 Found 818 hits with Last Name = 'francis' and Initial = 'l'
Found 818 hits with Last Name = 'francis' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82124
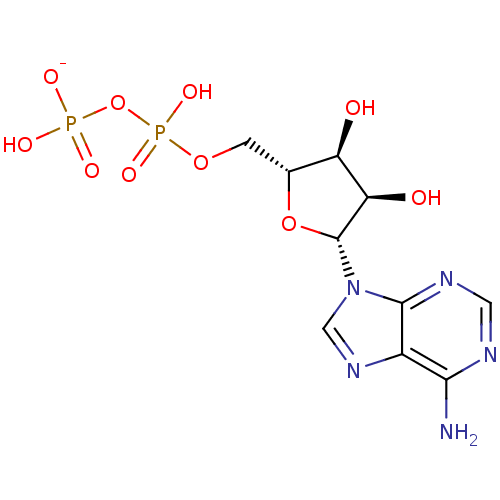
(adenosine-derived inhibitor (Grp78), 1)Show SMILES Nc1ncnc2n(cnc12)[C@@H]1O[C@H](COP(O)(=O)OP(O)([O-])=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H15N5O10P2/c11-8-5-9(13-2-12-8)15(3-14-5)10-7(17)6(16)4(24-10)1-23-27(21,22)25-26(18,19)20/h2-4,6-7,10,16-17H,1H2,(H,21,22)(H2,11,12,13)(H2,18,19,20)/p-1/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | -39.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32378
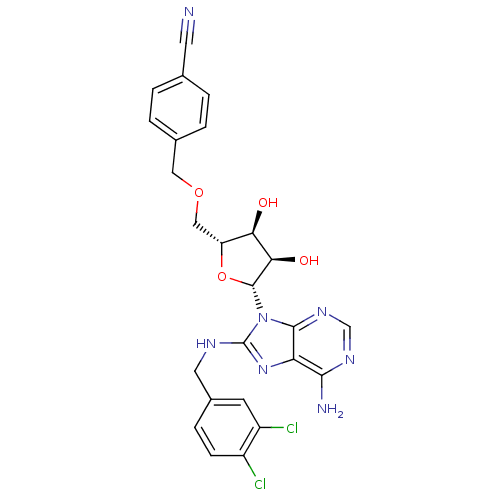
(adenosine-derived inhibitor (Grp78), 13 | adenosin...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](COCc4ccc(cc4)C#N)[C@@H](O)[C@H]3O)c(NCc3ccc(Cl)c(Cl)c3)nc12 |r| Show InChI InChI=1S/C25H23Cl2N7O4/c26-16-6-5-15(7-17(16)27)9-30-25-33-19-22(29)31-12-32-23(19)34(25)24-21(36)20(35)18(38-24)11-37-10-14-3-1-13(8-28)2-4-14/h1-7,12,18,20-21,24,35-36H,9-11H2,(H,30,33)(H2,29,31,32)/t18-,20-,21-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 120 | -39.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32381
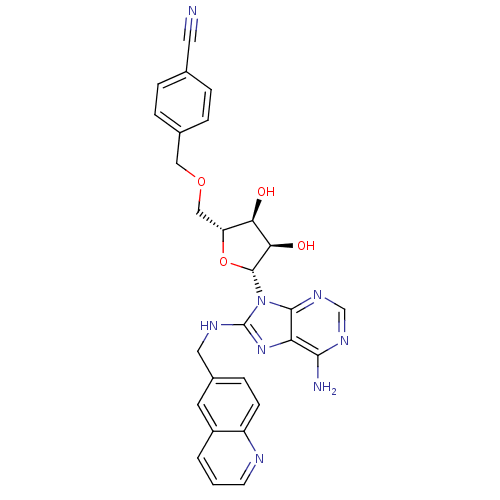
(adenosine-derived inhibitor (Grp78), 14 | adenosin...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](COCc4ccc(cc4)C#N)[C@@H](O)[C@H]3O)c(NCc3ccc4ncccc4c3)nc12 |r| Show InChI InChI=1S/C28H26N8O4/c29-11-16-3-5-17(6-4-16)13-39-14-21-23(37)24(38)27(40-21)36-26-22(25(30)33-15-34-26)35-28(36)32-12-18-7-8-20-19(10-18)2-1-9-31-20/h1-10,15,21,23-24,27,37-38H,12-14H2,(H,32,35)(H2,30,33,34)/t21-,23-,24-,27-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 310 | -37.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82127
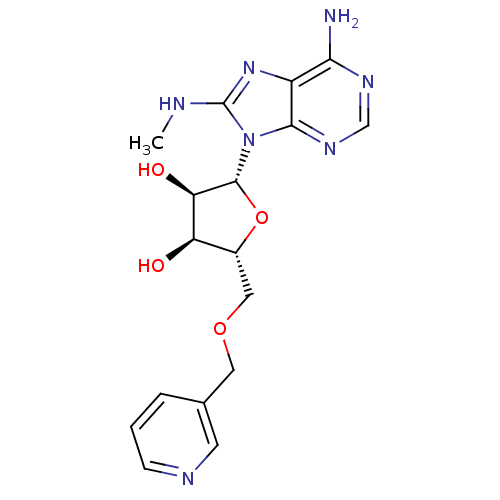
((2R,3R,4S,5R)-2-(6-Amino-8-methylaminopurin-9-yl)-...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCc2cccnc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C17H21N7O4/c1-19-17-23-11-14(18)21-8-22-15(11)24(17)16-13(26)12(25)10(28-16)7-27-6-9-3-2-4-20-5-9/h2-5,8,10,12-13,16,25-26H,6-7H2,1H3,(H,19,23)(H2,18,21,22)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 450 | -36.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32377
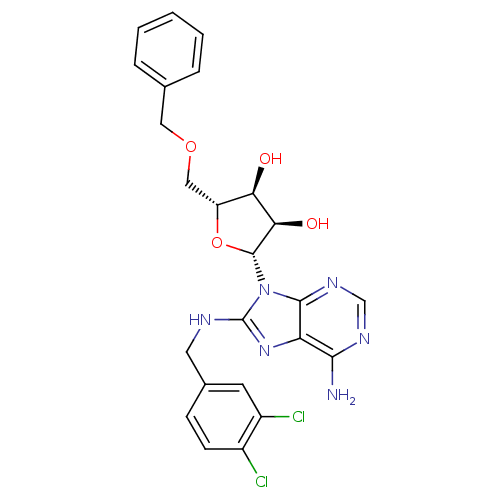
(adenosine-derived inhibitor (Grp78), 12 | adenosin...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](COCc4ccccc4)[C@@H](O)[C@H]3O)c(NCc3ccc(Cl)c(Cl)c3)nc12 |r| Show InChI InChI=1S/C24H24Cl2N6O4/c25-15-7-6-14(8-16(15)26)9-28-24-31-18-21(27)29-12-30-22(18)32(24)23-20(34)19(33)17(36-23)11-35-10-13-4-2-1-3-5-13/h1-8,12,17,19-20,23,33-34H,9-11H2,(H,28,31)(H2,27,29,30)/t17-,19-,20-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.05E+3 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82125
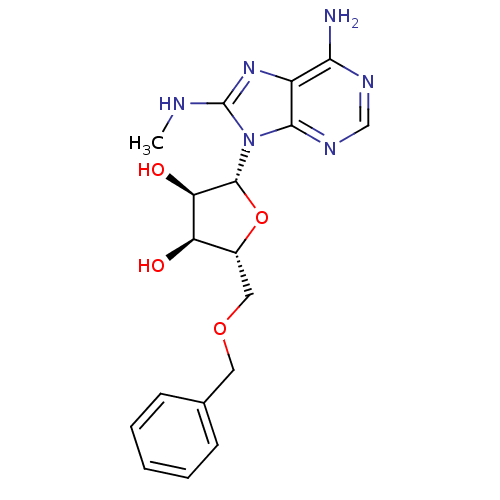
((2R,3R,4S,5R)-2-(6-Amino-8-methylaminopurin-9-yl)-...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCc2ccccc2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H22N6O4/c1-20-18-23-12-15(19)21-9-22-16(12)24(18)17-14(26)13(25)11(28-17)8-27-7-10-5-3-2-4-6-10/h2-6,9,11,13-14,17,25-26H,7-8H2,1H3,(H,20,23)(H2,19,21,22)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | -34.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32375
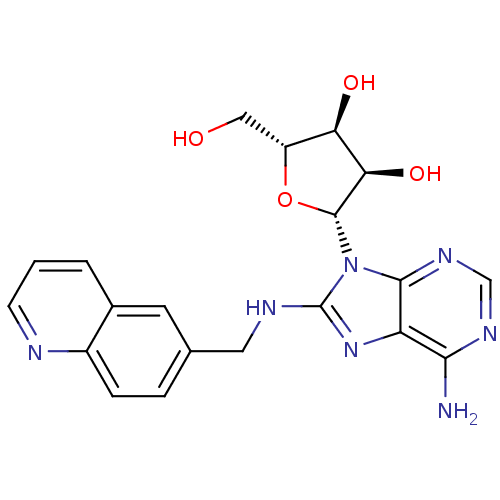
(adenosine-derived inhibitor (Grp78), 9 | adenosine...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(NCc3ccc4ncccc4c3)nc12 |r| Show InChI InChI=1S/C20H21N7O4/c21-17-14-18(25-9-24-17)27(19-16(30)15(29)13(8-28)31-19)20(26-14)23-7-10-3-4-12-11(6-10)2-1-5-22-12/h1-6,9,13,15-16,19,28-30H,7-8H2,(H,23,26)(H2,21,24,25)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 1.29E+3 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32376
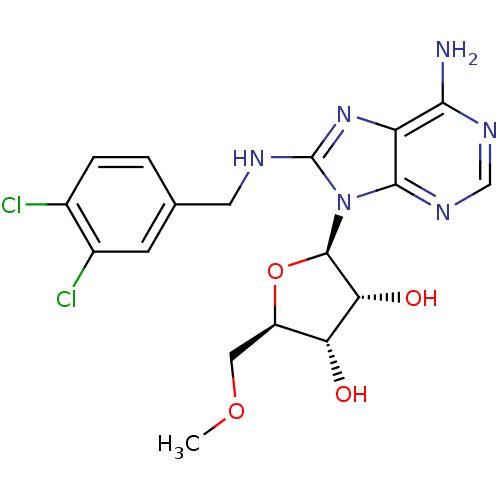
(adenosine-derived inhibitor (Grp78), 11 | adenosin...)Show SMILES COC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1c(NCc2ccc(Cl)c(Cl)c2)nc2c(N)ncnc12 |r| Show InChI InChI=1S/C18H20Cl2N6O4/c1-29-6-11-13(27)14(28)17(30-11)26-16-12(15(21)23-7-24-16)25-18(26)22-5-8-2-3-9(19)10(20)4-8/h2-4,7,11,13-14,17,27-28H,5-6H2,1H3,(H,22,25)(H2,21,23,24)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.44E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82128
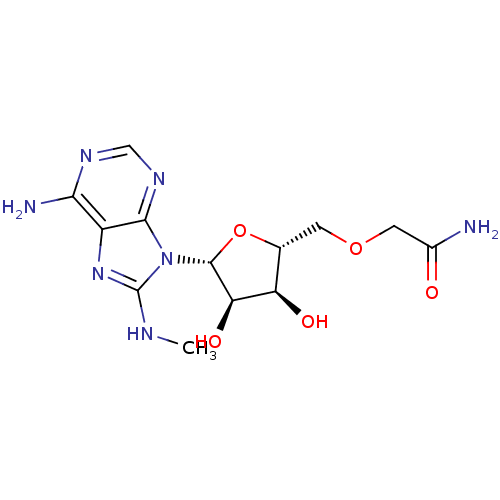
(2-[(2R,3S,4R,5R)-5-(6-Amino-8-methylaminopurin-9-y...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCC(N)=O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C13H19N7O5/c1-16-13-19-7-10(15)17-4-18-11(7)20(13)12-9(23)8(22)5(25-12)2-24-3-6(14)21/h4-5,8-9,12,22-23H,2-3H2,1H3,(H2,14,21)(H,16,19)(H2,15,17,18)/t5-,8-,9-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 1.85E+3 | -32.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82126

