 Found 359 hits with Last Name = 'frazee' and Initial = 'js'
Found 359 hits with Last Name = 'frazee' and Initial = 'js' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403349
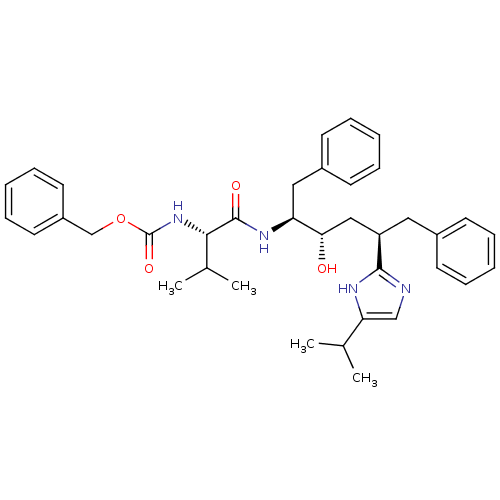
(CHEMBL407551)Show SMILES CC(C)[C@H](NC(=O)OCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C37H46N4O4/c1-25(2)32-23-38-35(39-32)30(20-27-14-8-5-9-15-27)22-33(42)31(21-28-16-10-6-11-17-28)40-36(43)34(26(3)4)41-37(44)45-24-29-18-12-7-13-19-29/h5-19,23,25-26,30-31,33-34,42H,20-22,24H2,1-4H3,(H,38,39)(H,40,43)(H,41,44)/t30-,31+,33+,34+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403357

(CHEMBL419286)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O3/c1-20(2)27-19-32-30(34-27)25(16-23-12-8-6-9-13-23)18-28(37)26(17-24-14-10-7-11-15-24)35-31(38)29(21(3)4)33-22(5)36/h6-15,19-21,25-26,28-29,37H,16-18H2,1-5H3,(H,32,34)(H,33,36)(H,35,38)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403360
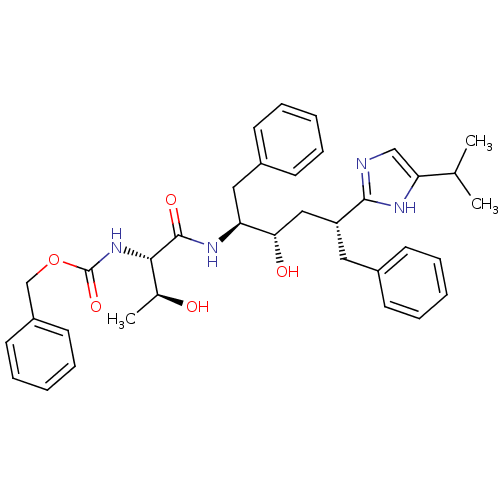
(CHEMBL1790592)Show SMILES CC(C)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)OCc1ccccc1)[C@H](C)O)Cc1ccccc1 Show InChI InChI=1S/C36H44N4O5/c1-24(2)31-22-37-34(38-31)29(19-26-13-7-4-8-14-26)21-32(42)30(20-27-15-9-5-10-16-27)39-35(43)33(25(3)41)40-36(44)45-23-28-17-11-6-12-18-28/h4-18,22,24-25,29-30,32-33,41-42H,19-21,23H2,1-3H3,(H,37,38)(H,39,43)(H,40,44)/t25-,29+,30-,32-,33-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403351
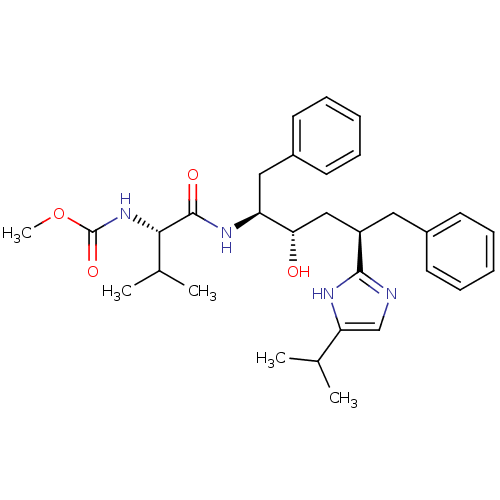
(CHEMBL79698)Show SMILES COC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O4/c1-20(2)26-19-32-29(33-26)24(16-22-12-8-6-9-13-22)18-27(36)25(17-23-14-10-7-11-15-23)34-30(37)28(21(3)4)35-31(38)39-5/h6-15,19-21,24-25,27-28,36H,16-18H2,1-5H3,(H,32,33)(H,34,37)(H,35,38)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403358
