

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminopeptidase B (Mus musculus) | BDBM50078120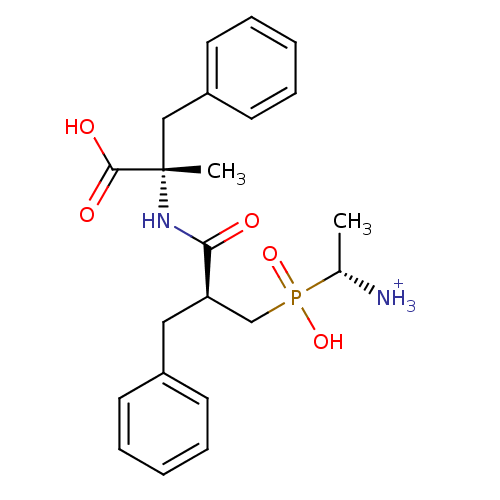 ((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on Aminopeptidase B using Arg p.NA as substrate | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Mus musculus) | BDBM50078122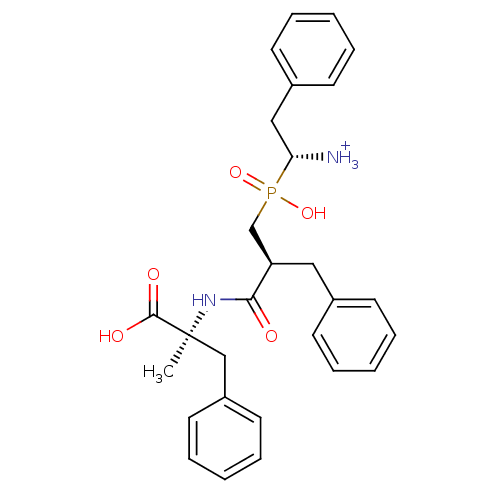 ((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | >0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on Aminopeptidase B using Arg p.NA as substrate | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50078122 ((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for C+D stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Sus scrofa) | BDBM50078120 ((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for A+B stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50078120 ((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for C+D stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Sus scrofa) | BDBM50078122 ((R)-1-{[(S)-2-((S)-1-Carboxy-1-methyl-2-phenyl-eth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on Aminopeptidase using GluNA as substrate | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM21653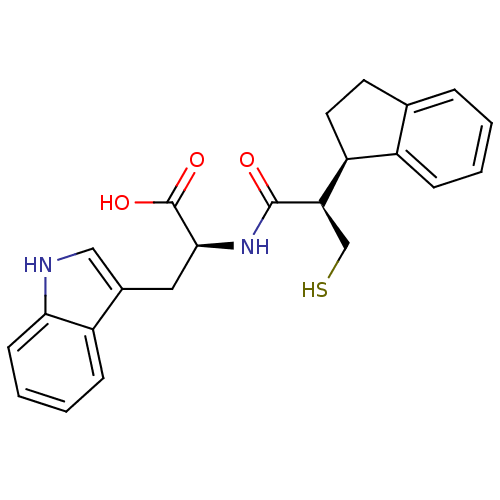 ((2S)-2-[(2R)-2-[(1R)-2,3-dihydro-1H-inden-1-yl]-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of Neutral endopeptidase. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407297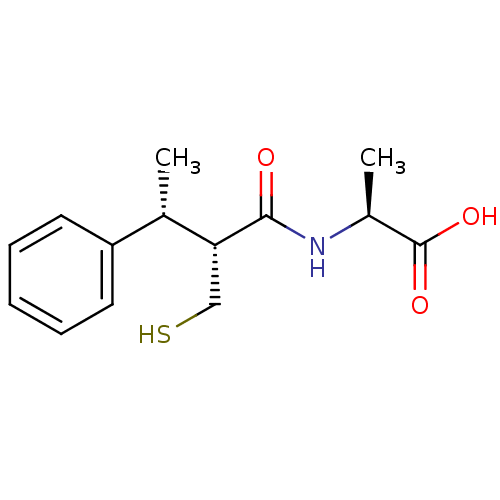 (CHEMBL2052008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407297 (CHEMBL2052008) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083386 (1-{[1-(2,3-Dicarboxy-pyrrolidine-1-carbonyl)-2-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.873 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase B (Homo sapiens (Human)) | BDBM50036831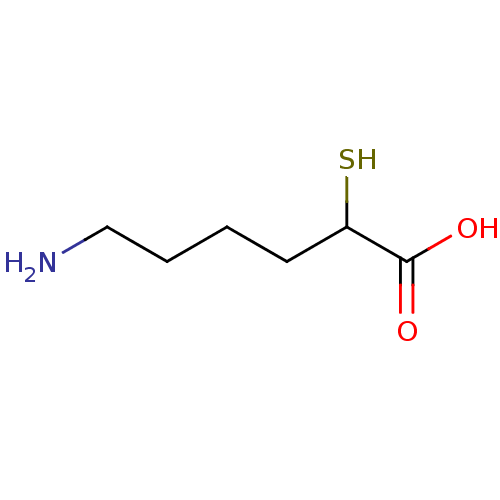 (6-Amino-2-mercapto-hexanoic acid | CHEMBL432852) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibition of arginylaminopeptidase (aminopeptidase B) | J Med Chem 37: 1339-46 (1994) BindingDB Entry DOI: 10.7270/Q2K074ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087104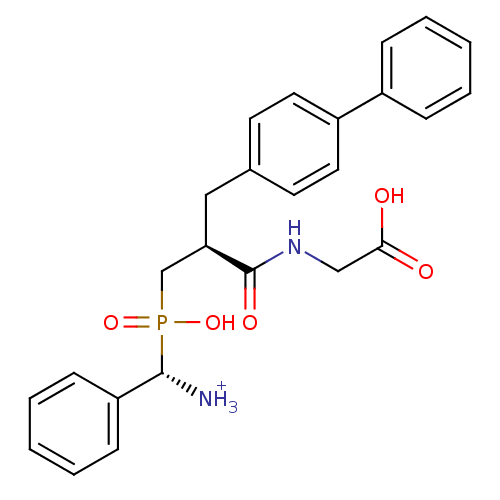 (C-{[3-Biphenyl-4-yl-2-(carboxymethyl-carbamoyl)-pr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105262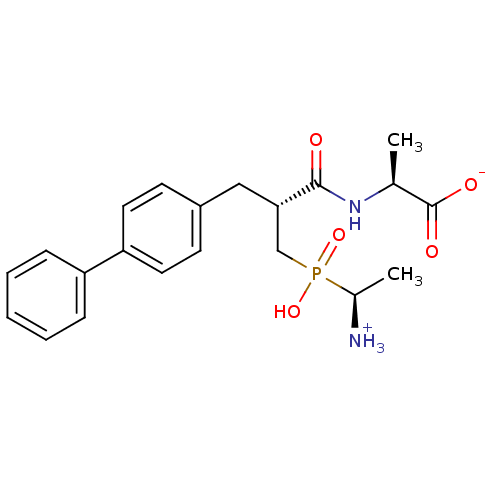 (2-{3-[(1-Amino-ethyl)-hydroxy-phosphinoyl]-2-biphe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087106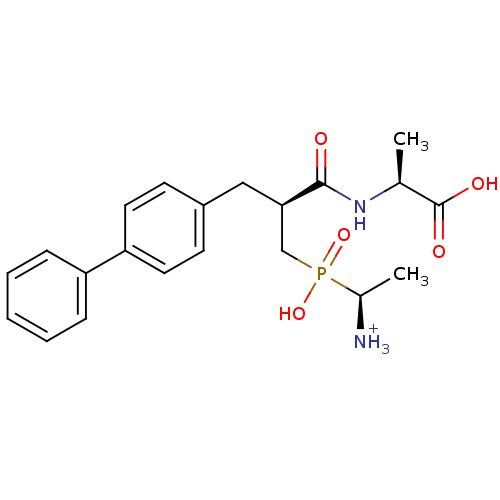 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumylethyl](hydroxy)ph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50115846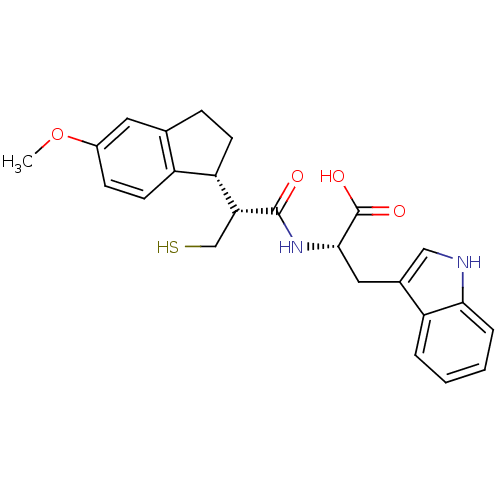 ((S)-3-(1H-Indol-3-yl)-2-[(R)-3-mercapto-2-((R)-5-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of Neutral endopeptidase. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087088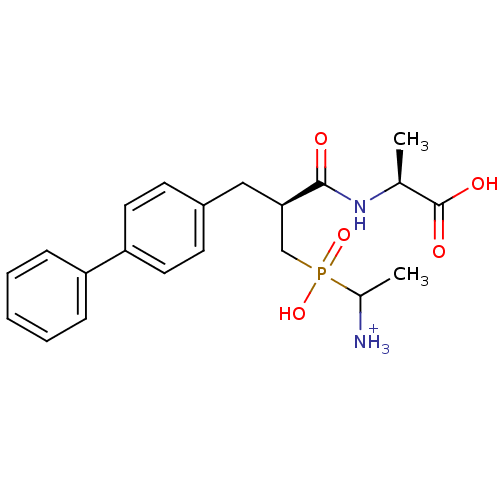 ((2S)-2-[(2S)-3-[(1-azaniumylethyl)(hydroxy)phospho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087089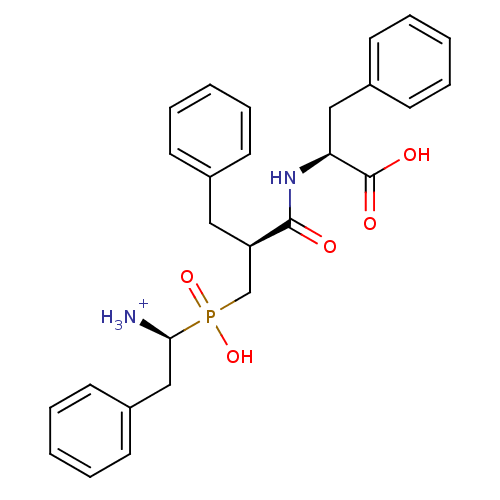 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407299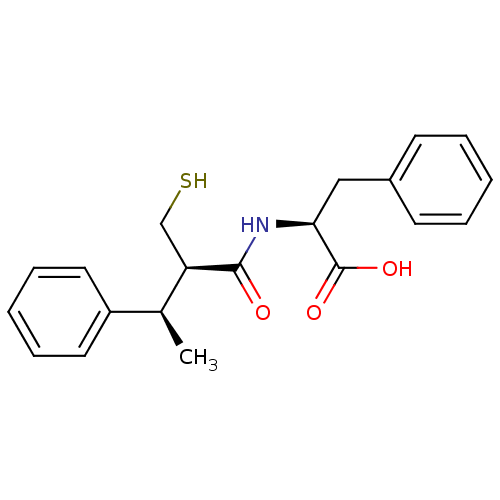 (CHEMBL2052007) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50407299 (CHEMBL2052007) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vivo inhibitory potency against neutral endopeptidase by displacement of [3H]-HACBOGly binding in mouse kidney | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50105264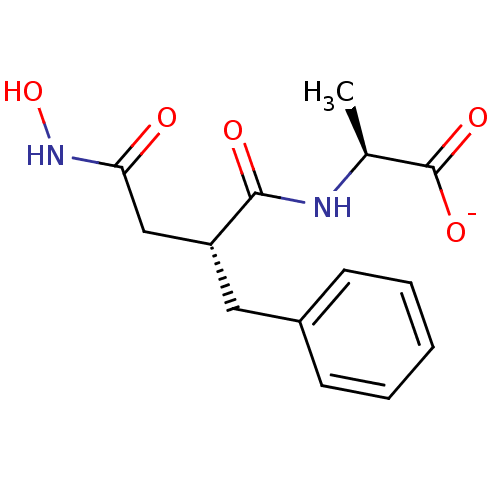 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% inhibitory activity