((2R,3R,4S,5R)-2-(6-Amino-8-methylaminopurin-9-yl)-...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](COCC2CCCCC2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C18H28N6O4/c1-20-18-23-12-15(19)21-9-22-16(12)24(18)17-14(26)13(25)11(28-17)8-27-7-10-5-3-2-4-6-10/h9-11,13-14,17,25-26H,2-8H2,1H3,(H,20,23)(H2,19,21,22)/t11-,13-,14-,17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.07E+3 | -30.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32373
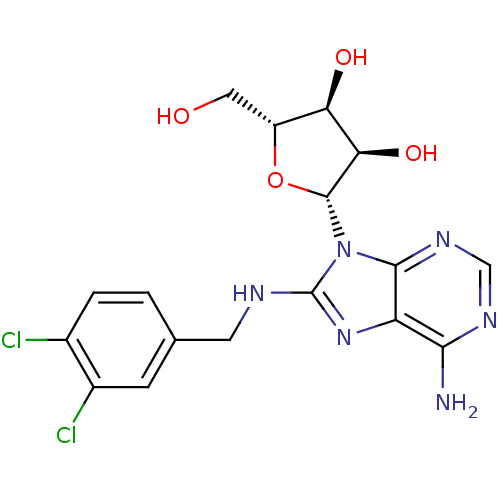
(adenosine-derived inhibitor (Grp78), 8 | adenosine...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(NCc3ccc(Cl)c(Cl)c3)nc12 |r| Show InChI InChI=1S/C17H18Cl2N6O4/c18-8-2-1-7(3-9(8)19)4-21-17-24-11-14(20)22-6-23-15(11)25(17)16-13(28)12(27)10(5-26)29-16/h1-3,6,10,12-13,16,26-28H,4-5H2,(H,21,24)(H2,20,22,23)/t10-,12-,13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.31E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM82129
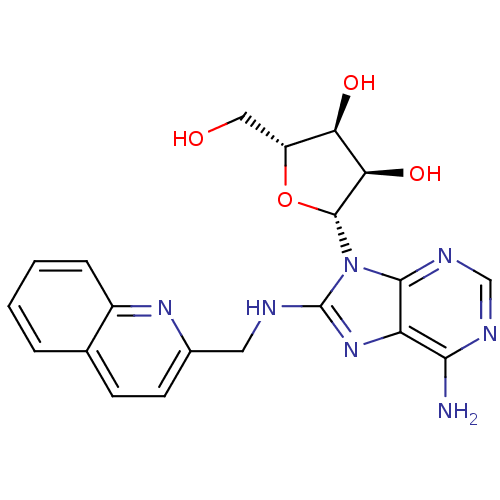
((2R,3R,4S,5R)-2-{6-Amino-8-[(quinolin-2-ylmethyl)a...)Show SMILES Nc1ncnc2n([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c(NCc3ccc4ccccc4n3)nc12 |r| Show InChI InChI=1S/C20H21N7O4/c21-17-14-18(24-9-23-17)27(19-16(30)15(29)13(8-28)31-19)20(26-14)22-7-11-6-5-10-3-1-2-4-12(10)25-11/h1-6,9,13,15-16,19,28-30H,7-8H2,(H,22,26)(H2,21,23,24)/t13-,15-,16-,19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 4.32E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32370
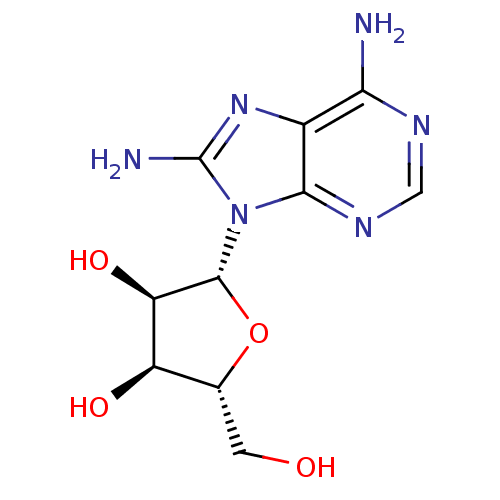
(adenosine-derived inhibitor (Grp78), 2 | adenosine...)Show SMILES Nc1nc2c(N)ncnc2n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C10H14N6O4/c11-7-4-8(14-2-13-7)16(10(12)15-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,15)(H2,11,13,14)/t3-,5-,6-,9-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 4.46E+3 | -30.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Heat shock 70 kDa protein 1A
(Homo sapiens (Human)) | BDBM32371
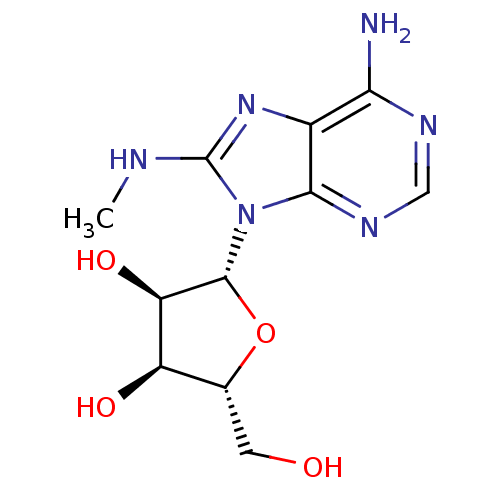
(adenosine-derived inhibitor (Grp78), 3 | adenosine...)Show SMILES CNc1nc2c(N)ncnc2n1[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C11H16N6O4/c1-13-11-16-5-8(12)14-3-15-9(5)17(11)10-7(20)6(19)4(2-18)21-10/h3-4,6-7,10,18-20H,2H2,1H3,(H,13,16)(H2,12,14,15)/t4-,6-,7-,10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Vernalis (R&D) Ltd.
| Assay Description
The assay was based on the fluorescence polarization of the unbound small binding partner will be low, and its binding to a larger binding partner wi... |
J Med Chem 54: 4034-41 (2011)
Article DOI: 10.1021/jm101625x
BindingDB Entry DOI: 10.7270/Q2R49P83 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM24996
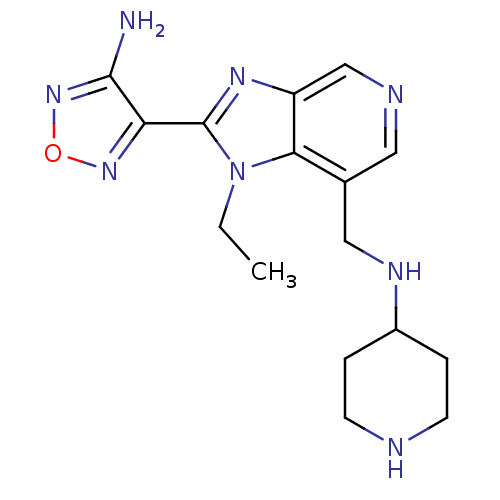
(4-{1-ethyl-7-[(piperidin-4-ylamino)methyl]-1H-imid...)Show InChI InChI=1S/C16H22N8O/c1-2-24-14-10(8-20-11-3-5-18-6-4-11)7-19-9-12(14)21-16(24)13-15(17)23-25-22-13/h7,9,11,18,20H,2-6,8H2,1H3,(H2,17,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168854
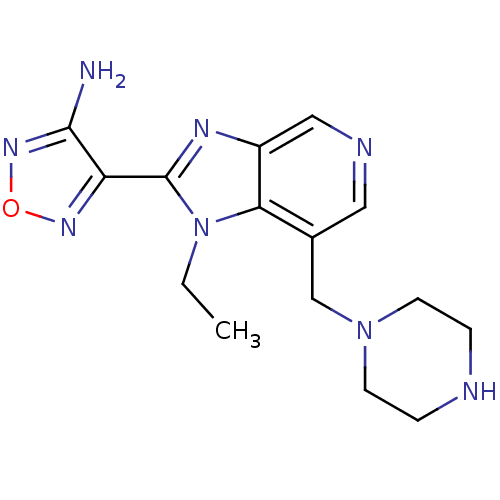
(4-(1-Ethyl-7-piperazin-1-ylmethyl-1H-imidazo[4,5-c...)Show InChI InChI=1S/C15H20N8O/c1-2-23-13-10(9-22-5-3-17-4-6-22)7-18-8-11(13)19-15(23)12-14(16)21-24-20-12/h7-8,17H,2-6,9H2,1H3,(H2,16,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168577
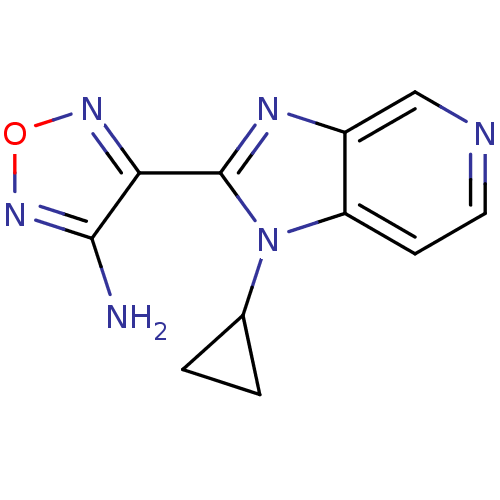
(4-(1-Cyclopropyl-1H-imidazo[4,5-c]pyridin-2-yl)-fu...)Show InChI InChI=1S/C11H10N6O/c12-10-9(15-18-16-10)11-14-7-5-13-4-3-8(7)17(11)6-1-2-6/h3-6H,1-2H2,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM11439

