

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM13066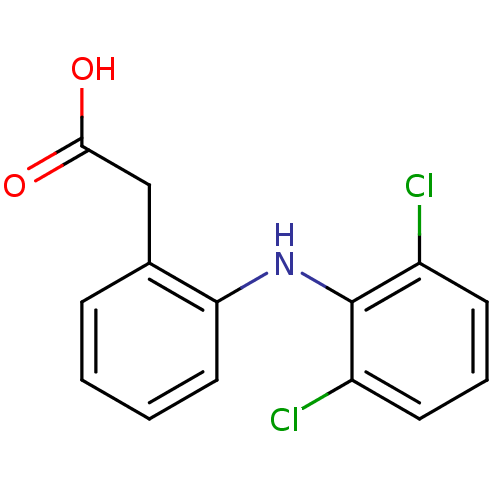 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2; (valus obtained by Kato et al.) | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM22369 (4-(4-methanesulfonylphenyl)-3-phenyl-2,5-dihydrofu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2; (valus obtained by Kato et al.) | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM13066 (2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic acid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2; (valus obtained by Kato et al.) | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50176068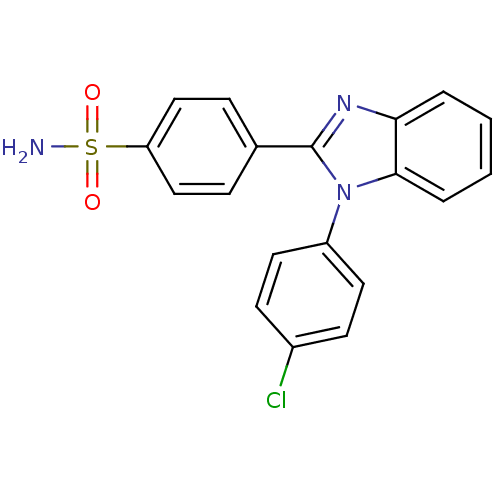 (4-[1-(4-Chloro-phenyl)-1H-benzoimidazol-2-yl]-benz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211482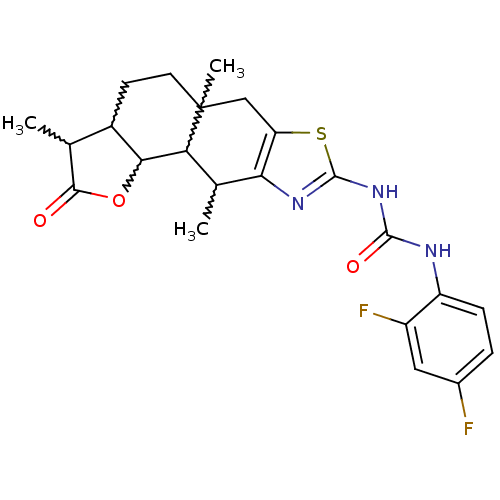 (1-(2,4-difluoro-phenyl)-3-(3,5a,10-trimethyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211478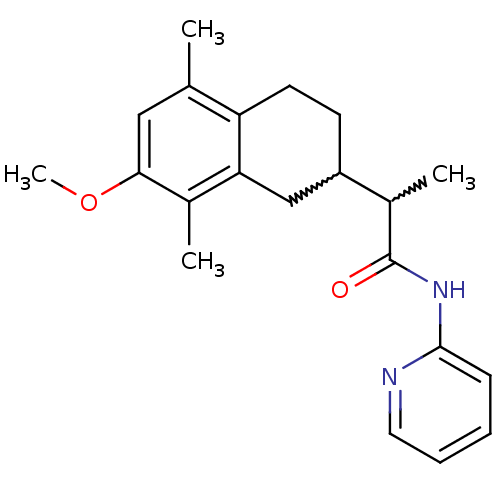 (2-(7-methoxy-5,8-dimethyl-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211491 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211496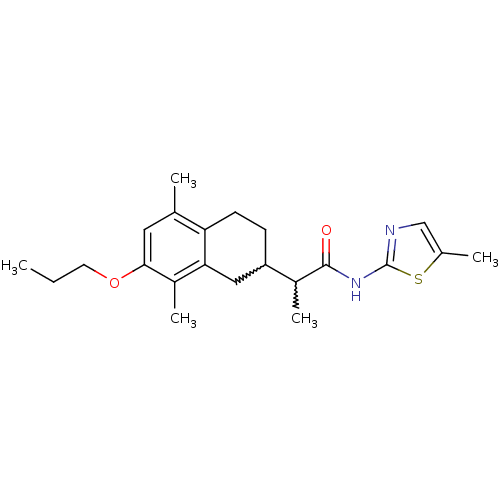 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211483 (3,4,5-trimethoxy-N-(3,5a,10-trimethyl-2-oxo-2,3,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211495 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211488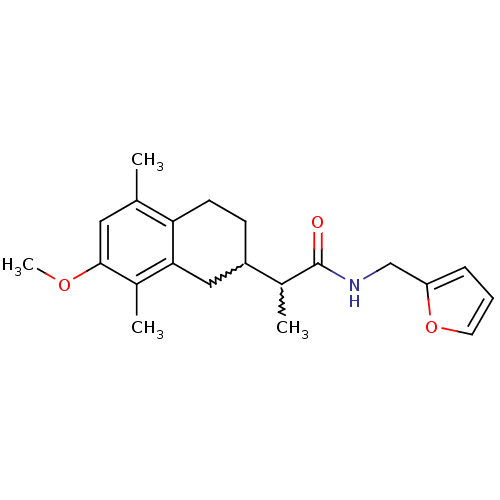 (CHEMBL376759 | N-(furan-2-ylmethyl)-2-(7-methoxy-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211489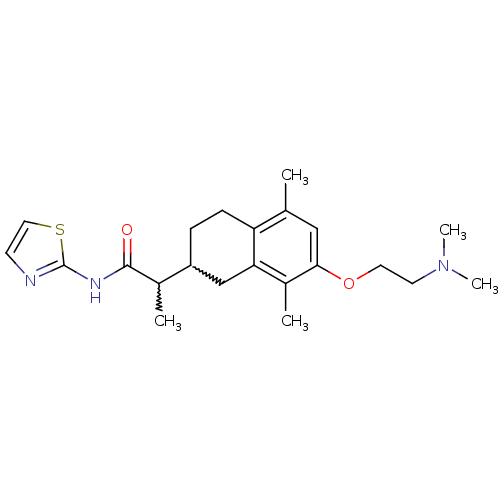 (2-(7-(2-(dimethylamino)ethoxy)-5,8-dimethyl-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211496 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211491 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211484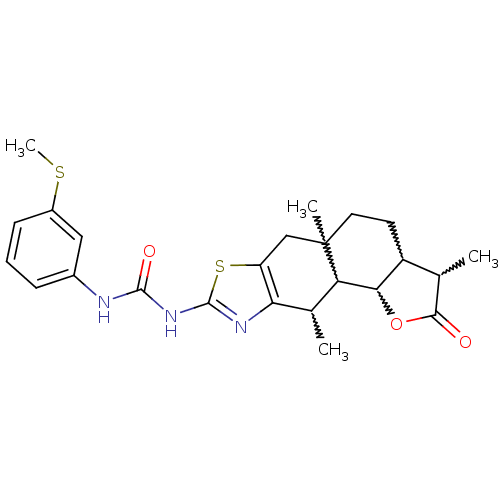 (1-(3-methylsulfanyl-phenyl)-3-(3,5a,10-trimethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211481 (CHEMBL226814 | benzo[1,3]dioxole-5-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211490 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211479 (1-benzyl-3-(3,5a,10-trimethyl-2-oxo-2,3,3a,4,5,5a,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211487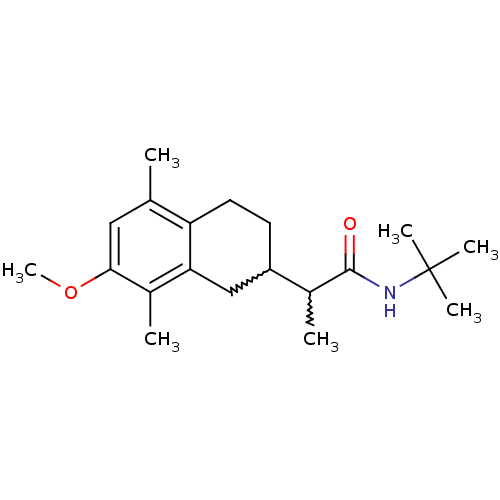 (CHEMBL227301 | N-tert-butyl-2-(7-methoxy-5,8-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211484 (1-(3-methylsulfanyl-phenyl)-3-(3,5a,10-trimethyl-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211485 (2-(2-methoxy-phenyl)-N-(3,5a,10-trimethyl-2-oxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211478 (2-(7-methoxy-5,8-dimethyl-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211480 (4-methoxy-N-(3,5a,10-trimethyl-2-oxo-2,3,3a,4,5,5a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211495 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211482 (1-(2,4-difluoro-phenyl)-3-(3,5a,10-trimethyl-2-oxo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211494 (2-(7-methoxy-5,8-dimethyl-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211492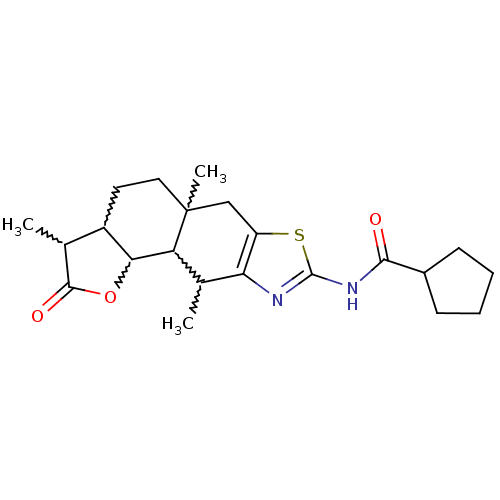 (CHEMBL227139 | cyclopentanecarboxylic acid (3,5a,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50176069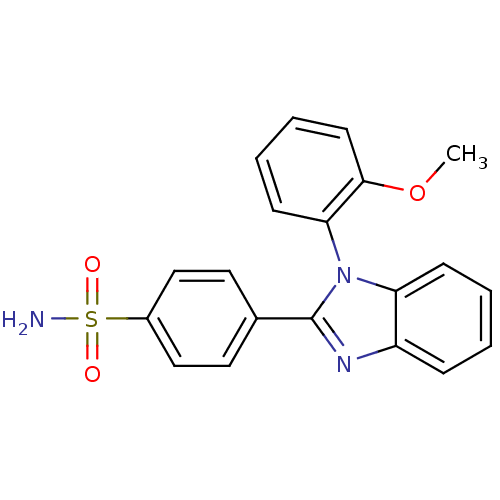 (4-[1-(2-Methoxy-phenyl)-1H-benzoimidazol-2-yl]-ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibitory concentration against COX-2 upon incubation for 15 minutes at 37 degree C | J Med Chem 48: 6997-7004 (2005) Article DOI: 10.1021/jm050619h BindingDB Entry DOI: 10.7270/Q2N29WH3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211490 (2-(5,8-dimethyl-7-propoxy-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211483 (3,4,5-trimethoxy-N-(3,5a,10-trimethyl-2-oxo-2,3,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211494 (2-(7-methoxy-5,8-dimethyl-1,2,3,4-tetrahydronaphth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211479 (1-benzyl-3-(3,5a,10-trimethyl-2-oxo-2,3,3a,4,5,5a,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211493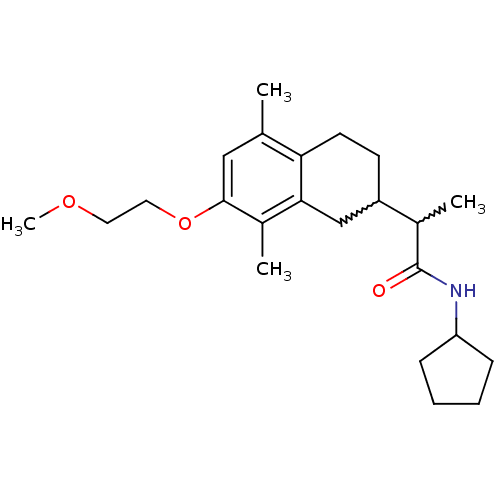 (CHEMBL373949 | N-cyclopentyl-2-[7-(2-methoxy-ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211488 (CHEMBL376759 | N-(furan-2-ylmethyl)-2-(7-methoxy-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211492 (CHEMBL227139 | cyclopentanecarboxylic acid (3,5a,1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211493 (CHEMBL373949 | N-cyclopentyl-2-[7-(2-methoxy-ethox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211480 (4-methoxy-N-(3,5a,10-trimethyl-2-oxo-2,3,3a,4,5,5a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211486 (4-methyl-[1,2,3]thiadiazole-5-carboxylic acid (3,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211489 (2-(7-(2-(dimethylamino)ethoxy)-5,8-dimethyl-1,2,3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211481 (CHEMBL226814 | benzo[1,3]dioxole-5-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211487 (CHEMBL227301 | N-tert-butyl-2-(7-methoxy-5,8-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of 5-LO activity in ionophore A23187 and arachidonic-stimulated human intact PMNL | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211485 (2-(2-methoxy-phenyl)-N-(3,5a,10-trimethyl-2-oxo-2,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50211486 (4-methyl-[1,2,3]thiadiazole-5-carboxylic acid (3,5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johann Wolfgang Goethe-Universität Curated by ChEMBL | Assay Description Inhibition of partially purified human 5-LO expressed in Escherichia coli | J Med Chem 50: 2640-6 (2007) Article DOI: 10.1021/jm060655w BindingDB Entry DOI: 10.7270/Q21N80T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||