against enkephalinase purified from rat kidney with [3H]D-Ala2-Leu-enkephalin (20 nM) as substrate. | J Med Chem 28: 1158-69 (1985) BindingDB Entry DOI: 10.7270/Q20C4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105264 (2-(2-Benzyl-3-hydroxycarbamoyl-propionylamino)-pro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50115843 ((2S)-2-[(2R)-2-[(1R)-5-bromo-2,3-dihydro-1H-inden-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of Neutral endopeptidase. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50115842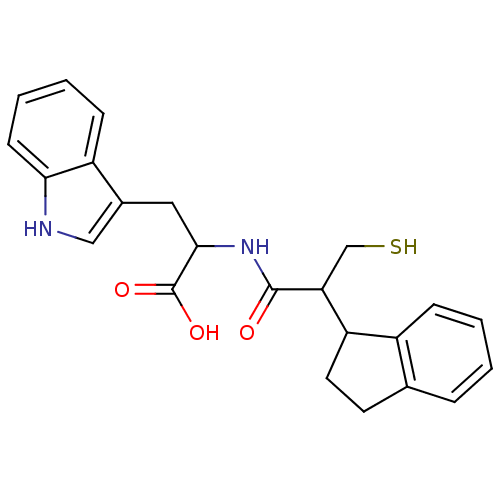 (2-(2-Indan-1-yl-3-mercapto-propionylamino)-3-(1H-i...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of Neutral endopeptidase. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Rattus norvegicus (Rat)) | BDBM50405292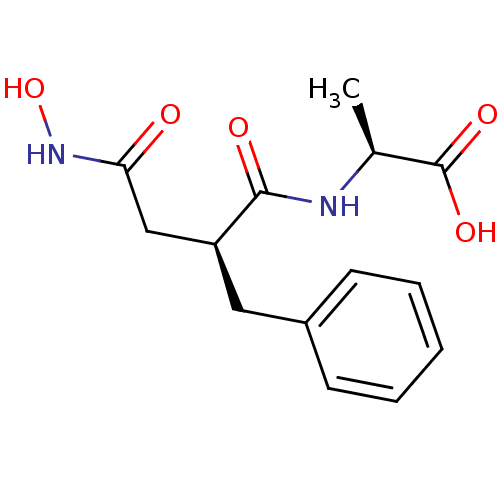 (CHEMBL2079630) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description 50% inhibitory activity against enkephalinase purified from rat kidney with [3H]D-Ala2-Leu-enkephalin (20 nM) as substrate. | J Med Chem 28: 1158-69 (1985) BindingDB Entry DOI: 10.7270/Q20C4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407299 (CHEMBL2052007) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087095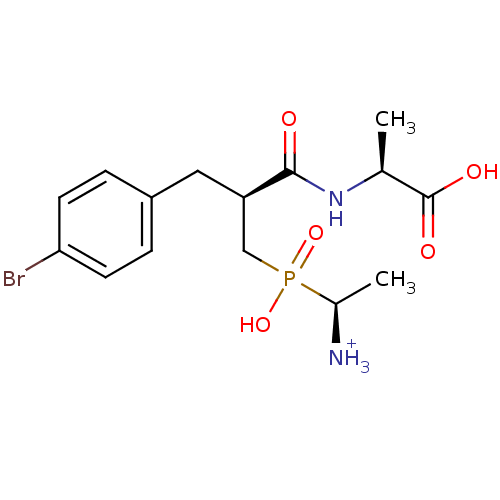 (1-{[3-(4-Bromo-phenyl)-2-(1-carboxy-ethylcarbamoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407299 (CHEMBL2052007) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105261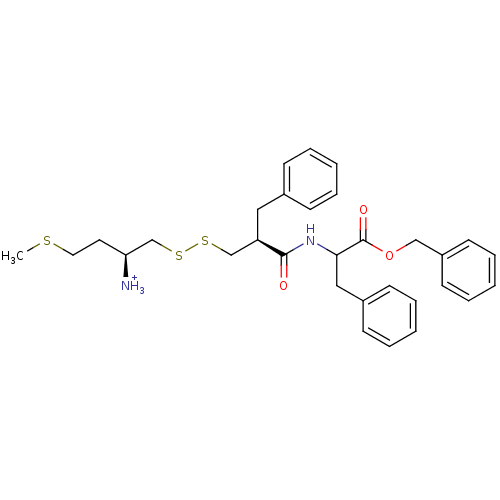 (1-[2-(1-Benzyloxycarbonyl-2-phenyl-ethylcarbamoyl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087113 ((2S)-2-[(2S)-3-{[(S)-azaniumyl(phenyl)methyl](hydr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM50105263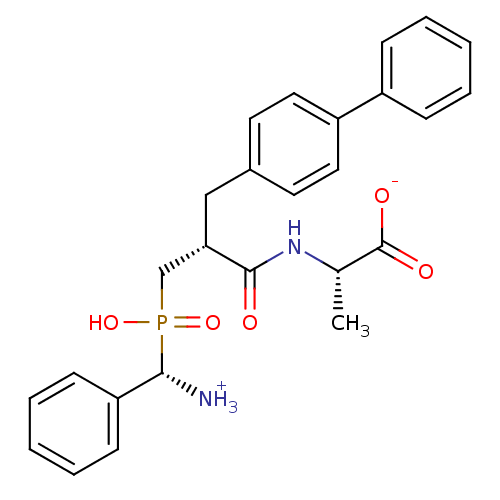 (2-{3-[(Amino-phenyl-methyl)-hydroxy-phosphinoyl]-2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, neutral endopeptidase (NEP) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087085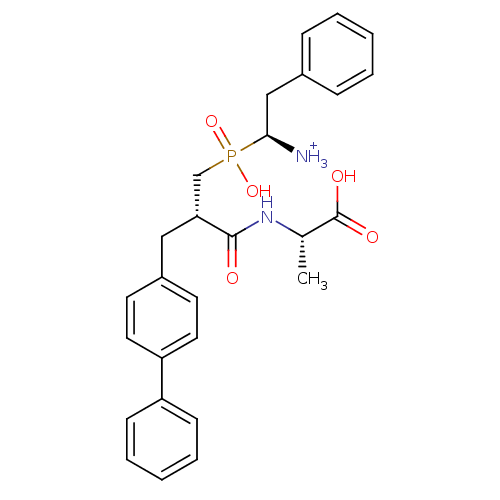 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087097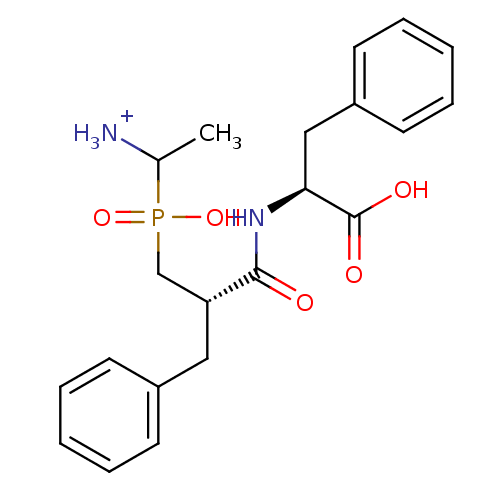 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087097 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for A+B stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087085 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087093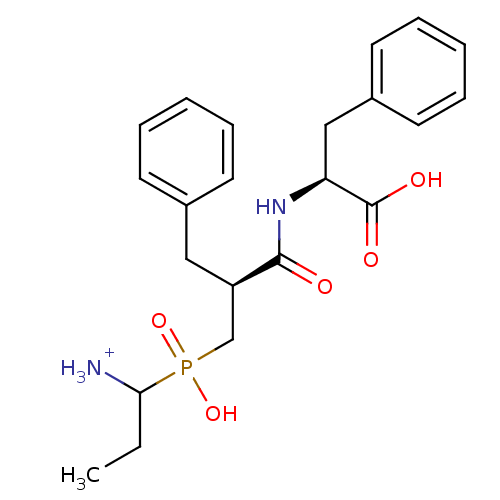 