(4-({5-[(4-aminocyclohexyl)amino]-3-bromopyrazolo[1...)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2cc(N[C@H]3CC[C@H](N)CC3)nc3c(Br)cnn23)cc1Cl |r,wU:18.18,wD:15.14,(-9.87,7.79,;-8.54,7.02,;-8.54,5.48,;-7.2,7.79,;-8.29,8.88,;-6.11,8.88,;-5.87,7.02,;-5.87,5.48,;-4.53,4.71,;-3.2,5.48,;-1.87,4.71,;-1.87,3.17,;-3.2,2.4,;-3.2,.86,;-4.53,.09,;-5.87,.86,;-5.87,2.4,;-7.2,3.17,;-8.53,2.4,;-9.87,3.17,;-8.53,.86,;-7.2,.09,;-1.87,.09,;-.53,.86,;.93,.39,;1.41,-1.08,;1.84,1.63,;.93,2.88,;-.53,2.4,;-3.2,7.02,;-4.53,7.79,;-4.53,9.33,)| Show InChI InChI=1S/C20H25BrClN7O2S/c1-28(2)32(30,31)17-8-7-14(9-16(17)22)26-19-10-18(25-13-5-3-12(23)4-6-13)27-20-15(21)11-24-29(19)20/h7-13,26H,3-6,23H2,1-2H3,(H,25,27)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 15: 863-7 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.073
BindingDB Entry DOI: 10.7270/Q2ZK5DWP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168858
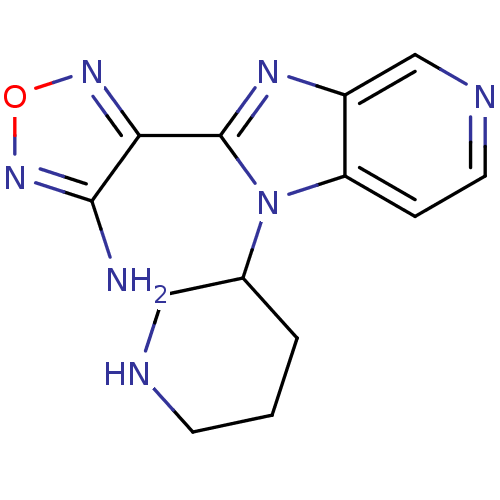
((R,S)-4-(1-Piperidin-3-yl-1H-imidazo[4,5-c]pyridin...)Show InChI InChI=1S/C13H15N7O/c14-12-11(18-21-19-12)13-17-9-7-16-5-3-10(9)20(13)8-2-1-4-15-6-8/h3,5,7-8,15H,1-2,4,6H2,(H2,14,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168856
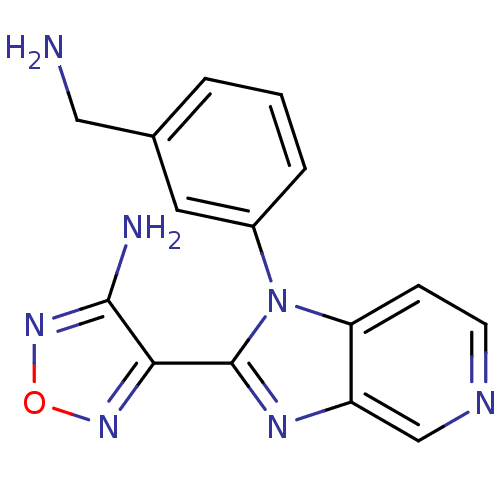
(4-[1-(3-Aminomethyl-phenyl)-1H-imidazo[4,5-c]pyrid...)Show InChI InChI=1S/C15H13N7O/c16-7-9-2-1-3-10(6-9)22-12-4-5-18-8-11(12)19-15(22)13-14(17)21-23-20-13/h1-6,8H,7,16H2,(H2,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168587

