

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Sus scrofa) | BDBM50452524 (CHEMBL2372291) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334215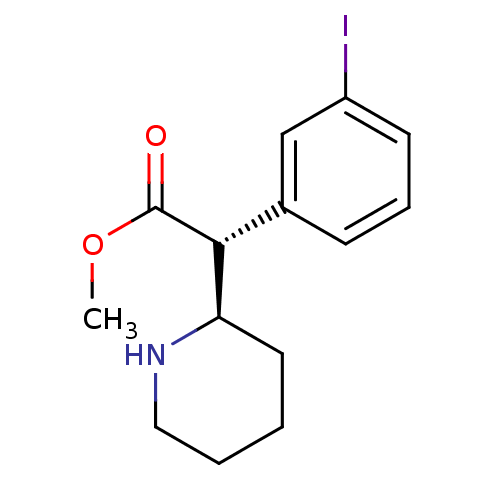 ((+/-)-threo-3-Iodomethylphenidate | CHEMBL1641691) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50293605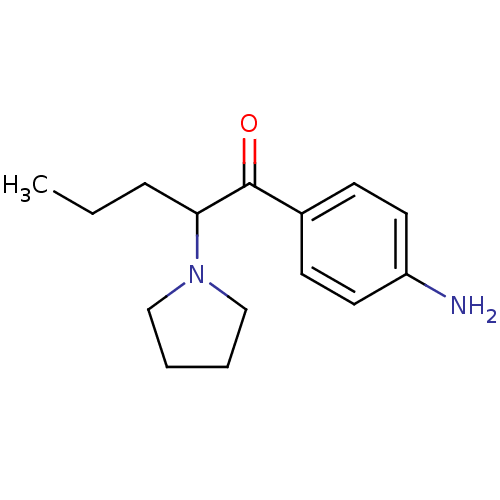 (1-(4-aminophenyl)-2-pyrrolidin-1-yl-pentan-1-one |...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human dopamine transporter expressed in mouse N2A cells by scintillation counting | Bioorg Med Chem 17: 3770-4 (2009) Article DOI: 10.1016/j.bmc.2009.04.057 BindingDB Entry DOI: 10.7270/Q2SJ1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50182555 (1-(4-methylphenyl)-2-pyrrolidin-1-yl-pentan-1-one ...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human dopamine transporter expressed in mouse N2A cells by scintillation counting | Bioorg Med Chem 17: 3770-4 (2009) Article DOI: 10.1016/j.bmc.2009.04.057 BindingDB Entry DOI: 10.7270/Q2SJ1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50327148 ((R)-methyl 2-(4-iodophenyl)-2-((R)-piperidin-2-yl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50062915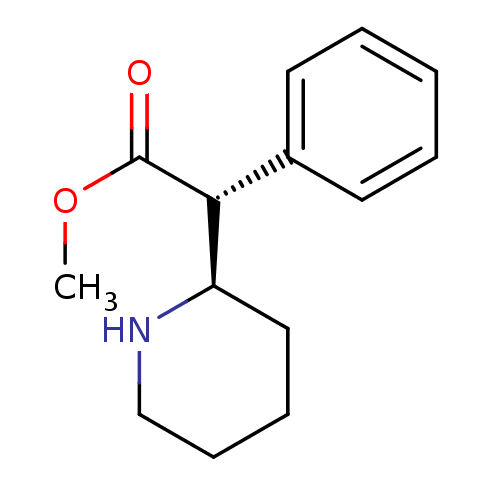 (CHEMBL827 | METHYLPHENIDATE | methyl (2R)-phenyl[(...