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50078127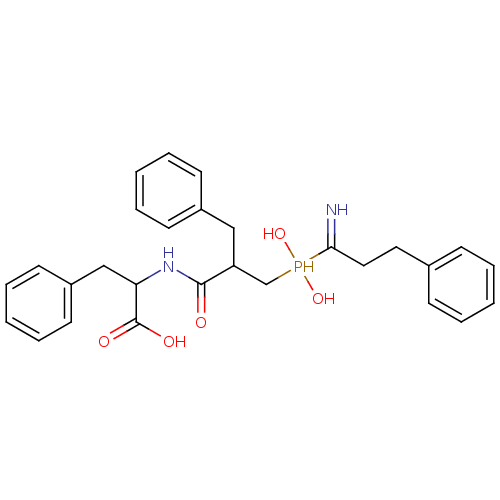 (2-{3-[(1-Amino-3-phenyl-propyl)-hydroxy-phosphinoy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for A+B stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087094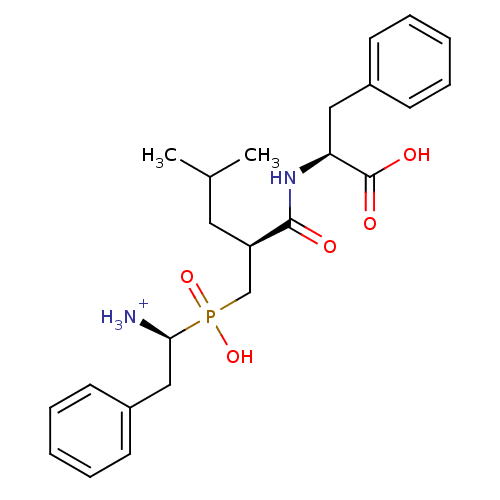 ((R,S,S)1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087084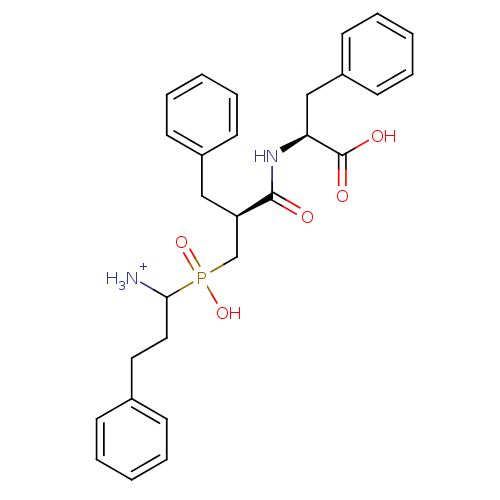 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50115850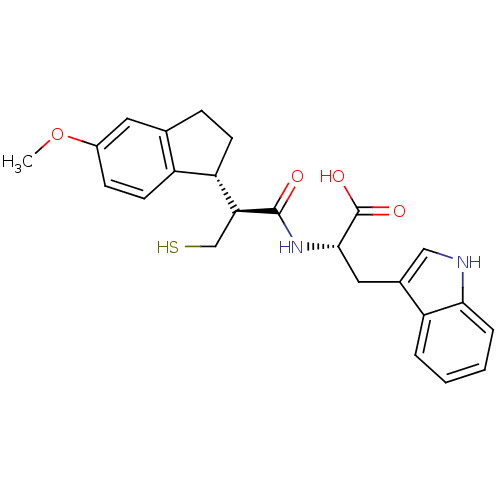 ((S)-3-(1H-Indol-3-yl)-2-[(S)-3-mercapto-2-((R)-5-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of Neutral endopeptidase. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50405292 (CHEMBL2079630) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against aminopeptidase using 10 nM of [3H]Leu-enkephalin as substrate | J Med Chem 28: 1158-69 (1985) BindingDB Entry DOI: 10.7270/Q20C4WB5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Oryctolagus cuniculus (rabbit)) | BDBM50087100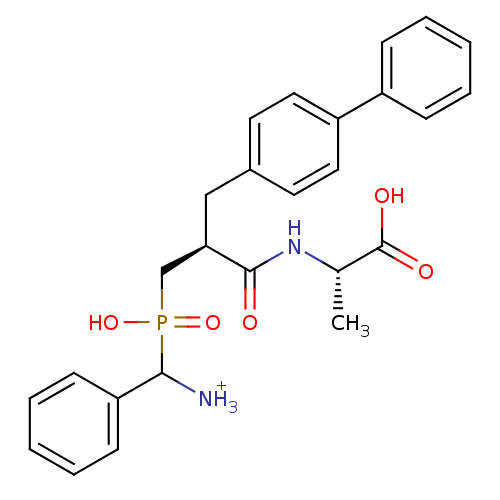 ((2S)-2-[(2S)-3-{[azaniumyl(phenyl)methyl](hydroxy)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on neutral endopeptidase (NEP) using DGNPA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087089 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumyl-2-phenylethyl](h...