(4-(1-Cyclohexyl-1H-imidazo[4,5-c]pyridin-2-yl)-fur...)Show InChI InChI=1S/C14H16N6O/c15-13-12(18-21-19-13)14-17-10-8-16-7-6-11(10)20(14)9-4-2-1-3-5-9/h6-9H,1-5H2,(H2,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168869
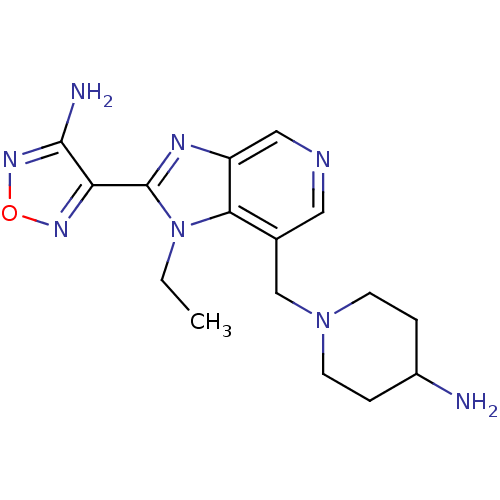
(1-[2-(4-Amino-furazan-3-yl)-1-ethyl-1H-imidazo[4,5...)Show InChI InChI=1S/C16H22N8O/c1-2-24-14-10(9-23-5-3-11(17)4-6-23)7-19-8-12(14)20-16(24)13-15(18)22-25-21-13/h7-8,11H,2-6,9,17H2,1H3,(H2,18,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM14032
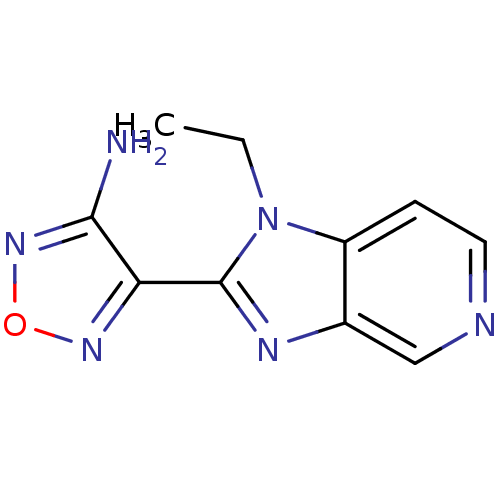
(4-(1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C10H10N6O/c1-2-16-7-3-4-12-5-6(7)13-10(16)8-9(11)15-17-14-8/h3-5H,2H2,1H3,(H2,11,15) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM11429
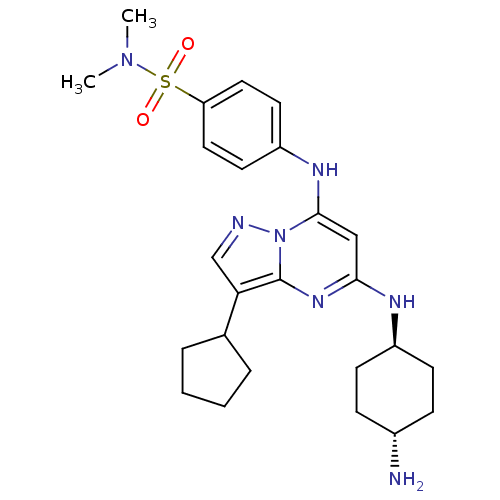
(4-({5-[(4-aminocyclohexyl)amino]-3-cyclopentylpyra...)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2cc(N[C@H]3CC[C@H](N)CC3)nc3c(cnn23)C2CCCC2)cc1 |r,wU:18.18,wD:15.14,(-8.53,5.48,;-8.53,7.02,;-9.87,7.79,;-7.2,7.79,;-8.29,8.88,;-6.11,8.88,;-5.87,7.02,;-5.87,5.48,;-4.53,4.71,;-3.2,5.48,;-1.87,4.71,;-1.87,3.17,;-3.2,2.4,;-3.2,.86,;-4.53,.09,;-5.87,.86,;-5.87,2.4,;-7.2,3.17,;-8.53,2.4,;-9.87,3.17,;-8.53,.86,;-7.2,.09,;-1.87,.09,;-.53,.86,;.93,.39,;1.84,1.63,;.93,2.88,;-.53,2.4,;1.41,-1.08,;.6,-2.39,;1.6,-3.56,;3.03,-2.97,;2.91,-1.44,;-3.2,7.02,;-4.53,7.79,)| Show InChI InChI=1S/C25H35N7O2S/c1-31(2)35(33,34)21-13-11-20(12-14-21)29-24-15-23(28-19-9-7-18(26)8-10-19)30-25-22(16-27-32(24)25)17-5-3-4-6-17/h11-19,29H,3-10,26H2,1-2H3,(H,28,30)/t18-,19- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 15: 863-7 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.073
BindingDB Entry DOI: 10.7270/Q2ZK5DWP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168861