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50293604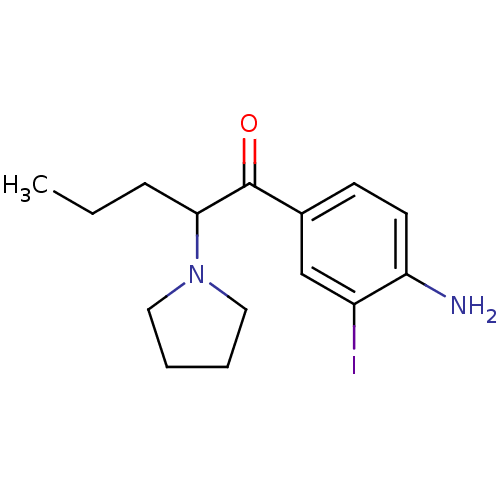 (1-(4-amino-3-iodophenyl)-2-pyrrolidin-1-yl-pentan-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human dopamine transporter expressed in mouse N2A cells by scintillation counting | Bioorg Med Chem 17: 3770-4 (2009) Article DOI: 10.1016/j.bmc.2009.04.057 BindingDB Entry DOI: 10.7270/Q2SJ1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (RAT) | BDBM50605085 (CHEMBL5204021) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50293606 (1-(4-azio-3-iodophenyl)-2-pyrroldin-1-yl-pentan-1-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human dopamine transporter expressed in mouse N2A cells by scintillation counting | Bioorg Med Chem 17: 3770-4 (2009) Article DOI: 10.1016/j.bmc.2009.04.057 BindingDB Entry DOI: 10.7270/Q2SJ1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334214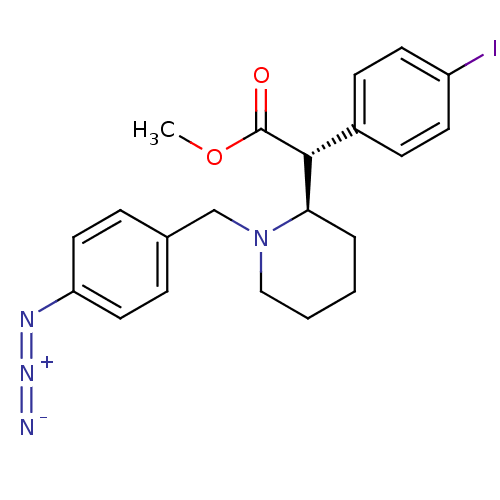 ((+/-)-threo-N-(p-Azido-benzyl)-4-iodomethylphenida...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 363 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50048392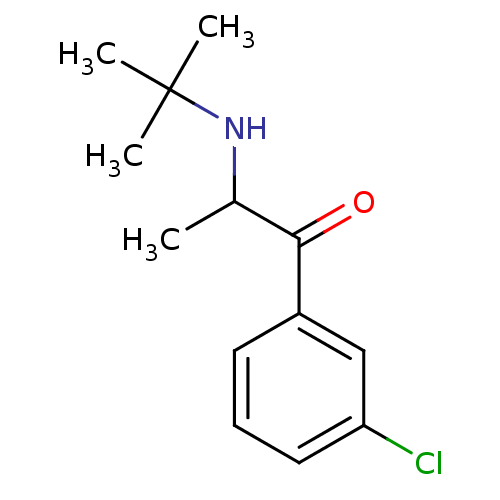 (2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | 441 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN35428 from human dopamine transporter expressed in mouse N2A cells by scintillation counting | Bioorg Med Chem 17: 3770-4 (2009) Article DOI: 10.1016/j.bmc.2009.04.057 BindingDB Entry DOI: 10.7270/Q2SJ1KN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334212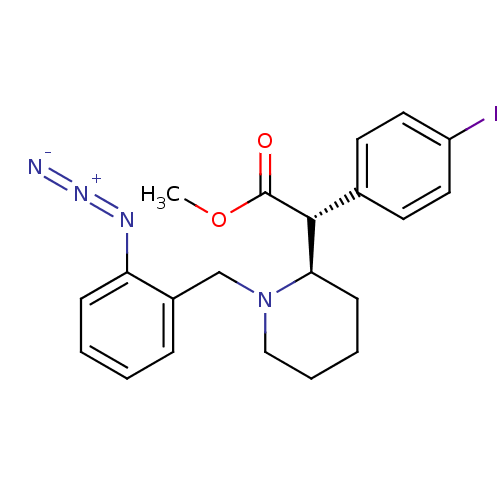 ((+/-)-threo-N-(o-Azido-benzyl)-4-iodomethylphenida...