

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0210 | -63.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.0400 | -61.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,I543V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.190 | -57.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,M535I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 0.5 | -55.2 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 0.700 | -54.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM518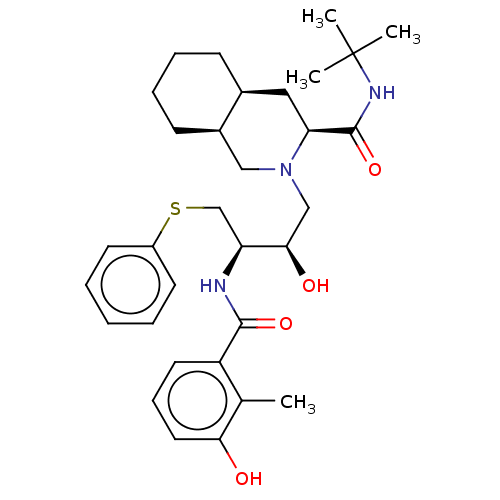 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 1.20 | -53.0 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,M535I,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132686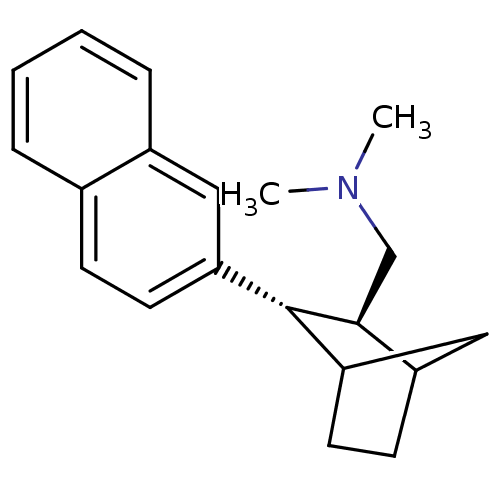 (CHEMBL326466 | Dimethyl-((2S,3S)-3-naphthalen-2-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132687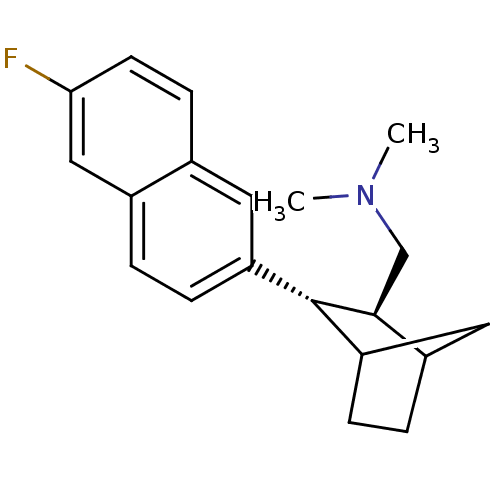 (CHEMBL324269 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132685 (CHEMBL109571 | Dimethyl-(3-naphthalen-2-yl-bicyclo...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055234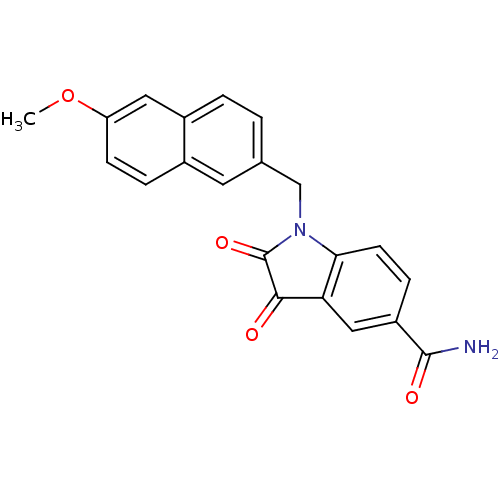 (1-(6-Methoxy-naphthalen-2-ylmethyl)-2,3-dioxo-2,3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM517 ((2S)-1-[(2S,4R)-4-benzyl-2-hydroxy-4-{[(1S,2R)-2-h...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 3.10 | -50.5 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132679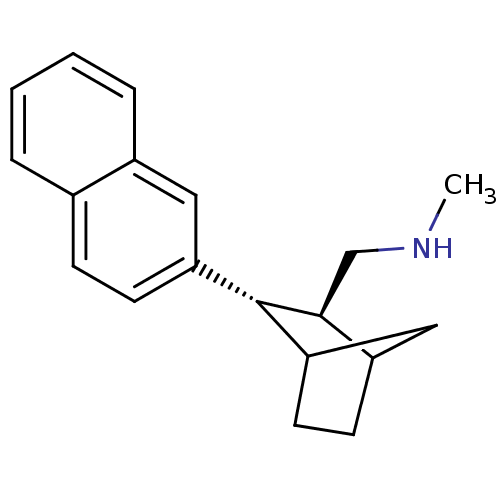 (CHEMBL111128 | Methyl-((2S,3S)-3-naphthalen-2-yl-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055218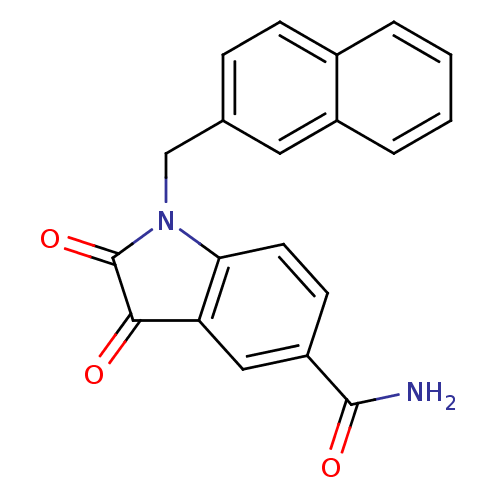 (1-(2-naphthlmethyl) isatin-5-carboxamide | 1-Napht...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055232 (1-(6-Hydroxy-naphthalen-2-ylmethyl)-2,3-dioxo-2,3-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055213 (1-(3,5-Dihydroxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132678 (CHEMBL432022 | [(2S,3S)-3-(6-Fluoro-naphthalen-2-y...