

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition constant of ritonavir towards HIV protease was determined | Bioorg Med Chem Lett 7: 699-704 (1997) Article DOI: 10.1016/S0960-894X(97)00080-2 BindingDB Entry DOI: 10.7270/Q23N23C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) assessed as midazolam 1'- hydroxylation | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50267297 (CHEMBL507731 | Methyl (S)-1-((2S,4S,5S)-5-((S)-2-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of cathepsin D (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50267295 (CHEMBL470508 | Methyl (S)-1-((2R,4S,5S)-4-Hydroxy-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of renin (unknown origin) | J Med Chem 52: 2571-86 (2009) Article DOI: 10.1021/jm900044w BindingDB Entry DOI: 10.7270/Q2G160Q0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50063071 (((1S,2S,4S)-1-Benzyl-2-hydroxy-4-{3-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062997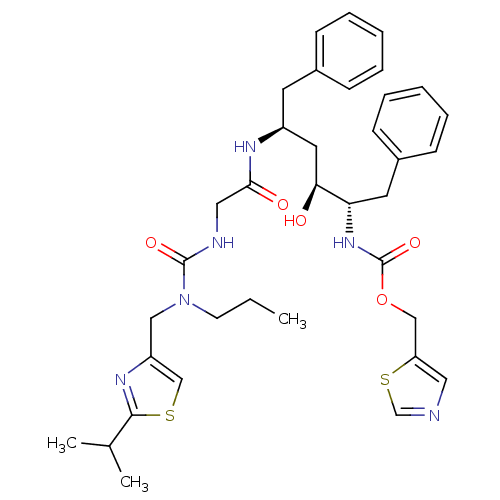 (((1S,2S,4S)-1-Benzyl-2-hydroxy-4-{2-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062946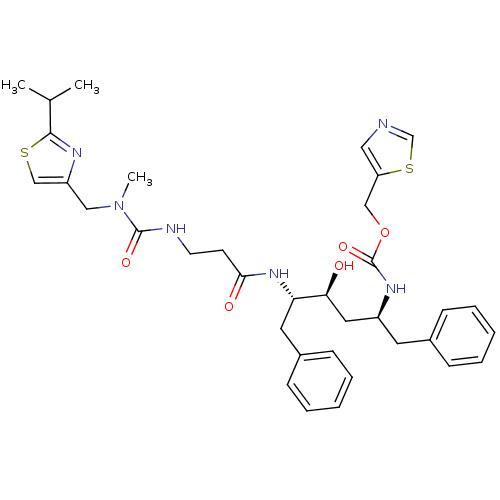 (((1S,3S,4S)-1-Benzyl-3-hydroxy-4-{3-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285916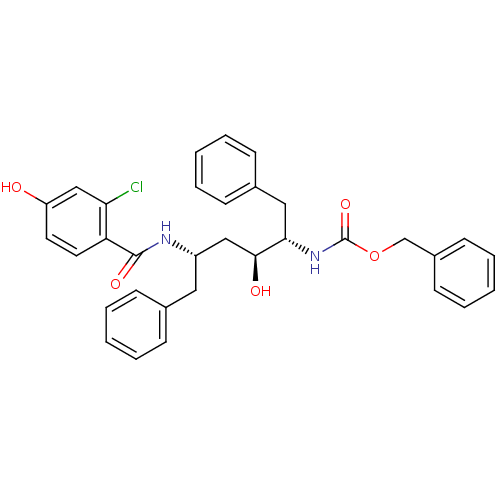 (CHEMBL90751 | [(1S,2S,4S)-1-Benzyl-4-(2-chloro-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285903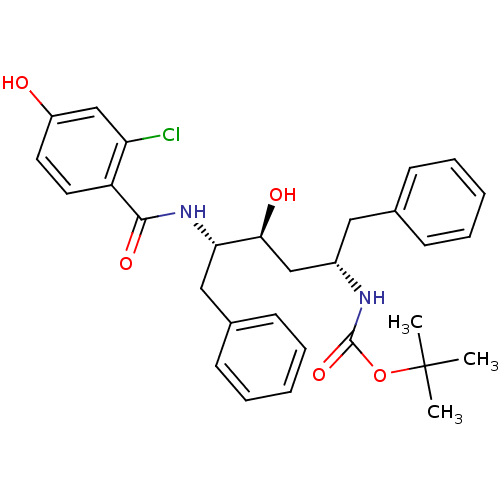 (CHEMBL93589 | [(1S,3S,4S)-1-Benzyl-4-(2-chloro-4-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062994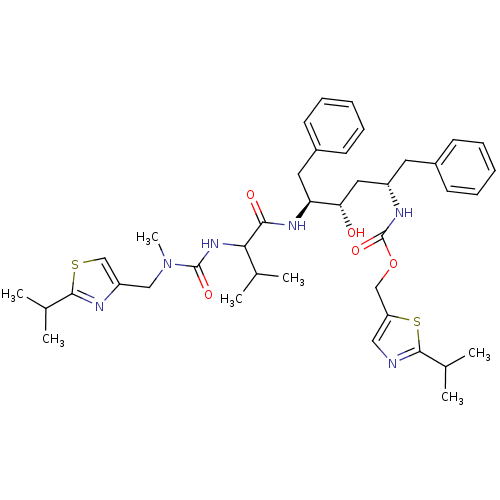 (((1S,3S,4S)-1-Benzyl-3-hydroxy-4-{2-[3-(2-isopropy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062954 (((1S,2S,4S)-1-Benzyl-2-hydroxy-4-{2-[3-isobutyl-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285910 (CHEMBL93718 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062969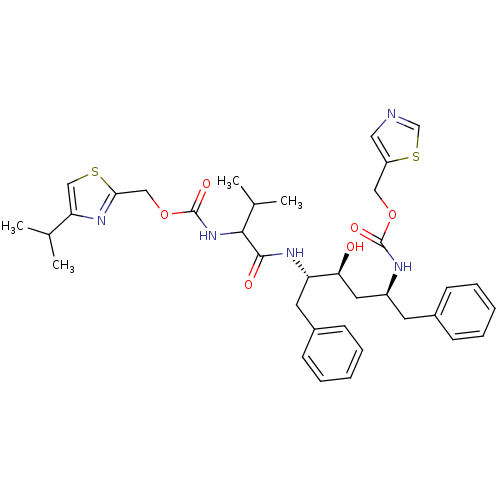 (CHEMBL346943 | {(1S,3S,4S)-1-Benzyl-3-hydroxy-4-[2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062941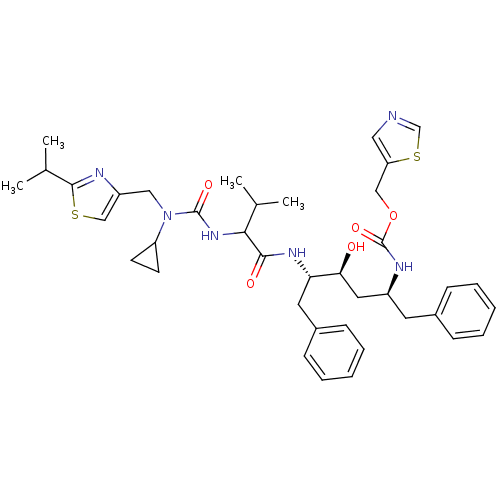 (((1S,3S,4S)-1-Benzyl-4-{2-[3-cyclopropyl-3-(2-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062939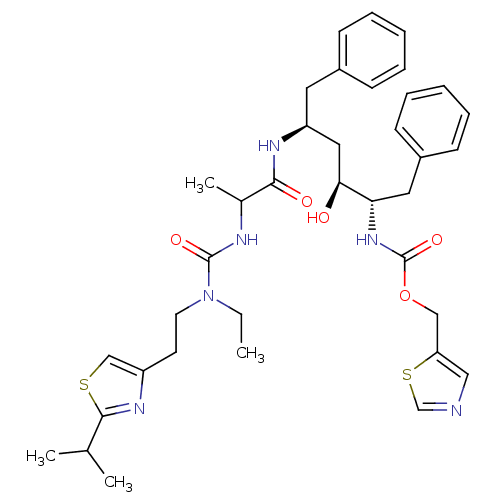 (CHEMBL347824 | [(1S,2S,4S)-1-Benzyl-4-(2-{3-ethyl-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285911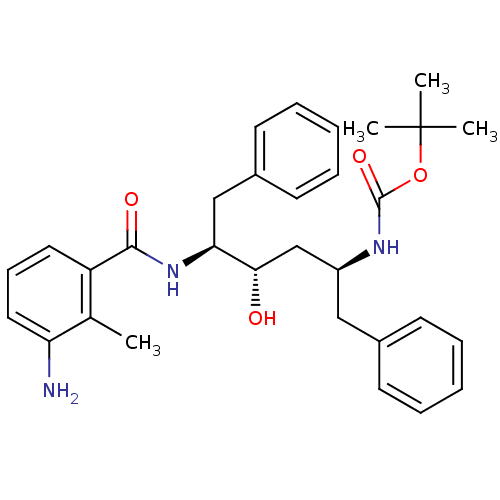 (CHEMBL90090 | [(1S,3S,4S)-4-(3-Amino-2-methyl-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285890 (CHEMBL93566 | [(1S,3S,4S)-4-(5-Amino-2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285913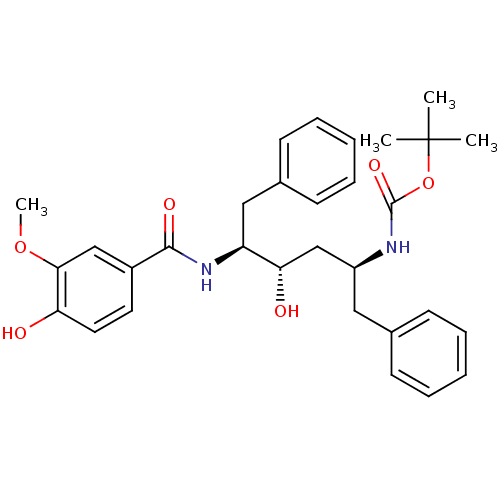 (CHEMBL327611 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(4...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285905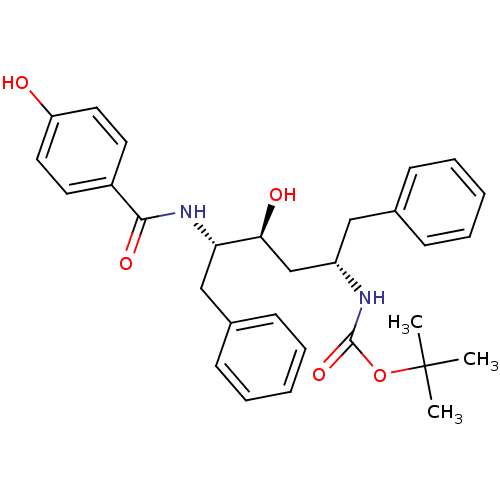 (CHEMBL93540 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(4-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285900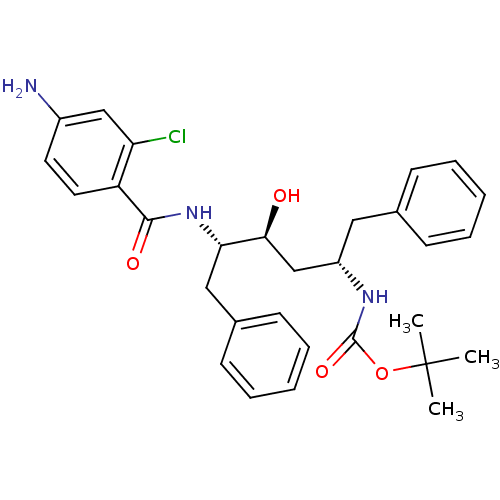 (CHEMBL93481 | [(1S,3S,4S)-4-(4-Amino-2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285897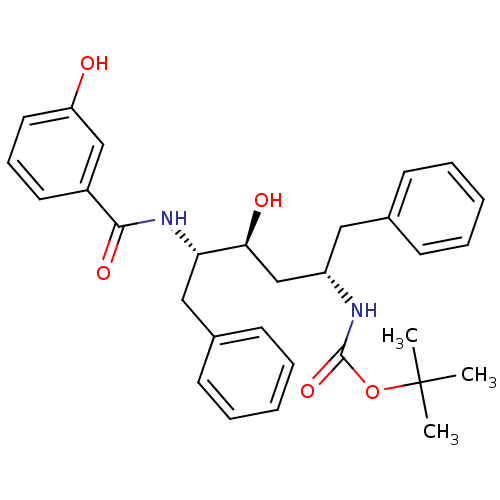 (CHEMBL273562 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285906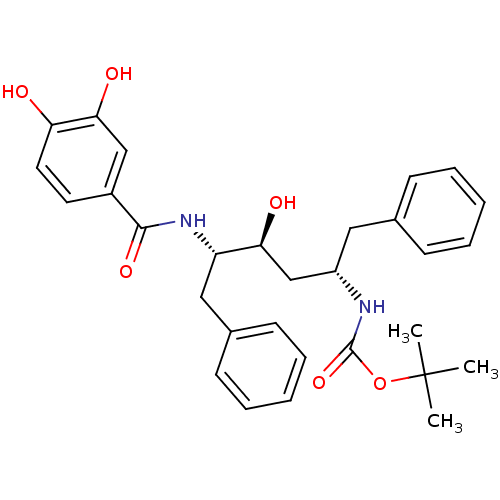 (CHEMBL328531 | [(1S,3S,4S)-1-Benzyl-4-(3,4-dihydro...