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 517 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334211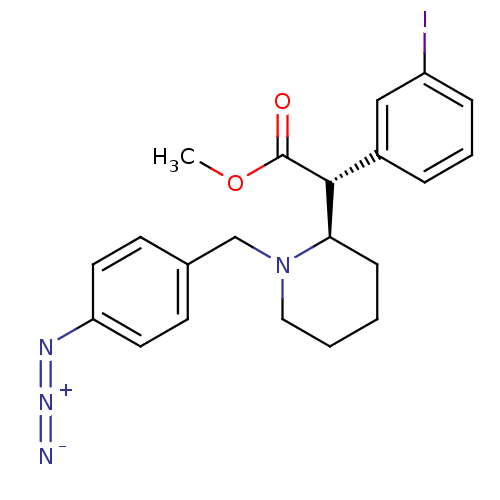 ((+/-)-threo-N-(p-Azido-benzyl)-3-iodomethylphenida...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 658 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334209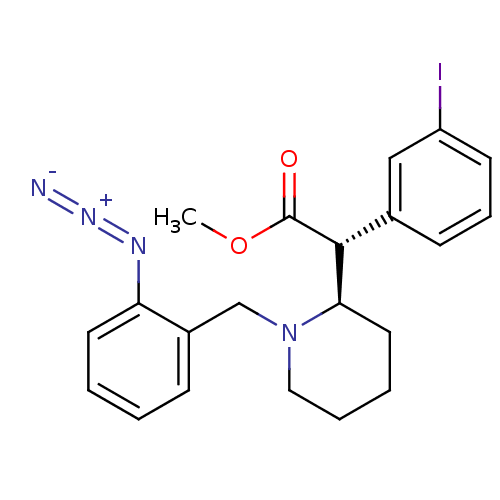 ((+/-)-threo-N-(o-Azido-benzyl)-3-iodomethylphenida...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334210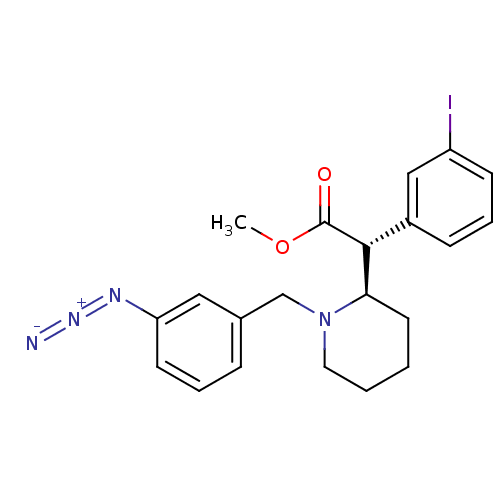 ((+/-)-threo-N-(m-Azido-benzyl)-3-iodomethylphenida...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M5 (Homo sapiens (Human)) | BDBM50426242 (CHEMBL2312346) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50334213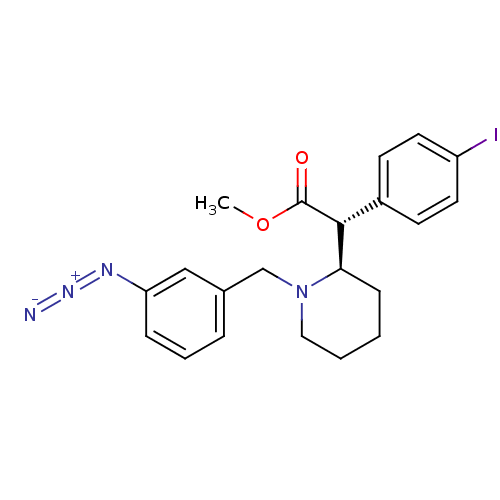 ((+/-)-threo-N-(m-Azido-benzyl)-4-iodomethylphenida...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Mylan School of Pharmacy Curated by ChEMBL | Assay Description Displacement of [3H]WIN-35428 from human DAT stably expressed in mouse N2A cells by scintillation countnig | Bioorg Med Chem 19: 504-12 (2011) Article DOI: 10.1016/j.bmc.2010.11.002 BindingDB Entry DOI: 10.7270/Q29Z9567 