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132684 (CHEMBL331799 | Methyl-(3-naphthalen-2-yl-bicyclo[2...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065588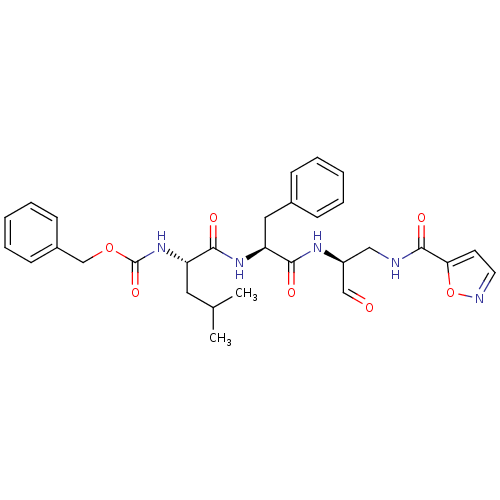 (CHEMBL96803 | [(S)-1-((S)-1-{(S)-1-Formyl-2-[(isox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065603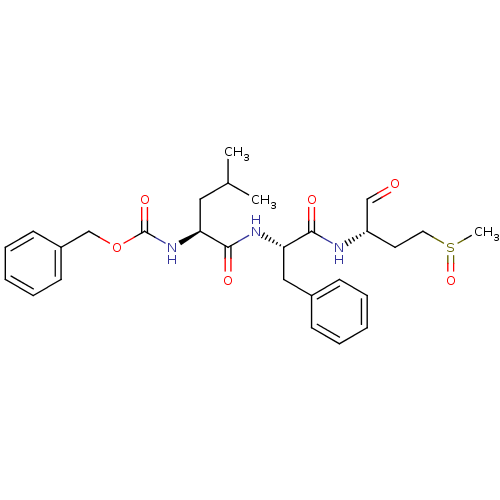 (CHEMBL96185 | {(S)-1-[(S)-1-((S)-1-Formyl-3-methan...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065598 (CHEMBL419332 | {(S)-1-[(S)-1-((S)-3-Dimethylcarbam...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,M535I,I543V,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132677 (CHEMBL109517 | Dimethyl-((2R,3R)-3-naphthalen-2-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50132683 (6-((2S,3S)-3-Dimethylaminomethyl-bicyclo[2.2.1]hep...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-WIN-35,428 from Dopamine transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065590 (((S)-1-{(S)-1-[(S)-1-(Acetylamino-methyl)-2-oxo-et...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055217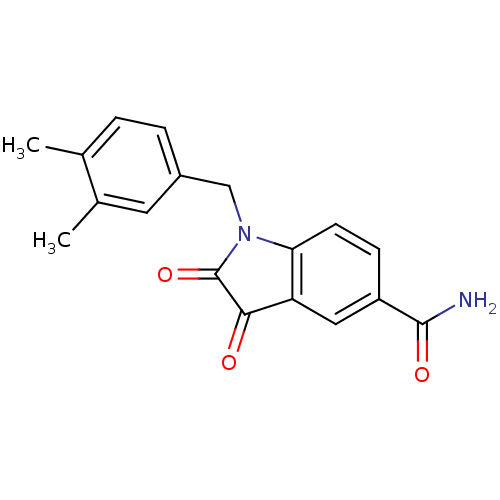 (1-(3,4-Dimethyl-benzyl)-2,3-dioxo-2,3-dihydro-1H-i...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065586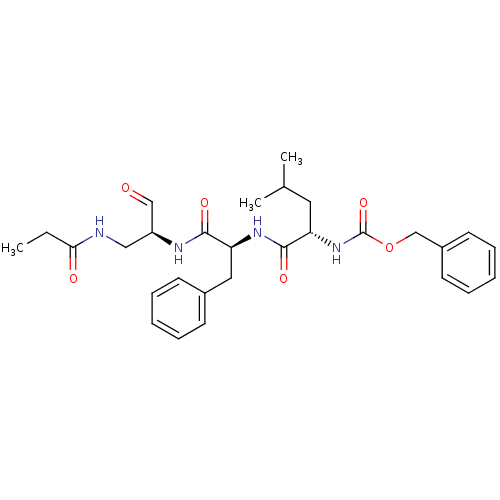 (((S)-3-Methyl-1-{(S)-1-[(S)-2-oxo-1-(propionylamin...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50026952 ((cis) [3-(3,4-Dichloro-phenyl)-bicyclo[2.2.2]oct-2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-NE re-uptake into synaptosome | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,I543V,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM580 ((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065595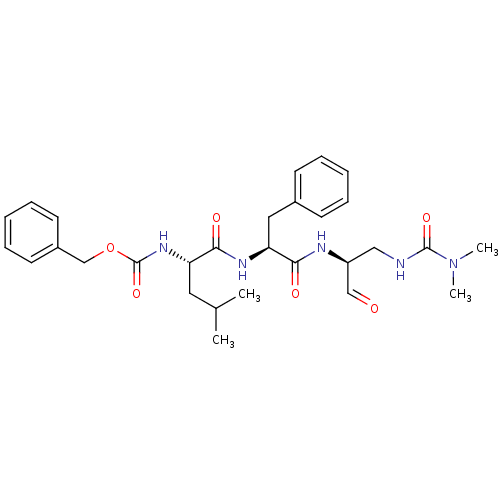 (((S)-1-{(S)-1-[(S)-2-(3,3-Dimethyl-ureido)-1-formy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055225 (1-(4-Methyl-benzyl)-2,3-dioxo-2,3-dihydro-1H-indol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055212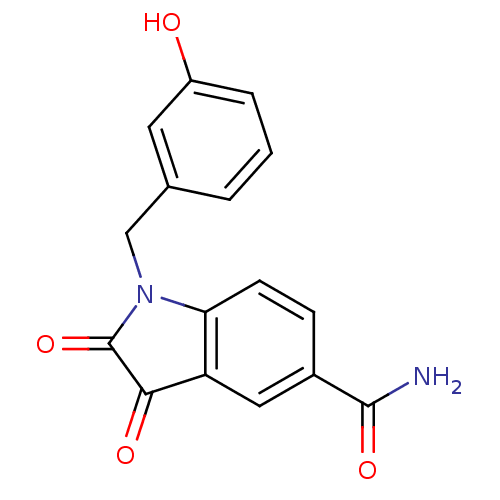 (1-(3-Hydroxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-indo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065599 (CHEMBL94652 | {(S)-1-[(S)-1-((S)-2-Benzoylamino-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132680 (CHEMBL322348 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 17 | -46.1 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065601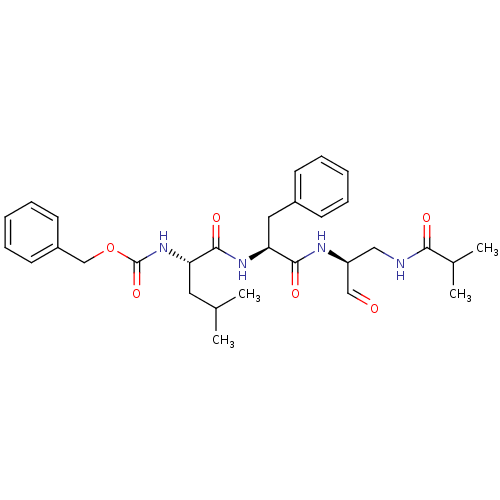 (CHEMBL95031 | {(S)-1-[(S)-1-((S)-1-Formyl-2-isobut...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Binding affinity against HRV-14 3C protease. | J Med Chem 41: 2786-805 (1998) Article DOI: 10.1021/jm980071x BindingDB Entry DOI: 10.7270/Q2F76BPF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50132685 (CHEMBL109571 | Dimethyl-(3-naphthalen-2-yl-bicyclo...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-WIN-35,428 from Dopamine transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055220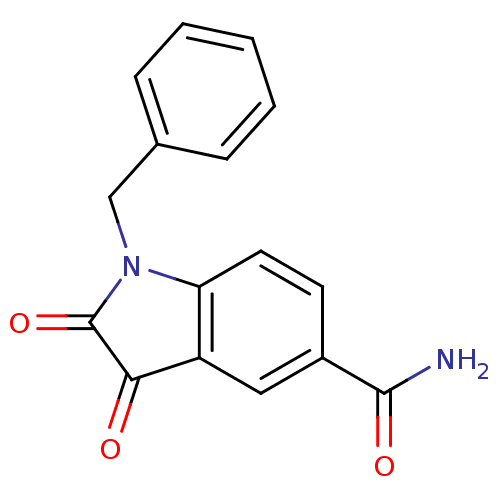 (1-Benzyl-2,3-dioxo-2,3-dihydro-1H-indole-5-carboxy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,I543V,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 23 | -45.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,I573V,L579M] (Human immunodeficiency virus type 1) | BDBM518 ((3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132681 (CHEMBL323678 | [(2S,3S)-3-(6-Methoxy-naphthalen-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from Serotonin transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50132682 (CHEMBL113136 | [(2S,3S)-3-(3-Chloro-phenyl)-bicycl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description In vitro ability of compound to inhibit 5-HT re-uptake of radiolabelled [3H]-tritium trasmitter into synaptosome | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50132686 (CHEMBL326466 | Dimethyl-((2S,3S)-3-naphthalen-2-yl...