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062925 (CHEMBL157673 | [(1S,2S,4S)-1-Benzyl-2-hydroxy-4-(2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285899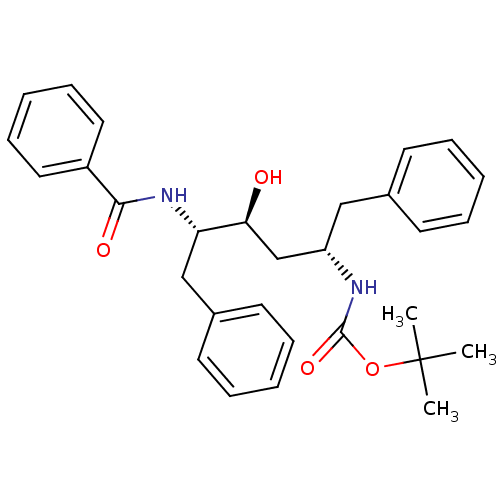 (((1S,3S,4S)-4-Benzoylamino-1-benzyl-3-hydroxy-5-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12210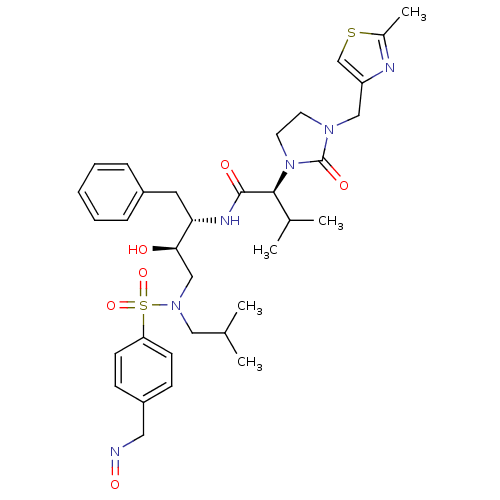 ((2S)-N-[(2S,3R)-3-hydroxy-4-({4-[(1E)-(hydroxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285889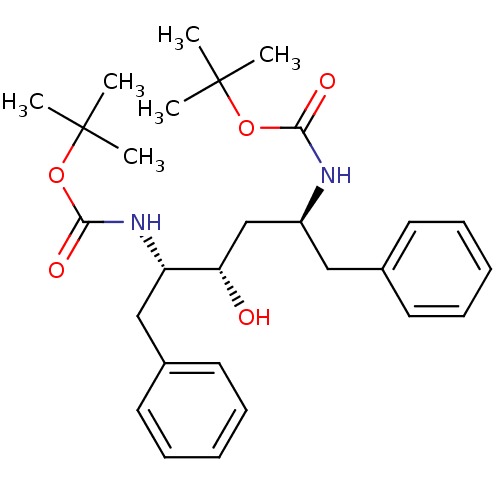 (((1S,3S,4S)-1-Benzyl-4-tert-butoxycarbonylamino-3-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285907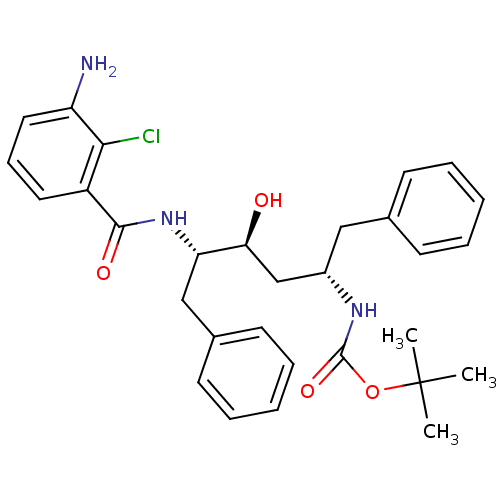 (CHEMBL93323 | [(1S,3S,4S)-4-(3-Amino-2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285892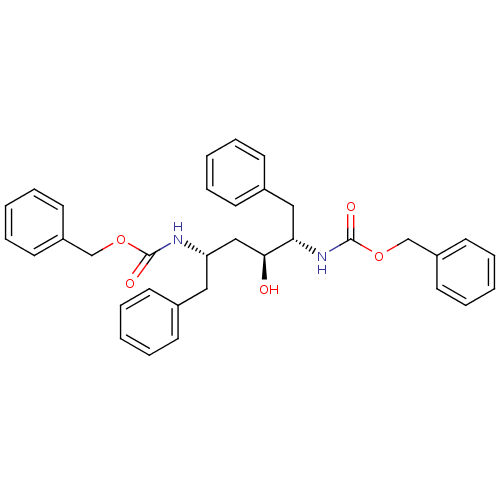 (((1S,3S,4S)-1-Benzyl-4-benzyloxycarbonylamino-3-hy...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285915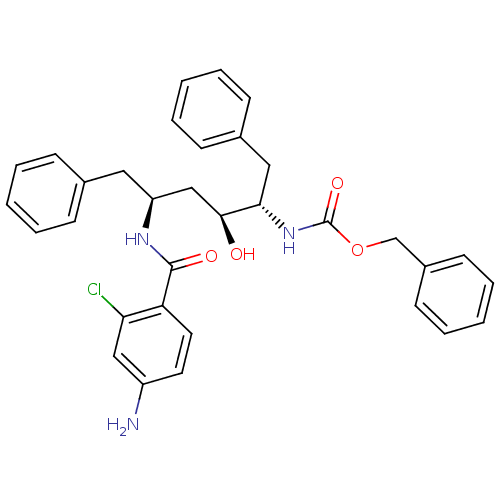 (CHEMBL91657 | [(1S,2S,4S)-4-(4-Amino-2-chloro-benz...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285891 (CHEMBL441065 | [(1S,3S,4S)-4-(4-Amino-3-methoxy-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285908 (CHEMBL90139 | [(1S,3S,4S)-4-(3-Amino-4-hydroxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50063021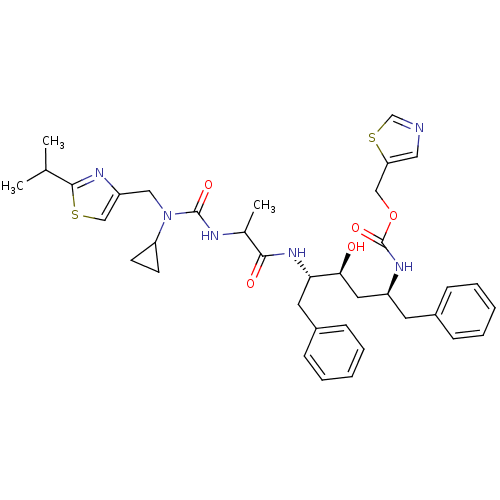 (((1S,3S,4S)-1-Benzyl-4-{2-[3-cyclopropyl-3-(2-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285893 (CHEMBL90699 | [(1S,3S,4S)-1-Benzyl-4-(3,4-diamino-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285909 (CHEMBL91443 | [(1S,3S,4S)-4-(4-Amino-3-hydroxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285904 (CHEMBL191849 | [(1S,2S,4S)-4-(4-Amino-3-methoxy-be...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285898 (CHEMBL91601 | [(1S,3S,4S)-1-Benzyl-4-(2-chloro-5-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50062933 (((1S,2S,4S)-1-Benzyl-4-{3-[3-cyclopropyl-3-(2-isop...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratory Curated by ChEMBL | Assay Description Inhibitory concentration against purified recombinant HIV-1 protease using fluorogenic substrate | J Med Chem 41: 602-17 (1998) Article DOI: 10.1021/jm970636+ BindingDB Entry DOI: 10.7270/Q2N58KGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50250183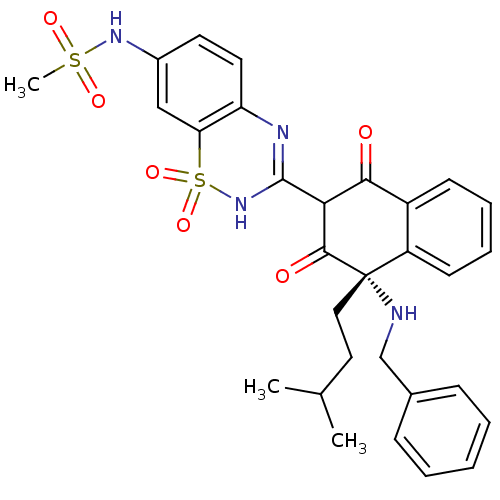 (CHEMBL503188 | N-{3-[(4S)-4-Benzylamino-1-hydroxy-...