 Found 84 hits with Last Name = 'fong' and Initial = 'hh'
Found 84 hits with Last Name = 'fong' and Initial = 'hh' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50057527
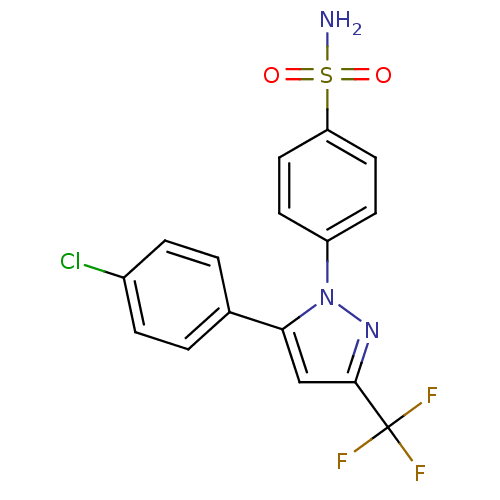
(4-(5-(4-chlorophenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES NS(=O)(=O)c1ccc(cc1)-n1nc(cc1-c1ccc(Cl)cc1)C(F)(F)F Show InChI InChI=1S/C16H11ClF3N3O2S/c17-11-3-1-10(2-4-11)14-9-15(16(18,19)20)22-23(14)12-5-7-13(8-6-12)26(21,24)25/h1-9H,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251003
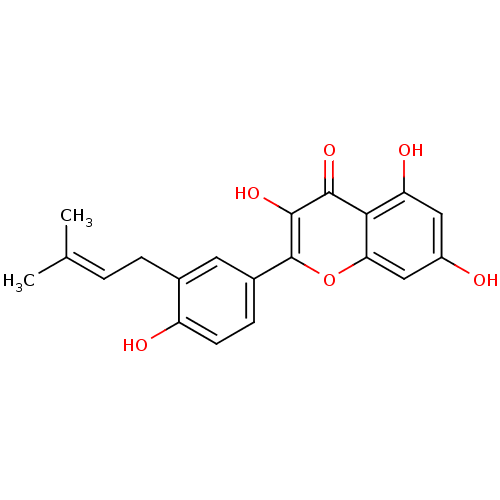
(CHEMBL457679 | cid_5318585 | isolicoflavonol)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-c1oc2cc(-[#8])cc(-[#8])c2c(=O)c1-[#8] Show InChI InChI=1S/C20H18O6/c1-10(2)3-4-11-7-12(5-6-14(11)22)20-19(25)18(24)17-15(23)8-13(21)9-16(17)26-20/h3,5-9,21-23,25H,4H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251002

((2S)-2',4'-dihydroxy-2' '-(1-hydroxy-1-methylethyl...)Show SMILES CC(C)(O)C1Cc2c(O1)ccc1C(=O)C[C@H](Oc21)c1ccc(O)cc1O |r| Show InChI InChI=1S/C20H20O6/c1-20(2,24)18-8-13-16(25-18)6-5-12-15(23)9-17(26-19(12)13)11-4-3-10(21)7-14(11)22/h3-7,17-18,21-22,24H,8-9H2,1-2H3/t17-,18?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50259771
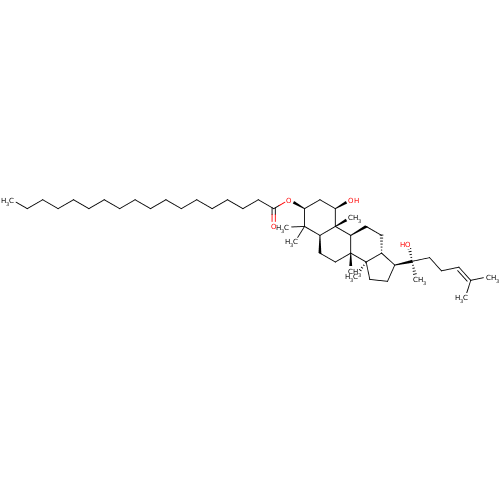
(1beta,20-(S)-dihydroxydammar-24(25)-ene-3beta-O-st...)Show SMILES [#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6]-[#6](=O)-[#8]-[#6@H]1-[#6]-[#6@@H](-[#8])[C@@]2([#6])[#6@@H](-[#6]-[#6][C@]3([#6])[#6@@H]2-[#6]-[#6]-[#6@@H]2-[#6@H](-[#6]-[#6][C@@]32[#6])[C@@]([#6])([#8])[#6]-[#6]\[#6]=[#6](\[#6])-[#6])C1([#6])[#6] |r| Show InChI InChI=1S/C48H86O4/c1-10-11-12-13-14-15-16-17-18-19-20-21-22-23-24-27-43(50)52-42-35-41(49)48(9)39(44(42,4)5)31-34-46(7)40(48)29-28-37-38(30-33-45(37,46)6)47(8,51)32-25-26-36(2)3/h26,37-42,49,51H,10-25,27-35H2,1-9H3/t37-,38+,39+,40+,41-,42+,45-,46-,47+,48+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Mahidol University
Curated by ChEMBL
| Assay Description
Inhibition of COX2 (unknown origin) |
J Nat Prod 69: 421-4 (2006)
Article DOI: 10.1021/np058112x
BindingDB Entry DOI: 10.7270/Q2XK8FBR |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251004
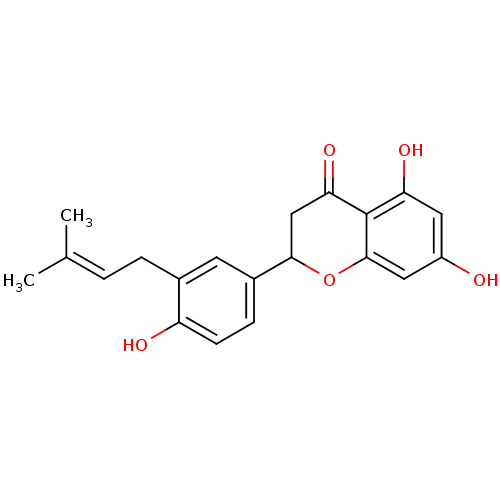
((2S)-abyssinone II | CHEMBL457680)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-[#6]-1-[#6]-[#6](=O)-c2c(-[#8])cc(-[#8])cc2-[#8]-1 Show InChI InChI=1S/C20H20O5/c1-11(2)3-4-12-7-13(5-6-15(12)22)18-10-17(24)20-16(23)8-14(21)9-19(20)25-18/h3,5-9,18,21-23H,4,10H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412080
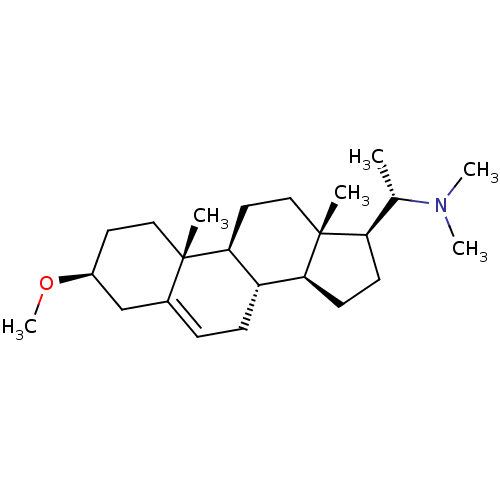