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1 (Homo sapiens (Human)) | BDBM50426242 (CHEMBL2312346) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50426242 (CHEMBL2312346) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (Homo sapiens (Human)) | BDBM50426242 (CHEMBL2312346) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50426242 (CHEMBL2312346) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acs.jmedchem.2c00192 BindingDB Entry DOI: 10.7270/Q23N27HG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023754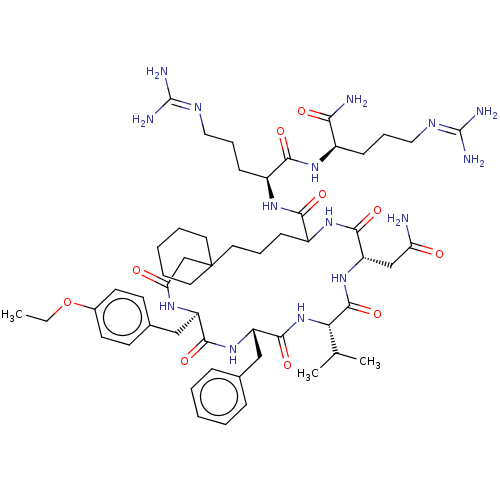 (13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023750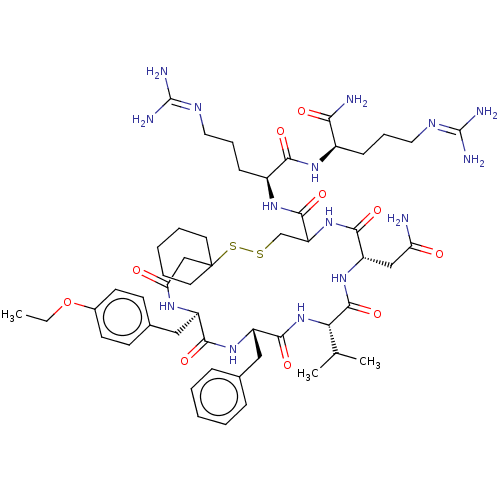 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50023750 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023752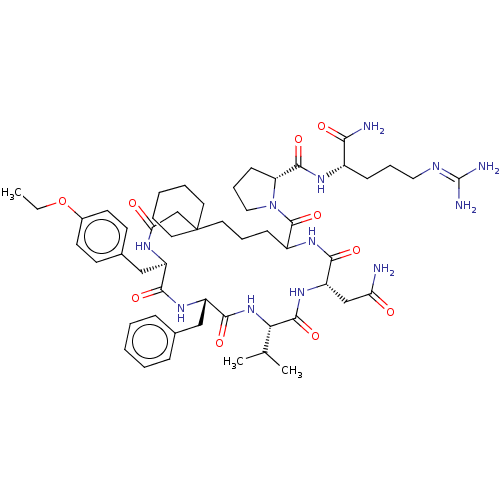 (1-[13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50452524 (CHEMBL2372291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50023751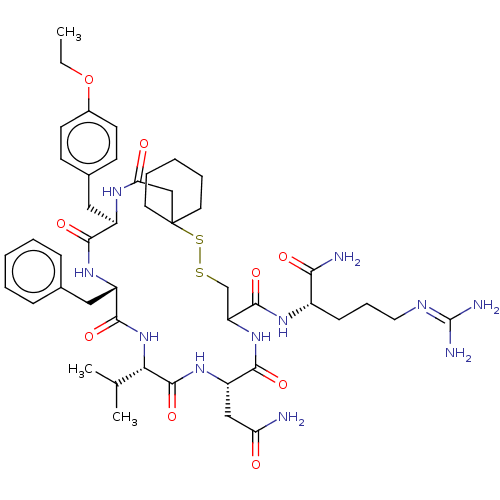 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023751 (19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023750 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50023752 (1-[13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023752 (1-[13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in human | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50452524 (CHEMBL2372291) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50023754 (13-Benzyl-19-carbamoylmethyl-10-(4-ethoxy-benzyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in human | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 1/2/3/4/5/6/7/8/9 (Homo sapiens (Human)) | BDBM50227472 (CHEMBL2372272) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.40E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smith Kline & French Laboratories Curated by ChEMBL | Assay Description In vitro antagonist activity of the compound was measured by inhibition of vasopressin-stimulated adenylate cyclase in renal medullary preparation in... | J Med Chem 31: 1487-9 (1988) BindingDB Entry DOI: 10.7270/Q2S46QZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil collagenase (Homo sapiens (Human)) | BDBM50082965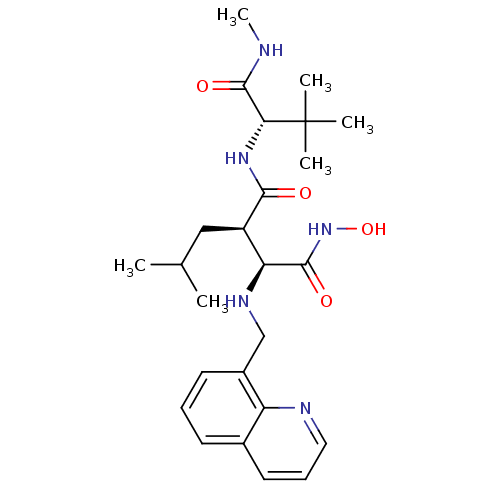 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibition of MMP-8 (matrix metalloproteinase-8) | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50070453 ((2R,3S)-2-Allyl-N*1*-hydroxy-3-isobutyl-N*4*-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082973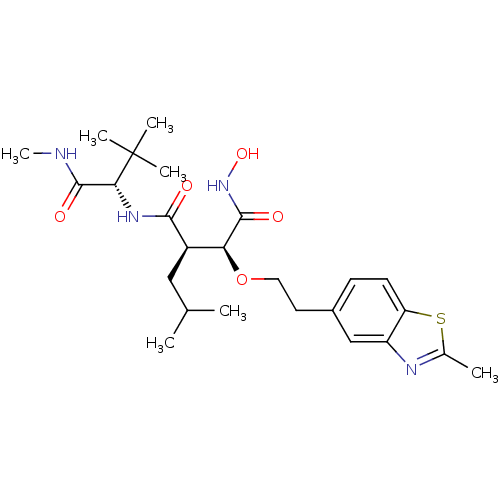 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082965 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082946 ((2R,3S)-2-(7-Bromo-2-methyl-4-oxo-3,4-dihydro-quin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082959 