(4-(4-Chloro-1-ethyl-1H-imidazo[4,5-c]pyridin-2-yl)...)Show InChI InChI=1S/C10H9ClN6O/c1-2-17-5-3-4-13-8(11)6(5)14-10(17)7-9(12)16-18-15-7/h3-4H,2H2,1H3,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168862
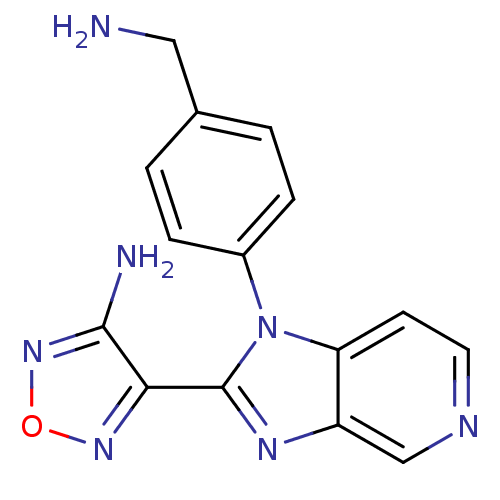
(4-[1-(4-Aminomethyl-phenyl)-1H-imidazo[4,5-c]pyrid...)Show InChI InChI=1S/C15H13N7O/c16-7-9-1-3-10(4-2-9)22-12-5-6-18-8-11(12)19-15(22)13-14(17)21-23-20-13/h1-6,8H,7,16H2,(H2,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530873