(CHEMBL342394)Show SMILES CO[C@H]1CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CC=C2C1)[C@H](C)N(C)C |r,c:20| Show InChI InChI=1S/C24H41NO/c1-16(25(4)5)20-9-10-21-19-8-7-17-15-18(26-6)11-13-23(17,2)22(19)12-14-24(20,21)3/h7,16,18-22H,8-15H2,1-6H3/t16-,18-,19-,20+,21-,22-,23-,24+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269597
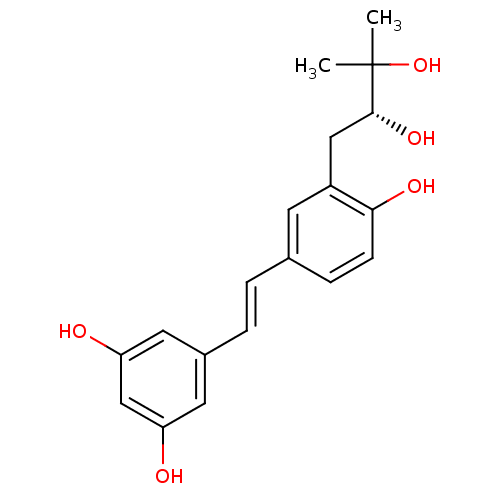
(3-(2,3-dihydroxy-3-methylbutyl)resveratrol | CHEMB...)Show SMILES CC(C)(O)[C@H](O)Cc1cc(\C=C\c2cc(O)cc(O)c2)ccc1O |r| Show InChI InChI=1S/C19H22O5/c1-19(2,24)18(23)10-14-7-12(5-6-17(14)22)3-4-13-8-15(20)11-16(21)9-13/h3-9,11,18,20-24H,10H2,1-2H3/b4-3+/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50250915
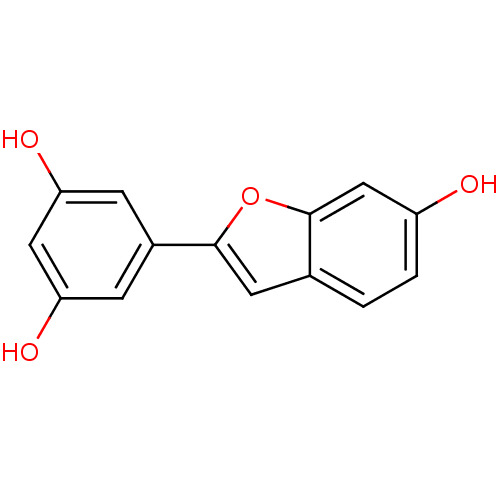
(CHEMBL512578 | moracin M)Show InChI InChI=1S/C14H10O4/c15-10-2-1-8-5-13(18-14(8)7-10)9-3-11(16)6-12(17)4-9/h1-7,15-17H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50250979

(3'-[gamma-hydroxymethyl-(E)-gamma-methylallyl]-2,4...)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2O)c1O Show InChI InChI=1S/C29H26O8/c1-18(17-37-28(35)15-5-19-3-8-21(30)9-4-19)2-11-23-26(33)14-12-24(29(23)36)25(32)13-7-20-6-10-22(31)16-27(20)34/h2-10,12-16,30-31,33-34,36H,11,17H2,1H3/b13-7+,15-5+,18-2+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412082
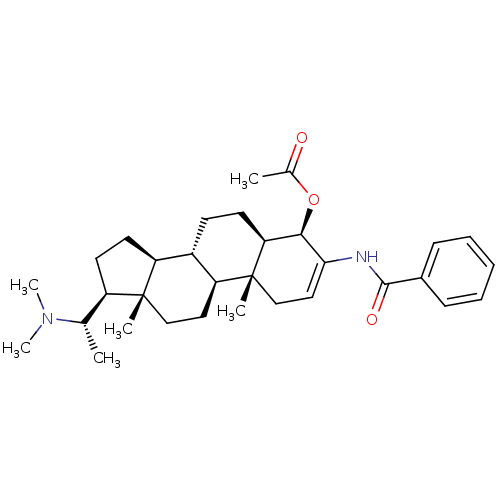
(CHEMBL455316)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4[C@@H](OC(C)=O)C(NC(=O)c5ccccc5)=CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C |r,c:25| Show InChI InChI=1S/C32H46N2O3/c1-20(34(5)6)24-14-15-25-23-12-13-27-29(37-21(2)35)28(33-30(36)22-10-8-7-9-11-22)17-19-32(27,4)26(23)16-18-31(24,25)3/h7-11,17,20,23-27,29H,12-16,18-19H2,1-6H3,(H,33,36)/t20-,23-,24+,25-,26-,27-,29+,31+,32+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412077
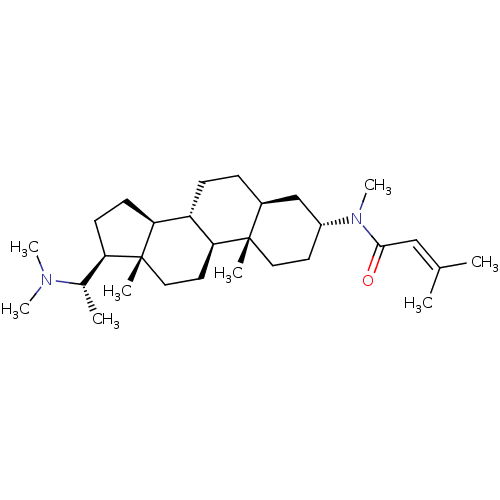
((+)-PACHYSAMINE B)Show SMILES [#6]-[#6@@H](-[#6@H]1-[#6]-[#6]-[#6@H]2-[#6@@H]-3-[#6]-[#6]-[#6@H]4-[#6]-[#6@@H](-[#6]-[#6][C@]4([#6])[#6@H]-3-[#6]-[#6][C@]12[#6])-[#7](-[#6])-[#6](=O)\[#6]=[#6](\[#6])-[#6])-[#7](-[#6])-[#6] |r| Show InChI InChI=1S/C29H50N2O/c1-19(2)17-27(32)31(8)22-13-15-28(4)21(18-22)9-10-23-25-12-11-24(20(3)30(6)7)29(25,5)16-14-26(23)28/h17,20-26H,9-16,18H2,1-8H3/t20-,21-,22+,23-,24+,25-,26-,28-,29+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269596
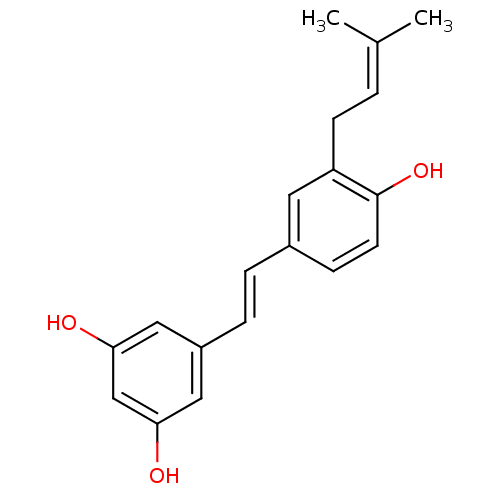
(3-(gamma,gamma-dimethylallyl)resveratrol | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)ccc1-[#8] Show InChI InChI=1S/C19H20O3/c1-13(2)3-7-16-9-14(6-8-19(16)22)4-5-15-10-17(20)12-18(21)11-15/h3-6,8-12,20-22H,7H2,1-2H3/b5-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM23926
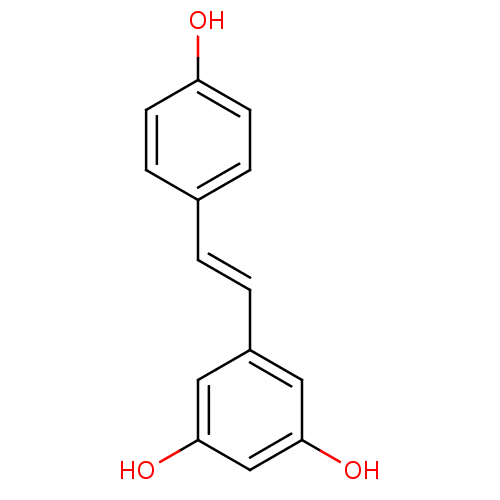
