[#7] |r,t:37| Show InChI InChI=1S/C26H44N6O10S/c1-14(2)8-17(30-24(36)22(41-3)13-42-43(38,39)40)25(37)32-18-11-21(34)20(33)10-16(18)9-19(32)23(35)29-6-4-15-5-7-31(12-15)26(27)28/h5,14,16-22,33-34H,4,6-13H2,1-3H3,(H6,27,28,29,30,35,36,38,39,40)/t16-,17-,18+,19+,20+,21+,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ de Montr£al
Curated by ChEMBL
| Assay Description
Inhibition of human thrombin |
Bioorg Med Chem Lett 19: 5429-32 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.107
BindingDB Entry DOI: 10.7270/Q2MP53BQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data