(CHEMBL4456215)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-4-3-5-29(13-16)14-17-10-18-19(34-17)21(30-6-8-33-9-7-30)27-20(26-18)15-11-24-22(23)25-12-15/h10-12,16H,3-9,13-14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530873

(CHEMBL4456215)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-4-3-5-29(13-16)14-17-10-18-19(34-17)21(30-6-8-33-9-7-30)27-20(26-18)15-11-24-22(23)25-12-15/h10-12,16H,3-9,13-14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168581
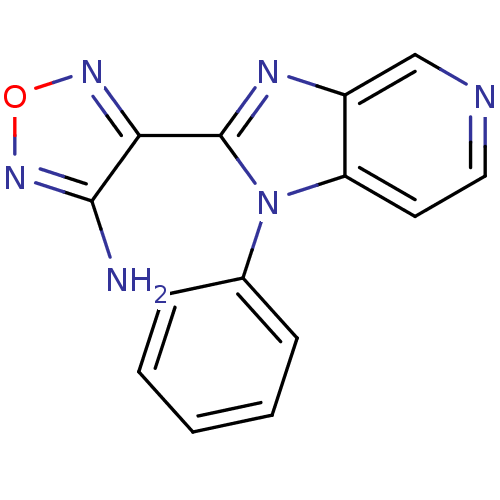
(4-(1-Phenyl-1H-imidazo[4,5-c]pyridin-2-yl)-furazan...)Show InChI InChI=1S/C14H10N6O/c15-13-12(18-21-19-13)14-17-10-8-16-7-6-11(10)20(14)9-4-2-1-3-5-9/h1-8H,(H2,15,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530894
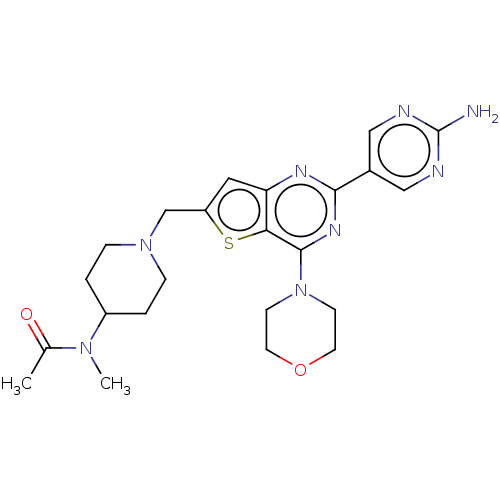
(CHEMBL4437468)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)C(C)=O Show InChI InChI=1S/C23H30N8O2S/c1-15(32)29(2)17-3-5-30(6-4-17)14-18-11-19-20(34-18)22(31-7-9-33-10-8-31)28-21(27-19)16-12-25-23(24)26-13-16/h11-13,17H,3-10,14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530894