((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) assessed as N-methyldihydronicotinamide oxidation per mg of protein a... |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50108046
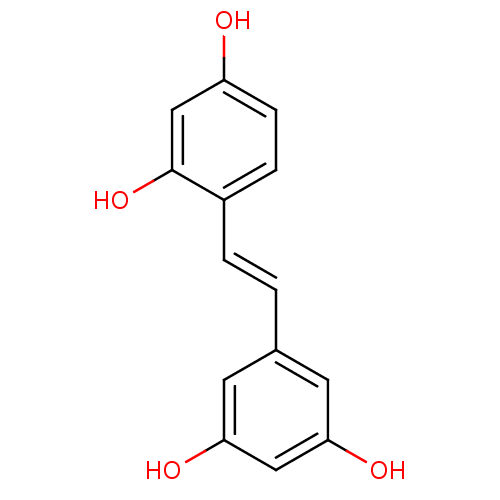
((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...)Show InChI InChI=1S/C14H12O4/c15-11-4-3-10(14(18)8-11)2-1-9-5-12(16)7-13(17)6-9/h1-8,15-18H/b2-1+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251005
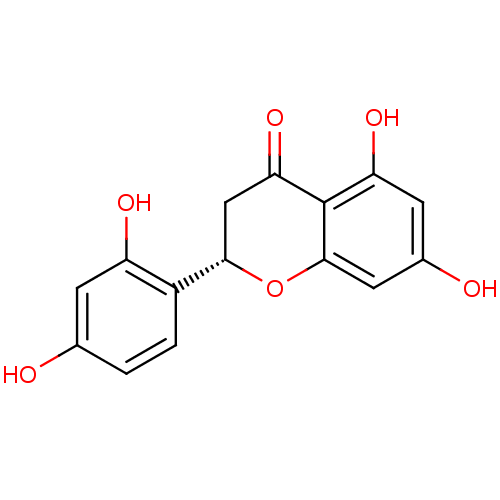
((2S)-5,7,2',4'-tetrahydroxyflavanone | (S)-2-(2,4-...)Show SMILES Oc1ccc([C@@H]2CC(=O)c3c(O)cc(O)cc3O2)c(O)c1 |r| Show InChI InChI=1S/C15H12O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-5,13,16-19H,6H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50292441
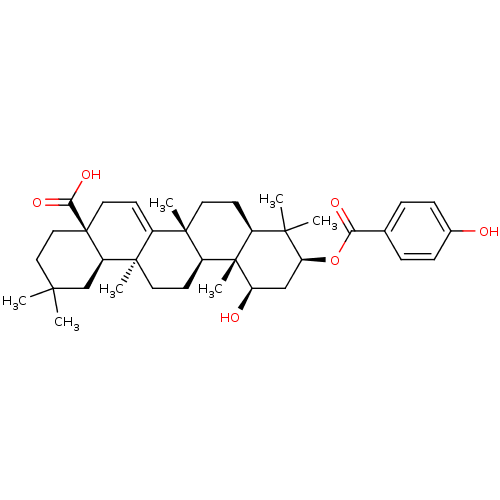
(1beta-hydroxyaleuritolic acid 3-p-hydroxybenzoate ...)Show SMILES CC1(C)CC[C@@]2(CC=C3[C@]4(C)CC[C@H]5C(C)(C)[C@H](C[C@@H](O)[C@]5(C)[C@H]4CC[C@@]3(C)[C@H]2C1)OC(=O)c1ccc(O)cc1)C(O)=O |r,t:7| Show InChI InChI=1S/C37H52O6/c1-32(2)18-19-37(31(41)42)17-14-25-34(5)15-12-24-33(3,4)29(43-30(40)22-8-10-23(38)11-9-22)20-28(39)36(24,7)26(34)13-16-35(25,6)27(37)21-32/h8-11,14,24,26-29,38-39H,12-13,15-21H2,1-7H3,(H,41,42)/t24-,26-,27+,28+,29-,34-,35+,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
J Nat Prod 58: 1024-1031 (1995)
Article DOI: 10.1021/np50121a006
BindingDB Entry DOI: 10.7270/Q2639PR2 |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412076
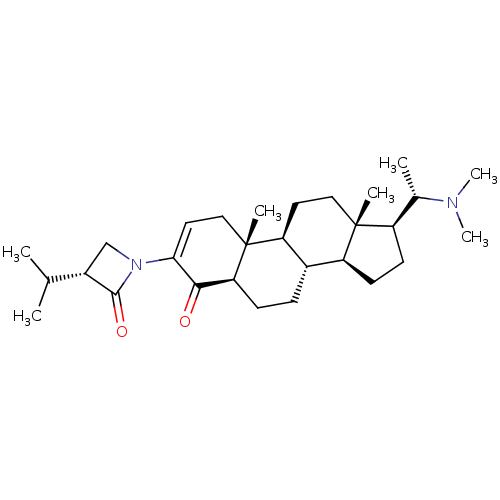
(CHEMBL457817)Show SMILES CC(C)[C@@H]1CN(C1=O)C1=CC[C@]2(C)[C@H]3CC[C@]4(C)[C@H](CC[C@H]4[C@@H]3CC[C@H]2C1=O)[C@H](C)N(C)C |r,t:9| Show InChI InChI=1S/C29H46N2O2/c1-17(2)20-16-31(27(20)33)25-13-15-29(5)23-12-14-28(4)21(18(3)30(6)7)10-11-22(28)19(23)8-9-24(29)26(25)32/h13,17-24H,8-12,14-16H2,1-7H3/t18-,19-,20-,21+,22-,23-,24-,28+,29+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251006
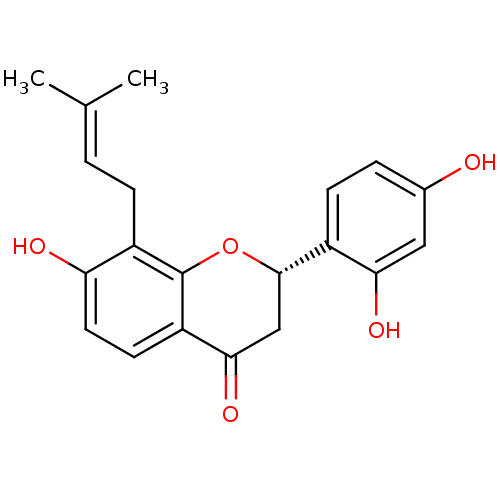
((2S)-Euchrenone a7 | CHEMBL457686)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2-[#6](=O)-[#6]-[#6@H](-[#8]-c12)-c1ccc(-[#8])cc1-[#8] |r| Show InChI InChI=1S/C20H20O5/c1-11(2)3-5-14-16(22)8-7-15-18(24)10-19(25-20(14)15)13-6-4-12(21)9-17(13)23/h3-4,6-9,19,21-23H,5,10H2,1-2H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478513
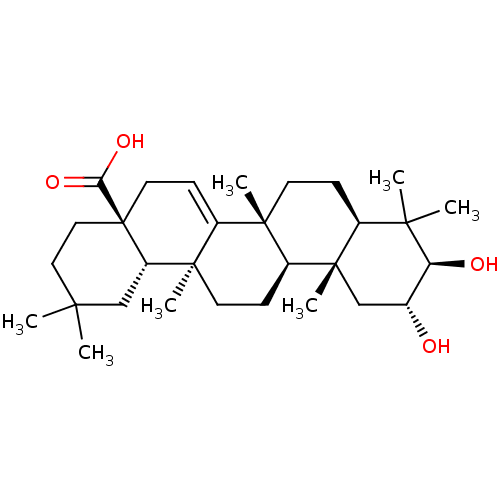
(2Alpha-Hydroxymaprounic Acid | CHEMBL469925)Show SMILES [H][C@@]12CC[C@@]3(C)C4=CC[C@]5(CCC(C)(C)C[C@@]5([H])[C@]4(C)CC[C@]3([H])[C@@]1(C)C[C@@H](O)[C@H](O)C2(C)C)C(O)=O |r,t:6| Show InChI InChI=1S/C30H48O4/c1-25(2)14-15-30(24(33)34)13-10-20-27(5)11-8-19-26(3,4)23(32)18(31)16-29(19,7)21(27)9-12-28(20,6)22(30)17-25/h10,18-19,21-23,31-32H,8-9,11-17H2,1-7H3,(H,33,34)/t18-,19+,21+,22+,23+,27+,28-,29+,30-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Nat Prod 57: 415-8 (1994)