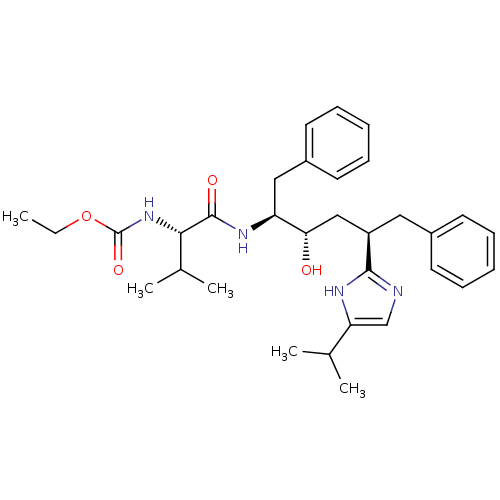
(CHEMBL78531)Show SMILES CCOC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C32H44N4O4/c1-6-40-32(39)36-29(22(4)5)31(38)35-26(18-24-15-11-8-12-16-24)28(37)19-25(17-23-13-9-7-10-14-23)30-33-20-27(34-30)21(2)3/h7-16,20-22,25-26,28-29,37H,6,17-19H2,1-5H3,(H,33,34)(H,35,38)(H,36,39)/t25-,26+,28+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403352

(CHEMBL81517)Show SMILES CC(C)[C@H](NC=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C30H40N4O3/c1-20(2)26-18-31-29(33-26)24(15-22-11-7-5-8-12-22)17-27(36)25(16-23-13-9-6-10-14-23)34-30(37)28(21(3)4)32-19-35/h5-14,18-21,24-25,27-28,36H,15-17H2,1-4H3,(H,31,33)(H,32,35)(H,34,37)/t24-,25+,27+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50009070
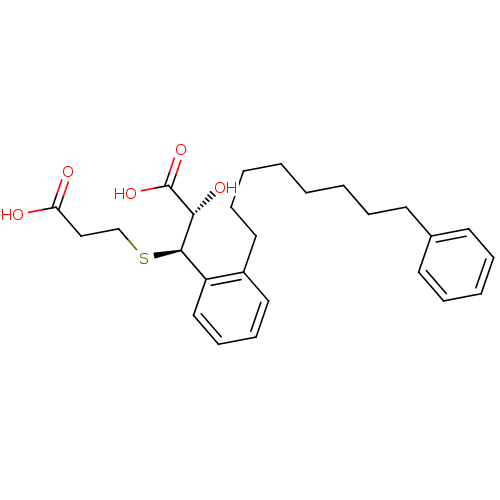
((2S,3R)-3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2...)Show SMILES O[C@H]([C@H](SCCC(O)=O)c1ccccc1CCCCCCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C26H34O5S/c27-23(28)18-19-32-25(24(29)26(30)31)22-17-11-10-16-21(22)15-9-4-2-1-3-6-12-20-13-7-5-8-14-20/h5,7-8,10-11,13-14,16-17,24-25,29H,1-4,6,9,12,15,18-19H2,(H,27,28)(H,30,31)/t24-,25-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. |
J Med Chem 30: 959-61 (1987)
BindingDB Entry DOI: 10.7270/Q2D50KZN |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50014978
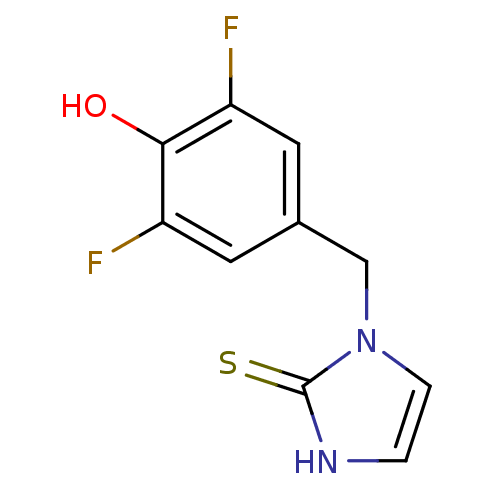
(1-(3,5-Difluoro-4-hydroxy-benzyl)-1,3-dihydro-imid...)Show InChI InChI=1S/C10H8F2N2OS/c11-7-3-6(4-8(12)9(7)15)5-14-2-1-13-10(14)16/h1-4,15H,5H2,(H,13,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate |