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV1b Con1 NS5B assessed as [3H]UTP incorporation into RNA by scintillation counting | J Med Chem 52: 3174-83 (2009) Article DOI: 10.1021/jm801485z BindingDB Entry DOI: 10.7270/Q2ZW1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12209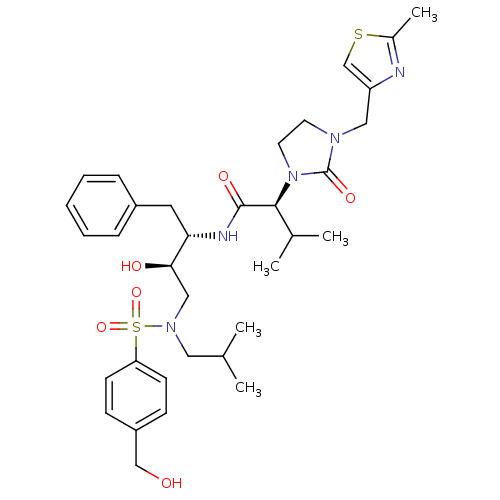 ((2S)-N-[(2S,3R)-3-hydroxy-4-{[4-(hydroxymethyl)ben...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12213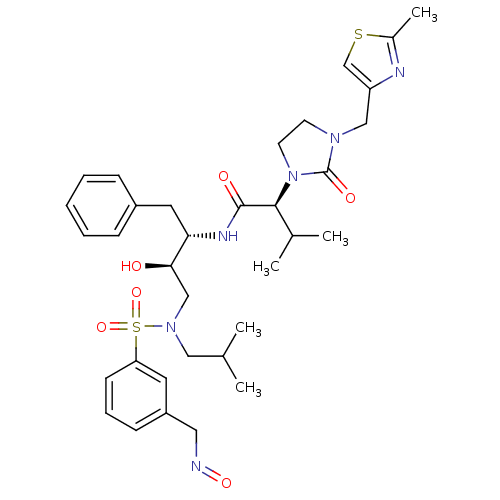 ((2S)-N-[(2S,3R)-3-hydroxy-4-({3-[(1E)-(hydroxyimin...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus (HCV)) | BDBM50249586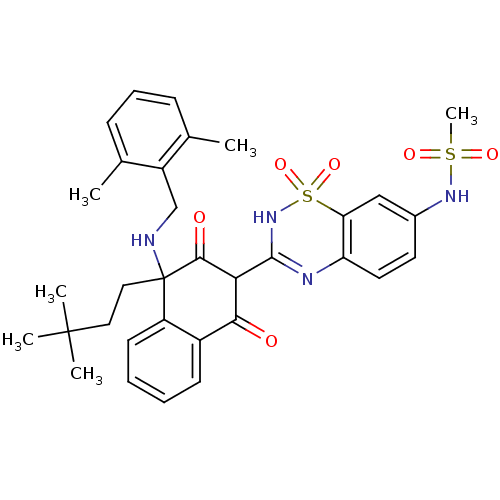 (CHEMBL504729 | N-{3-[4-[(2,6-Dimethylbenzyl)amino]...) | PDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV1b Con1 NS5B assessed as [3H]UTP incorporation into RNA by scintillation counting | J Med Chem 52: 3174-83 (2009) Article DOI: 10.1021/jm801485z BindingDB Entry DOI: 10.7270/Q2ZW1MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285896 (CHEMBL90510 | [(1S,2S,4S)-4-(4-Amino-3-hydroxy-ben...