Article DOI: 10.1021/np50105a017
BindingDB Entry DOI: 10.7270/Q2ZW1PNF |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412083

(CHEMBL458033)Show SMILES CC(C)[C@@H]1CN(C1=O)C1=CC[C@]2(C)[C@H]3CC[C@@]4(C)[C@@H](C[C@H](O)[C@@H]4[C@H](C)N(C)C)[C@@H]3CC[C@H]2C1=O |r,t:9| Show InChI InChI=1S/C29H46N2O3/c1-16(2)19-15-31(27(19)34)23-11-13-28(4)20-10-12-29(5)22(18(20)8-9-21(28)26(23)33)14-24(32)25(29)17(3)30(6)7/h11,16-22,24-25,32H,8-10,12-15H2,1-7H3/t17-,18+,19-,20-,21-,22-,24-,25-,28+,29-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478517
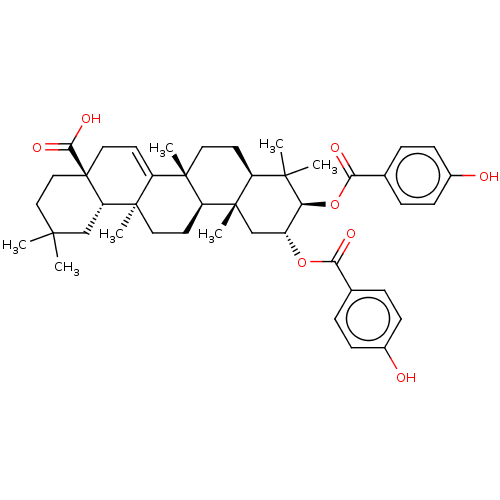
(CHEBI:66672 | CHEMBL472320)Show SMILES [H][C@@]12CC[C@@]3(C)C4=CC[C@]5(CCC(C)(C)C[C@@]5([H])[C@]4(C)CC[C@]3([H])[C@@]1(C)C[C@@H](OC(=O)c1ccc(O)cc1)[C@H](OC(=O)c1ccc(O)cc1)C2(C)C)C(O)=O |r,t:6| Show InChI InChI=1S/C44H56O8/c1-39(2)22-23-44(38(49)50)21-18-32-41(5)19-16-31-40(3,4)35(52-37(48)27-10-14-29(46)15-11-27)30(51-36(47)26-8-12-28(45)13-9-26)24-43(31,7)33(41)17-20-42(32,6)34(44)25-39/h8-15,18,30-31,33-35,45-46H,16-17,19-25H2,1-7H3,(H,49,50)/t30-,31+,33+,34+,35+,41+,42-,43+,44-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Nat Prod 57: 415-8 (1994)
Article DOI: 10.1021/np50105a017
BindingDB Entry DOI: 10.7270/Q2ZW1PNF |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478511
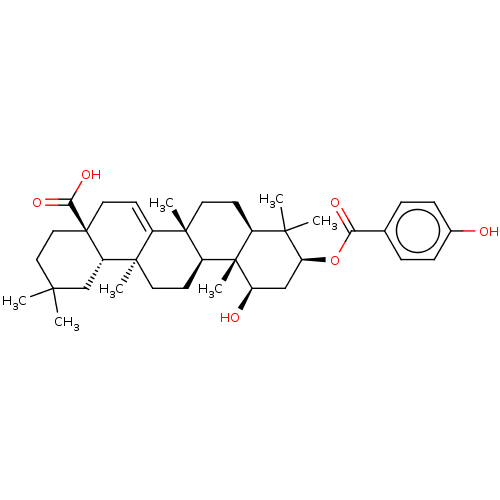
(CHEBI:66671 | CHEMBL449431)Show SMILES [H][C@@]12CC[C@@]3(C)C4=CC[C@]5(CCC(C)(C)C[C@@]5([H])[C@]4(C)CC[C@]3([H])[C@@]1(C)[C@H](O)C[C@H](OC(=O)c1ccc(O)cc1)C2(C)C)C(O)=O |r,t:6| Show InChI InChI=1S/C37H52O6/c1-32(2)18-19-37(31(41)42)17-14-25-34(5)15-12-24-33(3,4)29(43-30(40)22-8-10-23(38)11-9-22)20-28(39)36(24,7)26(34)13-16-35(25,6)27(37)21-32/h8-11,14,24,26-29,38-39H,12-13,15-21H2,1-7H3,(H,41,42)/t24-,26-,27-,28+,29-,34-,35+,36-,37+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Nat Prod 57: 415-8 (1994)
Article DOI: 10.1021/np50105a017
BindingDB Entry DOI: 10.7270/Q2ZW1PNF |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50339150

(5,7-dihydroxy-2-(4-hydroxyphenyl)-3-((2R,3S,4S,5S,...)Show SMILES C[C@H]1O[C@H](Oc2c(O)c3c(cc(O)cc3=O)oc2-c2ccc(O)cc2)[C@@H](O)[C@@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H20O10/c1-8-15(25)17(27)18(28)21(29-8)31-20-16(26)14-12(24)6-11(23)7-13(14)30-19(20)9-2-4-10(22)5-3-9/h2-8,15,17-18,21-23,25-28H,1H3/t8-,15-,17+,18+,21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Yunnan Normal University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 expressed in Escherichia coli BL21(DE3) assessed as N-methyldihydronicotinamide oxidation per mg of protein a... |