J Med Chem 29: 887-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K55 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403348
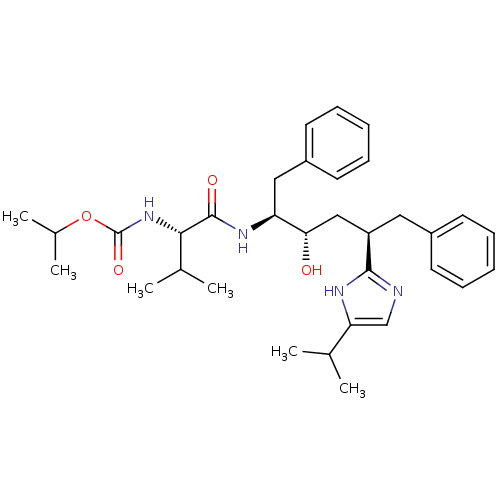
(CHEMBL421709)Show SMILES CC(C)OC(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C33H46N4O4/c1-21(2)28-20-34-31(35-28)26(17-24-13-9-7-10-14-24)19-29(38)27(18-25-15-11-8-12-16-25)36-32(39)30(22(3)4)37-33(40)41-23(5)6/h7-16,20-23,26-27,29-30,38H,17-19H2,1-6H3,(H,34,35)(H,36,39)(H,37,40)/t26-,27+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(Homo sapiens (Human)) | BDBM50009070

((2S,3R)-3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2...)Show SMILES O[C@H]([C@H](SCCC(O)=O)c1ccccc1CCCCCCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C26H34O5S/c27-23(28)18-19-32-25(24(29)26(30)31)22-17-11-10-16-21(22)15-9-4-2-1-3-6-12-20-13-7-5-8-14-20/h5,7-8,10-11,13-14,16-17,24-25,29H,1-4,6,9,12,15,18-19H2,(H,27,28)(H,30,31)/t24-,25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. |
J Med Chem 30: 959-61 (1987)
BindingDB Entry DOI: 10.7270/Q2D50KZN |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50014983
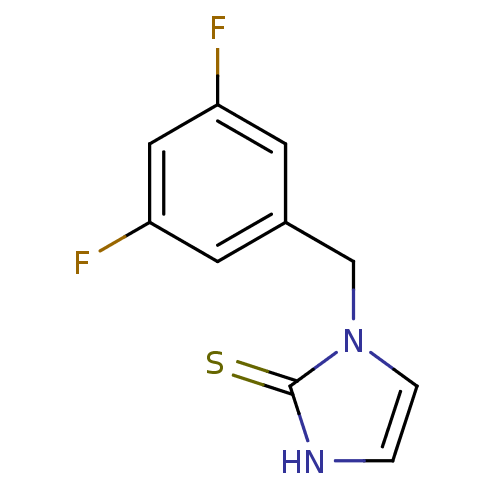
(1-(3,5-Difluoro-benzyl)-1,3-dihydro-imidazole-2-th...)Show InChI InChI=1S/C10H8F2N2S/c11-8-3-7(4-9(12)5-8)6-14-2-1-13-10(14)15/h1-5H,6H2,(H,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate |
J Med Chem 29: 887-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K55 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403345
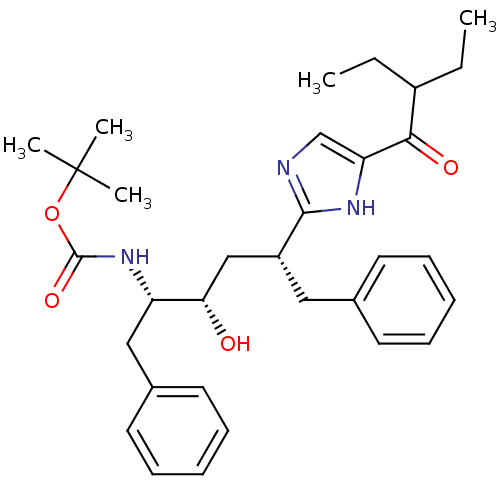