(CHEMBL4437468)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)C(C)=O Show InChI InChI=1S/C23H30N8O2S/c1-15(32)29(2)17-3-5-30(6-4-17)14-18-11-19-20(34-18)22(31-7-9-33-10-8-31)28-21(27-19)16-12-25-23(24)26-13-16/h11-13,17H,3-10,14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530887
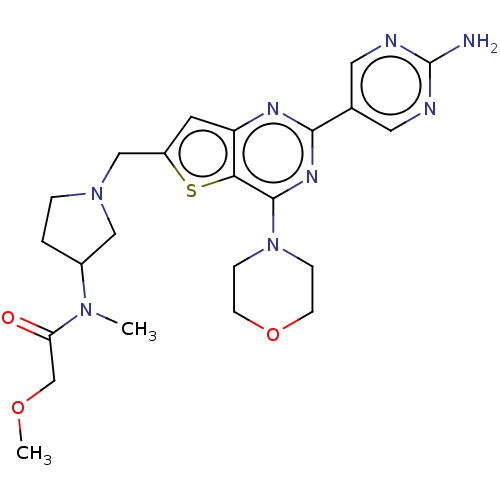
(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
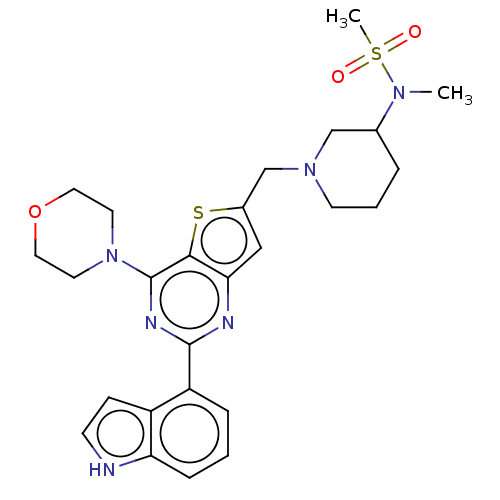
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530895
(CHEMBL4590897)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)S(C)(=O)=O Show InChI InChI=1S/C26H32N6O3S2/c1-30(37(2,33)34)18-5-4-10-31(16-18)17-19-15-23-24(36-19)26(32-11-13-35-14-12-32)29-25(28-23)21-6-3-7-22-20(21)8-9-27-22/h3,6-9,15,18,27H,4-5,10-14,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530887

(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
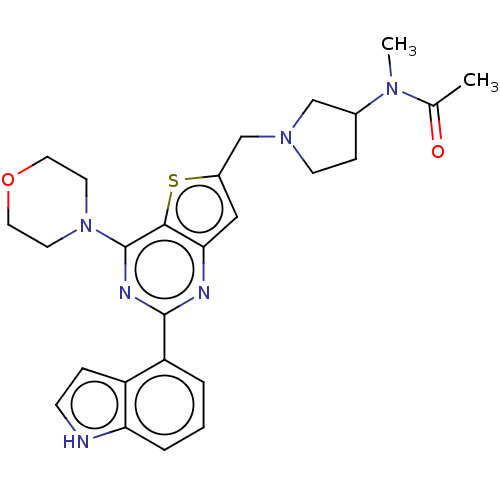
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530877
(CHEMBL4580111)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)C(C)=O Show InChI InChI=1S/C26H30N6O2S/c1-17(33)30(2)18-7-9-31(15-18)16-19-14-23-24(35-19)26(32-10-12-34-13-11-32)29-25(28-23)21-4-3-5-22-20(21)6-8-27-22/h3-6,8,14,18,27H,7,9-13,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530895

(CHEMBL4590897)Show SMILES CN(C1CCCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)S(C)(=O)=O Show InChI InChI=1S/C26H32N6O3S2/c1-30(37(2,33)34)18-5-4-10-31(16-18)17-19-15-23-24(36-19)26(32-11-13-35-14-12-32)29-25(28-23)21-6-3-7-22-20(21)8-9-27-22/h3,6-9,15,18,27H,4-5,10-14,16-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530877

(CHEMBL4580111)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)C1)C(C)=O Show InChI InChI=1S/C26H30N6O2S/c1-17(33)30(2)18-7-9-31(15-18)16-19-14-23-24(35-19)26(32-10-12-34-13-11-32)29-25(28-23)21-4-3-5-22-20(21)6-8-27-22/h3-6,8,14,18,27H,7,9-13,15-16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Cyclin-A2/Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM11430

(4-({5-[(4-aminocyclohexyl)amino]-3-cyanopyrazolo[1...)Show SMILES CN(C)S(=O)(=O)c1ccc(Nc2cc(N[C@H]3CC[C@H](N)CC3)nc3c(cnn23)C#N)cc1 |r,wU:18.18,wD:15.14,(-8.53,5.48,;-8.53,7.02,;-9.87,7.79,;-7.2,7.79,;-8.29,8.88,;-6.11,8.88,;-5.87,7.02,;-5.87,5.48,;-4.53,4.71,;-3.2,5.48,;-1.87,4.71,;-1.87,3.17,;-3.2,2.4,;-3.2,.86,;-4.53,.09,;-5.87,.86,;-5.87,2.4,;-7.2,3.17,;-8.53,2.4,;-9.87,3.17,;-8.53,.86,;-7.2,.09,;-1.87,.09,;-.53,.86,;.93,.39,;1.84,1.63,;.93,2.88,;-.53,2.4,;1.41,-1.08,;1.81,-2.56,;-3.2,7.02,;-4.53,7.79,)| Show InChI InChI=1S/C21H26N8O2S/c1-28(2)32(30,31)18-9-7-17(8-10-18)26-20-11-19(25-16-5-3-15(23)4-6-16)27-21-14(12-22)13-24-29(20)21/h7-11,13,15-16,26H,3-6,23H2,1-2H3,(H,25,27)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Vernalis (R&D) Ltd
| Assay Description
In vitro kinase assay using purified CDK2/Cyclin A was incubated at room temperature with substrate, and test compounds in the presence of 100 uM ATP... |
Bioorg Med Chem Lett 15: 863-7 (2005)
Article DOI: 10.1016/j.bmcl.2004.12.073
BindingDB Entry DOI: 10.7270/Q2ZK5DWP |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168853
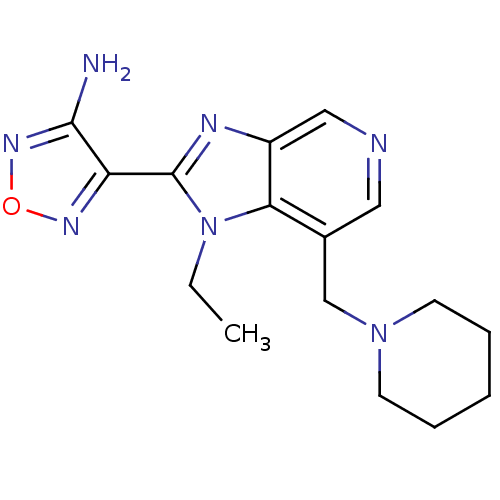
(4-(1-Ethyl-7-piperidin-1-ylmethyl-1H-imidazo[4,5-c...)Show InChI InChI=1S/C16H21N7O/c1-2-23-14-11(10-22-6-4-3-5-7-22)8-18-9-12(14)19-16(23)13-15(17)21-24-20-13/h8-9H,2-7,10H2,1H3,(H2,17,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530905