J Nat Prod 74: 129-36 (2011)
Article DOI: 10.1021/np100373f
BindingDB Entry DOI: 10.7270/Q20865MS |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269599
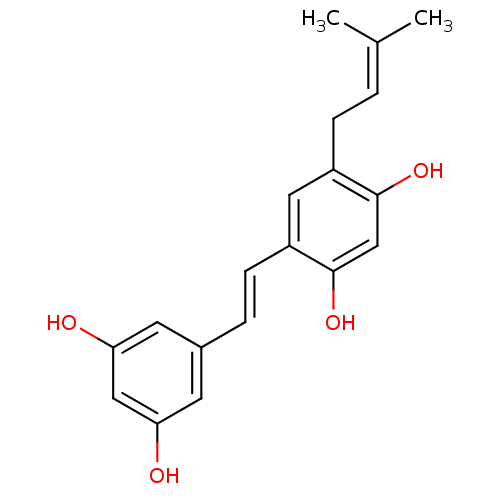
(5-(gamma,gamma-dimethylallyl)-oxyresveratrol | CHE...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)c(-[#8])cc1-[#8] Show InChI InChI=1S/C19H20O4/c1-12(2)3-5-14-9-15(19(23)11-18(14)22)6-4-13-7-16(20)10-17(21)8-13/h3-4,6-11,20-23H,5H2,1-2H3/b6-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251013
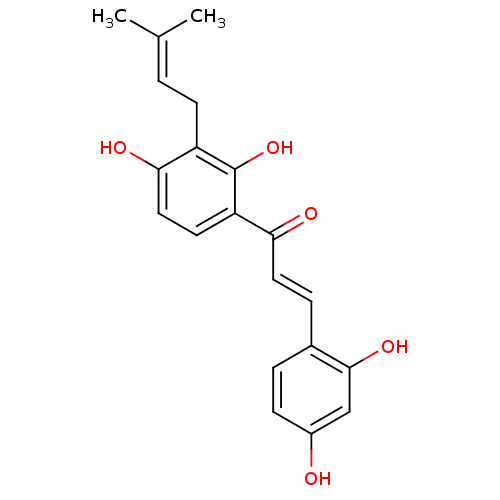
(2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc(-[#6](=O)\[#6]=[#6]\c2ccc(-[#8])cc2-[#8])c1-[#8] Show InChI InChI=1S/C20H20O5/c1-12(2)3-7-15-18(23)10-8-16(20(15)25)17(22)9-5-13-4-6-14(21)11-19(13)24/h3-6,8-11,21,23-25H,7H2,1-2H3/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50100939
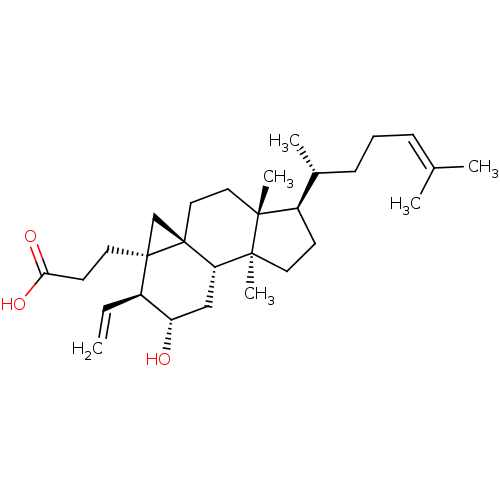
(3-[(2S,3S,3aR,4aS,6aR,7R,9aS,9bS)-7-((R)-1,5-Dimet...)Show SMILES [#6]-[#6@H](-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@H]1-[#6]-[#6][C@@]2([#6])[#6@@H]3-[#6]-[#6@H](-[#8])-[#6@@H](-[#6]=[#6])[C@@]4([#6]-[#6]-[#6](-[#8])=O)[#6][C@@]34[#6]-[#6][C@]12[#6] Show InChI InChI=1S/C29H46O3/c1-7-21-23(30)17-24-27(6)13-11-22(20(4)10-8-9-19(2)3)26(27,5)15-16-29(24)18-28(21,29)14-12-25(31)32/h7,9,20-24,30H,1,8,10-18H2,2-6H3,(H,31,32)/t20-,21-,22-,23+,24+,26-,27+,28-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Prostaglandin G/H synthase 2 |
Bioorg Med Chem Lett 11: 1565-8 (2001)
BindingDB Entry DOI: 10.7270/Q2862FQQ |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269598
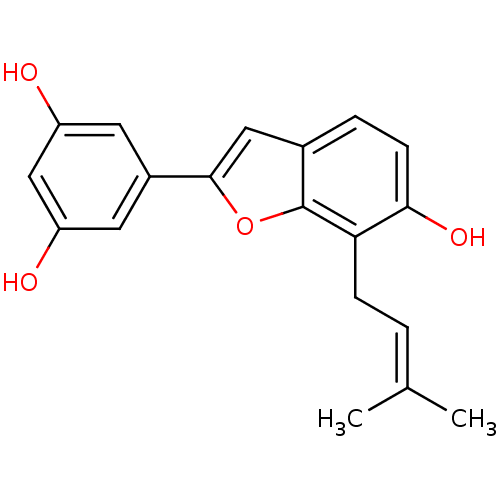
(3-(gamma,gamma-dimethylpropenyl)moracinM | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])ccc2cc(oc12)-c1cc(-[#8])cc(-[#8])c1 Show InChI InChI=1S/C19H18O4/c1-11(2)3-5-16-17(22)6-4-12-9-18(23-19(12)16)13-7-14(20)10-15(21)8-13/h3-4,6-10,20-22H,5H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478512

(1Beta-Hydroxymaprounic Acid | CHEMBL512314)Show SMILES [H][C@@]12CC[C@@]3(C)C4=CC[C@]5(CCC(C)(C)C[C@@]5([H])[C@]4(C)CC[C@]3([H])[C@@]1(C)[C@H](O)C[C@H](O)C2(C)C)C(O)=O |r,t:6| Show InChI InChI=1S/C30H48O4/c1-25(2)14-15-30(24(33)34)13-10-19-27(5)11-8-18-26(3,4)22(31)16-23(32)29(18,7)20(27)9-12-28(19,6)21(30)17-25/h10,18,20-23,31-32H,8-9,11-17H2,1-7H3,(H,33,34)/t18-,20-,21-,22-,23+,27-,28+,29-,30+/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Nat Prod 57: 415-8 (1994)
Article DOI: 10.1021/np50105a017
BindingDB Entry DOI: 10.7270/Q2ZW1PNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269605