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082983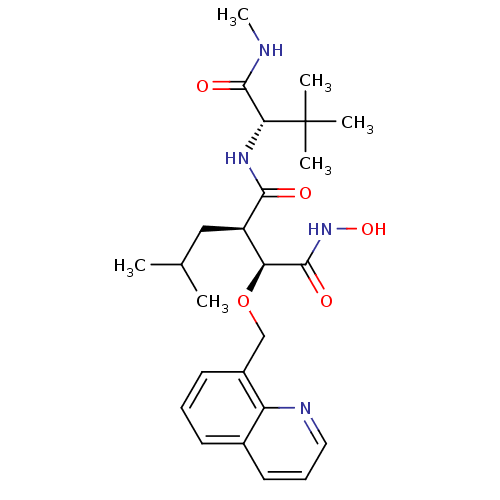 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082974 ((2S,3S)-2-(3,5-Dichloro-phenylsulfanyl)-N*4*-((S)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082993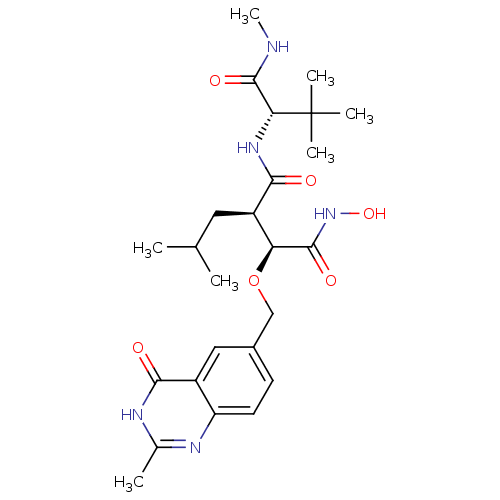 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082948 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082942 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082988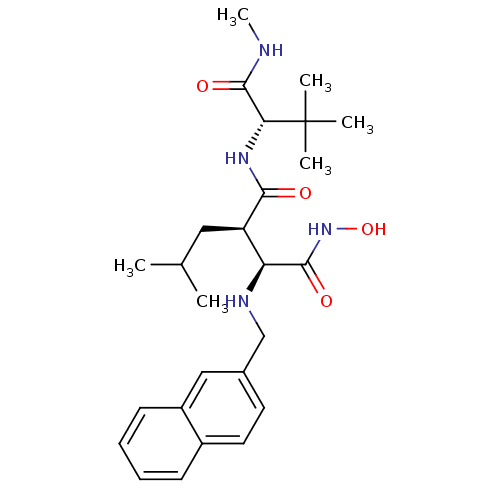 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082963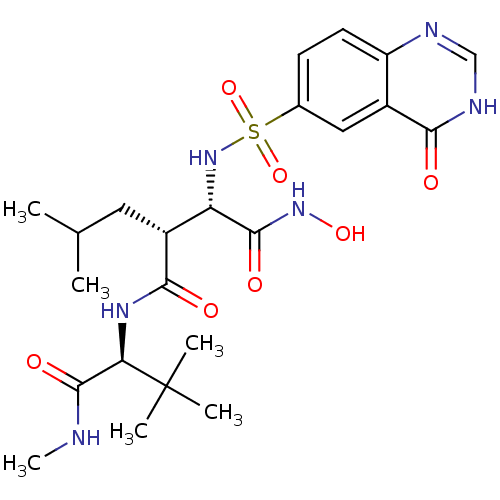 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082949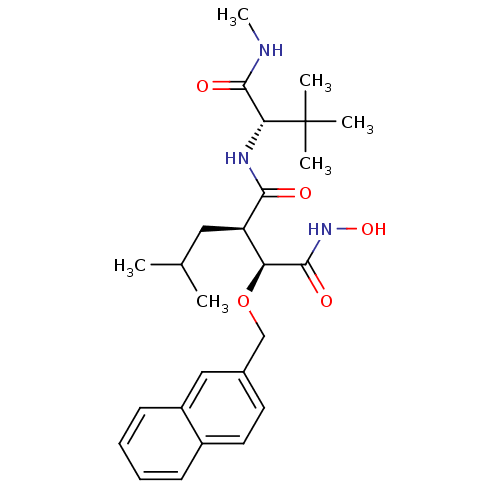 ((2R,3S)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082980 ((2R,3S)-2-(Benzenesulfonyl-methyl-amino)-N*4*-((S)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM50082939 ((2S,3S)-2-(4-Cyano-phenylsulfanyl)-N*4*-((S)-2,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a |
Centre de Recherches Curated by ChEMBL | Assay Description Inhibitory activity against Tumor necrosis factor alpha-converting enzyme (TACE) in blood. | J Med Chem 42: 4890-908 (1999) BindingDB Entry DOI: 10.7270/Q2HM57NS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 730 total ) | Next | Last >> |