(CHEMBL4453879)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-3-5-29(6-4-16)14-17-11-18-19(34-17)21(30-7-9-33-10-8-30)27-20(26-18)15-12-24-22(23)25-13-15/h11-13,16H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50530887

(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530872
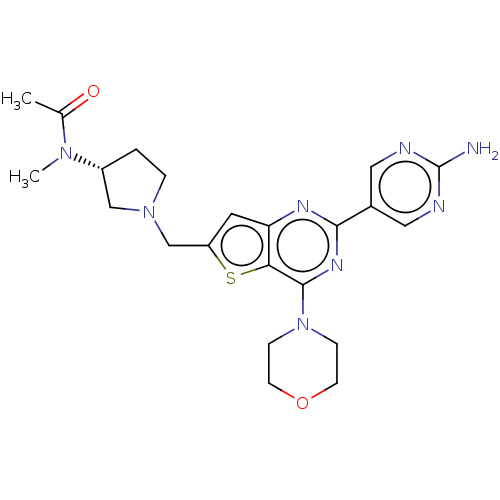
(CHEMBL4462986)Show SMILES CN([C@@H]1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)C(C)=O |r| Show InChI InChI=1S/C22H28N8O2S/c1-14(31)28(2)16-3-4-29(12-16)13-17-9-18-19(33-17)21(30-5-7-32-8-6-30)27-20(26-18)15-10-24-22(23)25-11-15/h9-11,16H,3-8,12-13H2,1-2H3,(H2,23,24,25)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530872

(CHEMBL4462986)Show SMILES CN([C@@H]1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)C(C)=O |r| Show InChI InChI=1S/C22H28N8O2S/c1-14(31)28(2)16-3-4-29(12-16)13-17-9-18-19(33-17)21(30-5-7-32-8-6-30)27-20(26-18)15-10-24-22(23)25-11-15/h9-11,16H,3-8,12-13H2,1-2H3,(H2,23,24,25)/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50530928

(CHEMBL4534127)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1)S(C)(=O)=O Show InChI InChI=1S/C21H28N8O3S2/c1-27(34(2,30)31)15-3-4-28(12-15)13-16-9-17-18(33-16)20(29-5-7-32-8-6-29)26-19(25-17)14-10-23-21(22)24-11-14/h9-11,15H,3-8,12-13H2,1-2H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50530887

(CHEMBL4538734)Show SMILES COCC(=O)N(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)C1 Show InChI InChI=1S/C23H30N8O3S/c1-29(19(32)14-33-2)16-3-4-30(12-16)13-17-9-18-20(35-17)22(31-5-7-34-8-6-31)28-21(27-18)15-10-25-23(24)26-11-15/h9-11,16H,3-8,12-14H2,1-2H3,(H2,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50530905

(CHEMBL4453879)Show SMILES CN(C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cnc(N)nc2)CC1)S(C)(=O)=O Show InChI InChI=1S/C22H30N8O3S2/c1-28(35(2,31)32)16-3-5-29(6-4-16)14-17-11-18-19(34-17)21(30-7-9-33-10-8-30)27-20(26-18)15-12-24-22(23)25-13-15/h11-13,16H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nottingham
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using biotin-PIP3 as substrate preincubated for 15 mins followed by substrate addition and measured after 60... |
J Med Chem 62: 10402-10422 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01499
BindingDB Entry DOI: 10.7270/Q27H1P12 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha-5
(Homo sapiens (Human)) | BDBM50168866
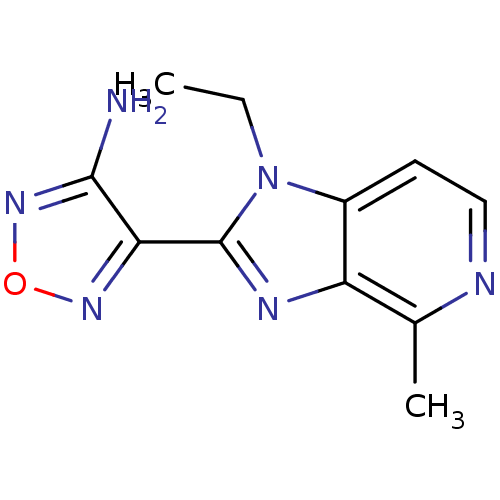
(4-(1-Ethyl-4-methyl-1H-imidazo[4,5-c]pyridin-2-yl)...)Show InChI InChI=1S/C11H12N6O/c1-3-17-7-4-5-13-6(2)8(7)14-11(17)9-10(12)16-18-15-9/h4-5H,3H2,1-2H3,(H2,12,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibitory concentration against MSK-1 |
Bioorg Med Chem Lett 15: 3407-11 (2005)
Article DOI: 10.1016/j.bmcl.2005.05.020
BindingDB Entry DOI: 10.7270/Q2S75FVN |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data