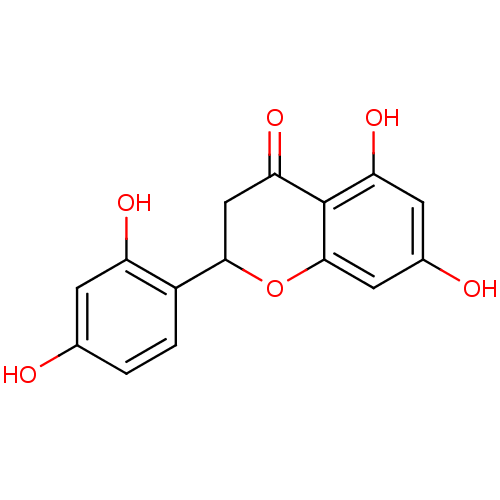
(CHEMBL465194 | steppogenin)Show InChI InChI=1S/C15H12O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-5,13,16-19H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50240892

((-)-epiafzelechin | (2R,3R)-2-(4-Hydroxy-phenyl)-c...)Show SMILES O[C@@H]1Cc2c(O)cc(O)cc2O[C@@H]1c1ccc(O)cc1 |r| Show InChI InChI=1S/C15H14O5/c16-9-3-1-8(2-4-9)15-13(19)7-11-12(18)5-10(17)6-14(11)20-15/h1-6,13,15-19H,7H2/t13-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412081

(CHEMBL505436)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4[C@@H](OC(C)=O)[C@H]([C@H](O)C[C@]4(C)[C@H]3CC[C@]12C)N1C(=O)c2ccccc2C1=O)N(C)C |r| Show InChI InChI=1S/C33H46N2O5/c1-18(34(5)6)23-13-14-24-22-11-12-26-29(40-19(2)36)28(35-30(38)20-9-7-8-10-21(20)31(35)39)27(37)17-33(26,4)25(22)15-16-32(23,24)3/h7-10,18,22-29,37H,11-17H2,1-6H3/t18-,22-,23+,24-,25-,26-,27+,28-,29+,32+,33+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50250978
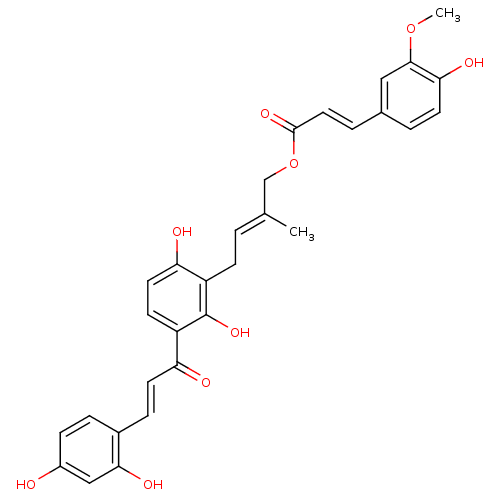
(CHEMBL463638 | isogemichalcone C)Show SMILES COc1cc(\C=C\C(=O)OC\C(C)=C\Cc2c(O)ccc(C(=O)\C=C\c3ccc(O)cc3O)c2O)ccc1O Show InChI InChI=1S/C30H28O9/c1-18(17-39-29(36)14-5-19-4-11-26(34)28(15-19)38-2)3-9-22-25(33)13-10-23(30(22)37)24(32)12-7-20-6-8-21(31)16-27(20)35/h3-8,10-16,31,33-35,37H,9,17H2,1-2H3/b12-7+,14-5+,18-3+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50412079
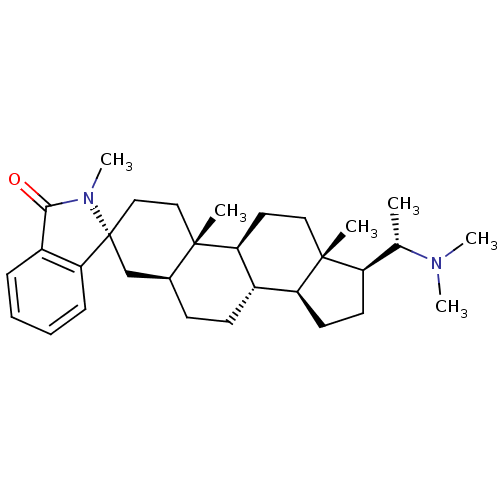
(CHEMBL456512)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C[C@@]5(CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C(=O)c1ccccc51)N(C)C |r| Show InChI InChI=1S/C31H46N2O/c1-20(32(4)5)24-13-14-25-22-12-11-21-19-31(27-10-8-7-9-23(27)28(34)33(31)6)18-17-29(21,2)26(22)15-16-30(24,25)3/h7-10,20-22,24-26H,11-19H2,1-6H3/t20-,21-,22-,24+,25-,26-,29-,30+,31+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
DNA polymerase beta
(Homo sapiens (Human)) | BDBM50250356
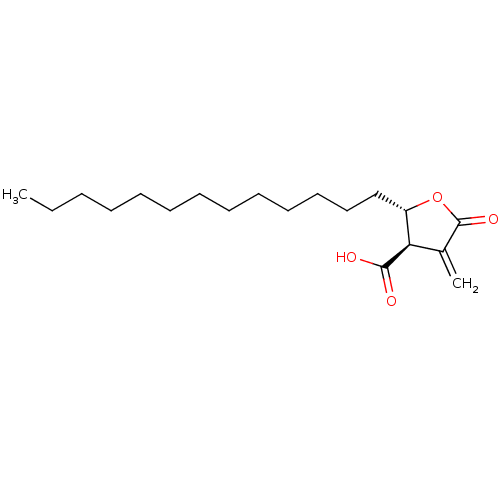
((+)-protolichesterinic acid | CHEMBL490329 | proto...)Show SMILES CCCCCCCCCCCCC[C@@H]1OC(=O)C(=C)[C@H]1C(O)=O |r| Show InChI InChI=1S/C19H32O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-16-17(18(20)21)15(2)19(22)23-16/h16-17H,2-14H2,1H3,(H,20,21)/t16-,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of DNA polymerase beta |
J Nat Prod 58: 1024-1031 (1995)
Article DOI: 10.1021/np50121a006
BindingDB Entry DOI: 10.7270/Q2639PR2 |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50251015
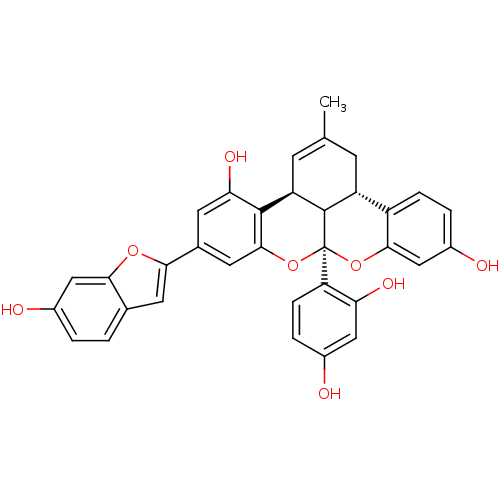