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087106 ((2S)-2-[(2S)-3-{[(1S)-1-azaniumylethyl](hydroxy)ph...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50078134 (2-{3-[(1-Amino-2-phenyl-ethyl)-hydroxy-phosphinoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for A+B stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Homo sapiens (Human)) | BDBM50105262 (2-{3-[(1-Amino-ethyl)-hydroxy-phosphinoyl]-2-biphe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of enkephalin degrading enzyme, aminopeptidase N(APN) | J Med Chem 44: 3523-30 (2001) BindingDB Entry DOI: 10.7270/Q2QN67HK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Rattus norvegicus) | BDBM50115837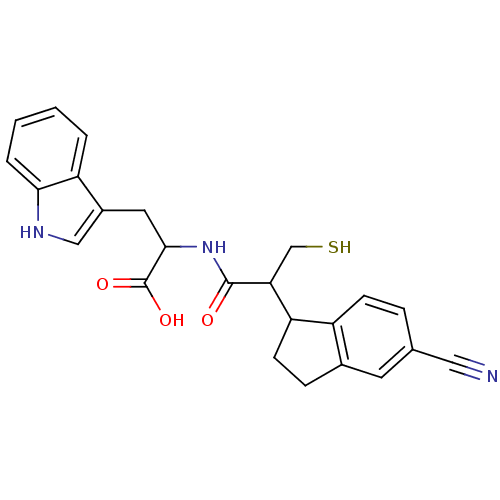 (2-[2-(5-Cyano-indan-1-yl)-3-mercapto-propionylamin...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme. | Bioorg Med Chem Lett 12: 2001-5 (2002) BindingDB Entry DOI: 10.7270/Q27080S4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087114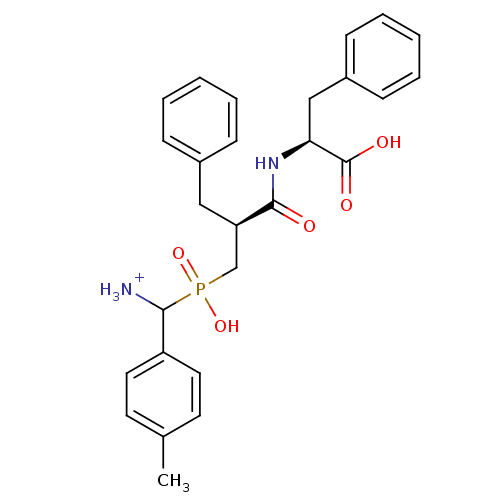 (C-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamyl aminopeptidase (Homo sapiens (Human)) | BDBM50083394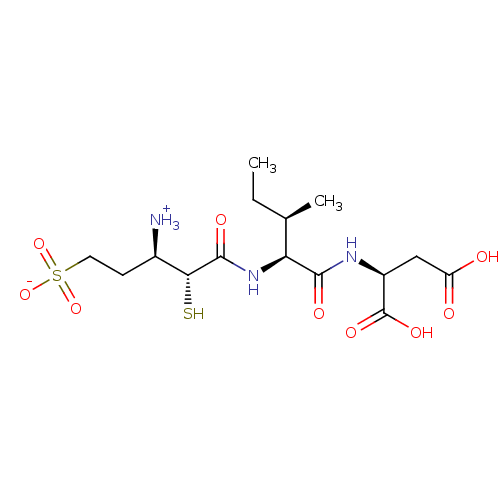 (1-{[1-(1,2-Dicarboxy-ethylcarbamoyl)-2-methyl-buty...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Binding affinity against recombinant Aminopeptidase A | J Med Chem 42: 5197-211 (2000) BindingDB Entry DOI: 10.7270/Q2028S8F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50078131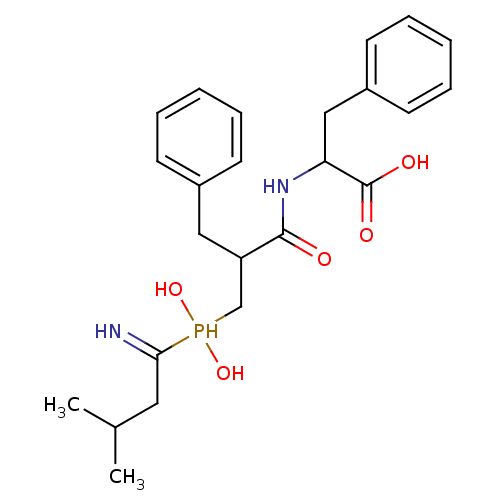 (2-{3-[(1-Amino-3-methyl-butyl)-hydroxy-phosphinoyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity was measured on pig kidney Aminopeptidase N (activity for A+B stereoisomer) | Bioorg Med Chem Lett 9: 1511-6 (1999) BindingDB Entry DOI: 10.7270/Q2H132JR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aminopeptidase N (Sus scrofa (Pig)) | BDBM50087096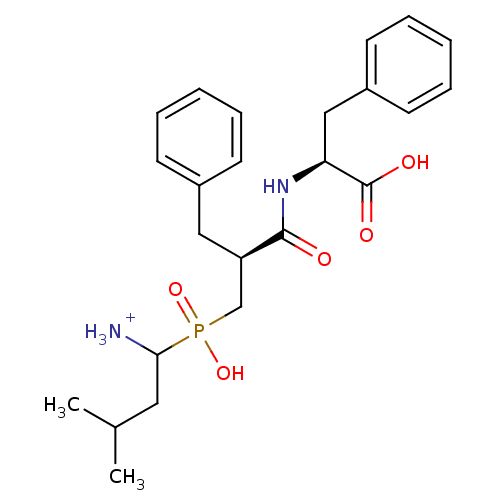 (1-{[2-(1-Carboxy-2-phenyl-ethylcarbamoyl)-3-phenyl...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory activity on aminopeptidase N (APN) using Ala-pNA as substrate. | J Med Chem 43: 1398-408 (2001) BindingDB Entry DOI: 10.7270/Q2TQ627J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 572 total ) | Next | Last >> |