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285894 (CHEMBL541982 | [(1S,3S,4S)-1-Benzyl-3-hydroxy-4-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM12198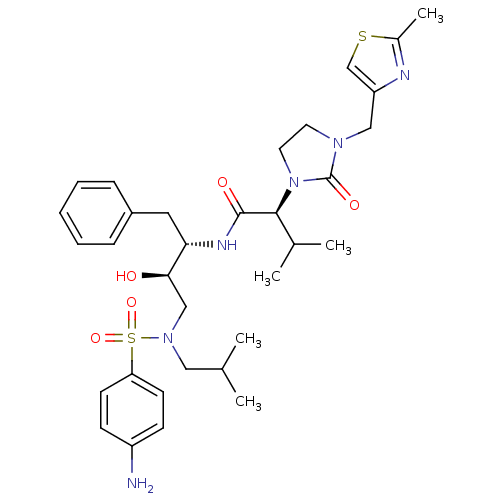 ((2S)-N-[(2S,3R)-4-[(4-aminobenzene)(2-methylpropyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | 4.5 | 30 |
Abbott Laboratories | Assay Description HIV-1 protease inhibition was measured by a fluorescence assay using recombinant HIV-1 protease. The reactions were initiated by the addition of 1 nM... | Bioorg Med Chem Lett 15: 2275-8 (2005) Article DOI: 10.1016/j.bmcl.2005.03.008 BindingDB Entry DOI: 10.7270/Q2SQ8XNQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285888 (((1S,2S,4S)-4-Benzoylamino-1-benzyl-2-hydroxy-5-ph...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50285902 (CHEMBL93120 | [(1S,3S,4S)-4-(3-Amino-2,6-dichloro-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against purified HIV protease | Bioorg Med Chem Lett 5: 2725-2728 (1995) Article DOI: 10.1016/0960-894X(95)00462-3 BindingDB Entry DOI: 10.7270/Q26973JN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 116 total ) | Next | Last >> |