(CHEMBL454705 | albanol A)Show SMILES CC1=C[C@H]2C3[C@H](C1)c1ccc(O)cc1O[C@]3(Oc1cc(cc(O)c21)-c1cc2ccc(O)cc2o1)c1ccc(O)cc1O |r,t:1| Show InChI InChI=1S/C34H26O8/c1-16-8-23-22-6-4-21(37)15-30(22)41-34(25-7-5-19(35)13-26(25)38)33(23)24(9-16)32-27(39)10-18(12-31(32)42-34)28-11-17-2-3-20(36)14-29(17)40-28/h2-7,9-15,23-24,33,35-39H,8H2,1H3/t23-,24-,33?,34+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 7.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
7-dehydrocholesterol reductase
(Homo sapiens (Human)) | BDBM50135147
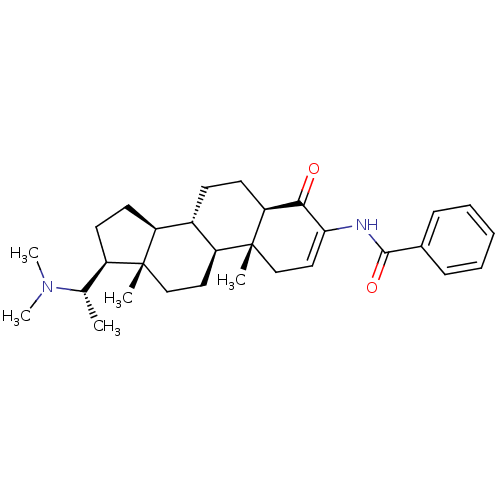
((+)-axillaridine A | 14-(1-dimethylaminoethyl)-2,1...)Show SMILES C[C@@H]([C@H]1CC[C@H]2[C@@H]3CC[C@H]4C(=O)C(NC(=O)c5ccccc5)=CC[C@]4(C)[C@H]3CC[C@]12C)N(C)C |r,c:22| Show InChI InChI=1S/C30H42N2O2/c1-19(32(4)5)22-13-14-23-21-11-12-25-27(33)26(31-28(34)20-9-7-6-8-10-20)16-18-30(25,3)24(21)15-17-29(22,23)2/h6-10,16,19,21-25H,11-15,17-18H2,1-5H3,(H,31,34)/t19-,21-,22+,23-,24-,25-,29+,30+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Displacement of [3H]tamoxifen from antiestrogen binding site in Sprague-Dawley rat liver by liquid scintillation counting |
J Nat Prod 61: 1257-62 (1998)
Article DOI: 10.1021/np980162x
BindingDB Entry DOI: 10.7270/Q2B27WJ0 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269600
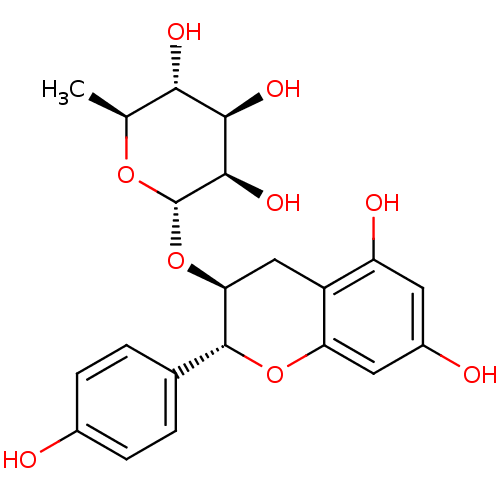
(CHEMBL517149 | afzelechin-3-O-alpha-Lrhamnopyranos...)Show SMILES C[C@@H]1O[C@@H](O[C@H]2Cc3c(O)cc(O)cc3O[C@@H]2c2ccc(O)cc2)[C@H](O)[C@H](O)[C@H]1O |r| Show InChI InChI=1S/C21H24O9/c1-9-17(25)18(26)19(27)21(28-9)30-16-8-13-14(24)6-12(23)7-15(13)29-20(16)10-2-4-11(22)5-3-10/h2-7,9,16-27H,8H2,1H3/t9-,16-,17-,18+,19+,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269596

(3-(gamma,gamma-dimethylallyl)resveratrol | CHEMBL4...)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(\[#6]=[#6]\c2cc(-[#8])cc(-[#8])c2)ccc1-[#8] Show InChI InChI=1S/C19H20O3/c1-13(2)3-7-16-9-14(6-8-19(16)22)4-5-15-10-17(20)12-18(21)11-15/h3-6,8-12,20-22H,7H2,1-2H3/b5-4+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM50242016
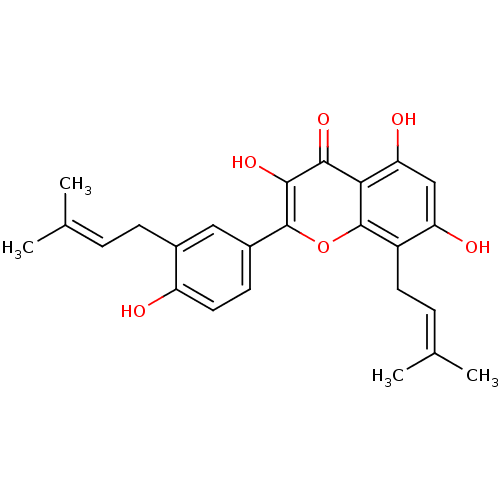
(CHEMBL464007 | broussoflavonol F)Show SMILES [#6]\[#6](-[#6])=[#6]\[#6]-c1cc(ccc1-[#8])-c1oc2c(-[#6]\[#6]=[#6](\[#6])-[#6])c(-[#8])cc(-[#8])c2c(=O)c1-[#8] Show InChI InChI=1S/C25H26O6/c1-13(2)5-7-15-11-16(8-10-18(15)26)24-23(30)22(29)21-20(28)12-19(27)17(25(21)31-24)9-6-14(3)4/h5-6,8,10-12,26-28,30H,7,9H2,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269606
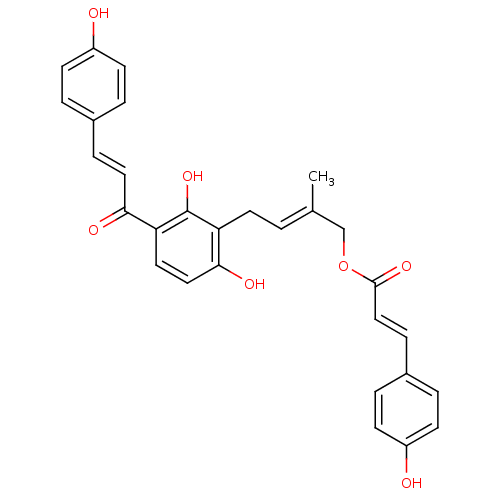
(CHEMBL517334 | isogemichalcone B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478514
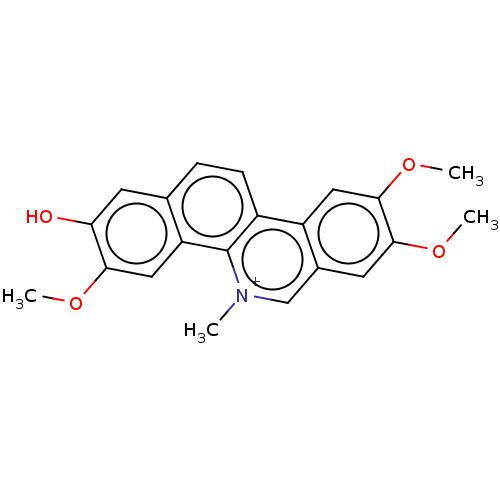