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description Displacement of [3H]-WIN-35,428 from Dopamine transporter | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50132684 (CHEMBL331799 | Methyl-(3-naphthalen-2-yl-bicyclo[2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Centre Curated by ChEMBL | Assay Description In vitro inhibition of DA re-uptake into synaptosome | Bioorg Med Chem Lett 13: 3277-80 (2003) BindingDB Entry DOI: 10.7270/Q29W0DXF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055229 (1-(3,5-Dimethoxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055226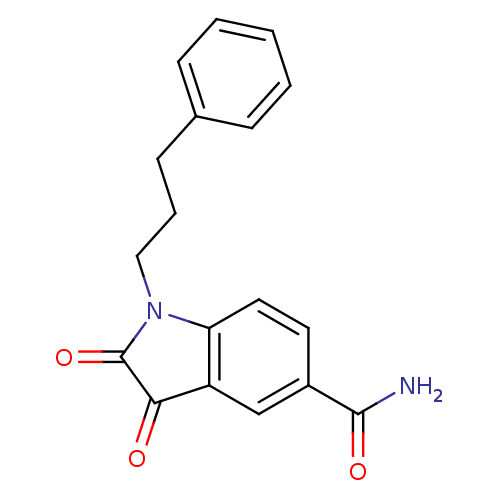 (2,3-Dioxo-1-(3-phenyl-propyl)-2,3-dihydro-1H-indol...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50055214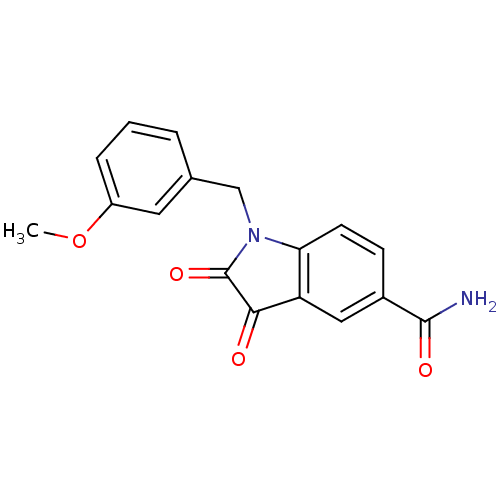 (1-(3-Methoxy-benzyl)-2,3-dioxo-2,3-dihydro-1H-indo...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human rhinovirus 3C protease | J Med Chem 39: 5072-82 (1997) Article DOI: 10.1021/jm960603e BindingDB Entry DOI: 10.7270/Q2K936MP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587,K509R,V521I,L522F,M525I,L552P,A560V,V571A,L579M] (Human immunodeficiency virus type 1) | BDBM520 (1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 30 | -44.7 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
University of Florida College of Medicine | Assay Description The inhibition constants Ki were determined by monitoring the inhibition of hydrolysis of the chromogenic substrate using a Hewlett-Packard 8452A spe... | Biochemistry 43: 12141-51 (2004) Article DOI: 10.1021/bi049459m BindingDB Entry DOI: 10.7270/Q2V69GTD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Human rhinovirus B) | BDBM50065621 (CHEMBL94688 | [(S)-1-((S)-1-{(S)-3-Carbamoyl-1-[2-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Agouron Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Catalytic rate constant (Kobs/[I]) of the compound was evaluated against human rhinovirus (HRV) serotype 14 3C Protease (3CP) | J Med Chem 41: 2806-18 (1998) Article DOI: 10.1021/jm980068d BindingDB Entry DOI: 10.7270/Q29G5KZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1074 total ) | Next | Last >> |