(Fagaronine | Fagaronine Chloride)Show SMILES [Cl-].COc1cc2c(ccc3c4cc(OC)c(OC)cc4c[n+](C)c23)cc1O Show InChI InChI=1S/C21H19NO4/c1-22-11-13-8-19(25-3)20(26-4)9-15(13)14-6-5-12-7-17(23)18(24-2)10-16(12)21(14)22/h5-11H,1-4H3/p+1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Nat Prod 57: 415-8 (1994)
Article DOI: 10.1021/np50105a017
BindingDB Entry DOI: 10.7270/Q2ZW1PNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269597

(3-(2,3-dihydroxy-3-methylbutyl)resveratrol | CHEMB...)Show SMILES CC(C)(O)[C@H](O)Cc1cc(\C=C\c2cc(O)cc(O)c2)ccc1O |r| Show InChI InChI=1S/C19H22O5/c1-19(2,24)18(23)10-14-7-12(5-6-17(14)22)3-4-13-8-15(20)11-16(21)9-13/h3-9,11,18,20-24H,10H2,1-2H3/b4-3+/t18-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50478516
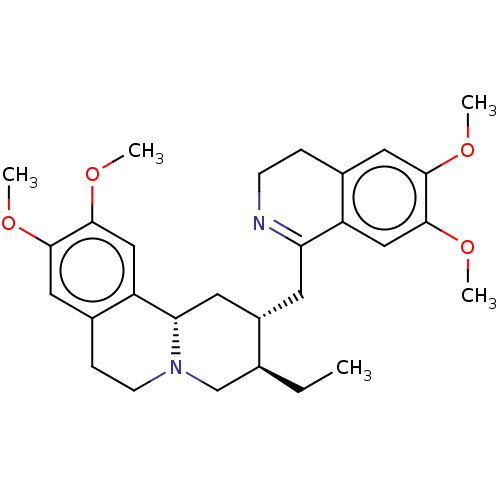
(CHEBI:81067 | CHEMBL471812 | O-Methylpsychotrine)Show SMILES [H][C@]1(CC2=NCCc3cc(OC)c(OC)cc23)C[C@]2([H])N(CCc3cc(OC)c(OC)cc23)C[C@@H]1CC |r,t:3| Show InChI InChI=1S/C29H38N2O4/c1-6-18-17-31-10-8-20-14-27(33-3)29(35-5)16-23(20)25(31)12-21(18)11-24-22-15-28(34-4)26(32-2)13-19(22)7-9-30-24/h13-16,18,21,25H,6-12,17H2,1-5H3/t18-,21-,25-/m0/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 reverse transcriptase |
J Nat Prod 57: 415-8 (1994)
Article DOI: 10.1021/np50105a017
BindingDB Entry DOI: 10.7270/Q2ZW1PNF |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269277
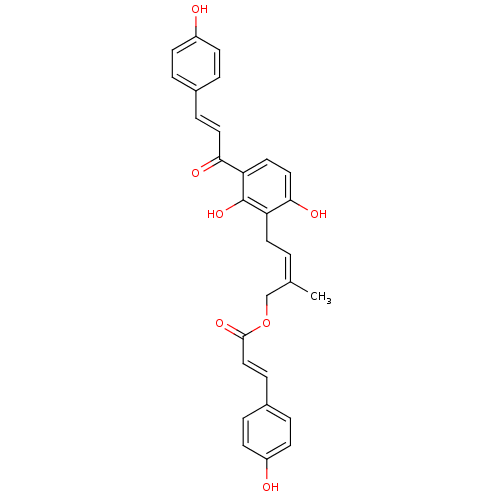
(CHEMBL497716 | gemichalcone B | gemichalcones B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C\Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50269559
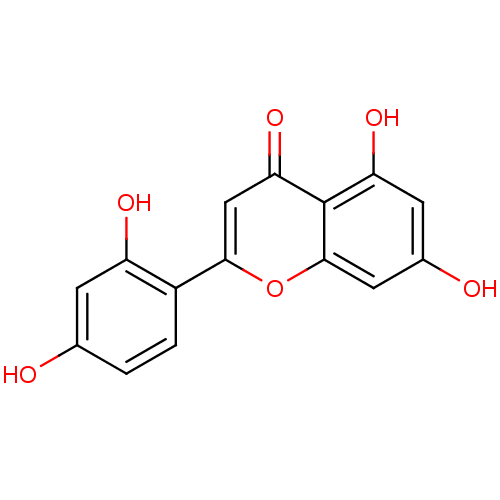
(2-(2,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...)Show InChI InChI=1S/C15H10O6/c16-7-1-2-9(10(18)3-7)13-6-12(20)15-11(19)4-8(17)5-14(15)21-13/h1-6,16-19H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50269606

(CHEMBL517334 | isogemichalcone B)Show SMILES C\C(COC(=O)\C=C\c1ccc(O)cc1)=C/Cc1c(O)ccc(C(=O)\C=C\c2ccc(O)cc2)c1O Show InChI InChI=1S/C29H26O7/c1-19(18-36-28(34)17-8-21-5-11-23(31)12-6-21)2-13-24-27(33)16-14-25(29(24)35)26(32)15-7-20-3-9-22(30)10-4-20/h2-12,14-17,30-31,33,35H,13,18H2,1H3/b15-7+,17-8+,19-2+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
J Nat Prod 65: 163-9 (2002)
BindingDB Entry DOI: 10.7270/Q2J38TGV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM23419

((2S)-5,7-dihydroxy-2-(4-hydroxyphenyl)-3,4-dihydro...)Show SMILES Oc1ccc(cc1)[C@@H]1CC(=O)c2c(O)cc(O)cc2O1 |r| Show InChI InChI=1S/C15H12O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-6,13,16-18H,7H2/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of aromatase from human placental microsomes |
J Nat Prod 64: 1286-93 (2001)
BindingDB Entry DOI: 10.7270/Q2RB74C4 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data