(CHEMBL83739)Show SMILES CCC(CC)C(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C32H43N3O4/c1-6-24(7-2)29(37)27-21-33-30(34-27)25(18-22-14-10-8-11-15-22)20-28(36)26(19-23-16-12-9-13-17-23)35-31(38)39-32(3,4)5/h8-17,21,24-26,28,36H,6-7,18-20H2,1-5H3,(H,33,34)(H,35,38)/t25-,26+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403350
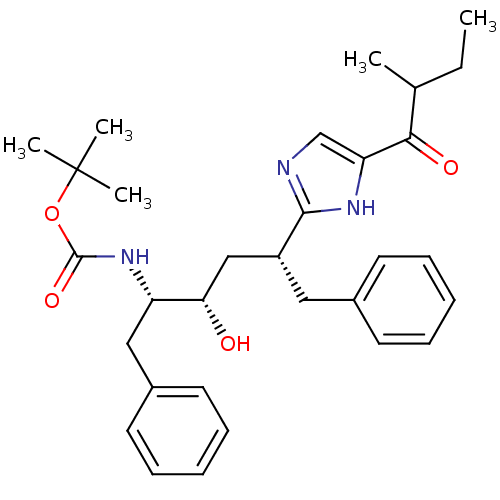
(CHEMBL81190)Show SMILES CCC(C)C(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C31H41N3O4/c1-6-21(2)28(36)26-20-32-29(33-26)24(17-22-13-9-7-10-14-22)19-27(35)25(18-23-15-11-8-12-16-23)34-30(37)38-31(3,4)5/h7-16,20-21,24-25,27,35H,6,17-19H2,1-5H3,(H,32,33)(H,34,37)/t21?,24-,25+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50014968
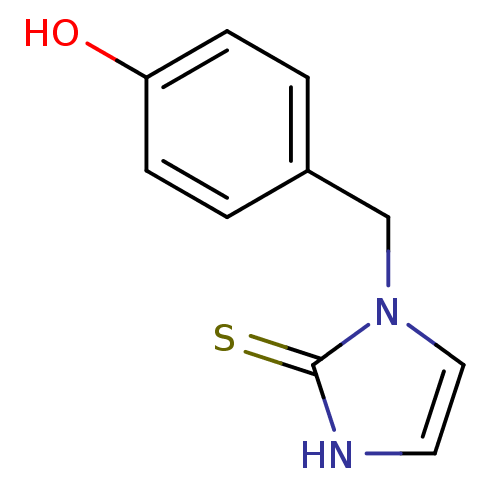
(1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...)Show InChI InChI=1S/C10H10N2OS/c13-9-3-1-8(2-4-9)7-12-6-5-11-10(12)14/h1-6,13H,7H2,(H,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 |
J Med Chem 29: 2465-72 (1987)
BindingDB Entry DOI: 10.7270/Q2BR8R5P |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50014968

(1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...)Show InChI InChI=1S/C10H10N2OS/c13-9-3-1-8(2-4-9)7-12-6-5-11-10(12)14/h1-6,13H,7H2,(H,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate |
J Med Chem 29: 887-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K55 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50021780
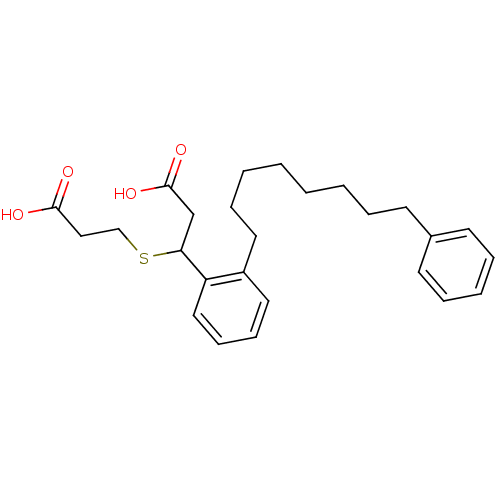
(3-(2-Carboxy-ethylsulfanyl)-3-[2-(8-phenyl-octyl)-...)Show SMILES OC(=O)CCSC(CC(O)=O)c1ccccc1CCCCCCCCc1ccccc1 Show InChI InChI=1S/C26H34O4S/c27-25(28)18-19-31-24(20-26(29)30)23-17-11-10-16-22(23)15-9-4-2-1-3-6-12-21-13-7-5-8-14-21/h5,7-8,10-11,13-14,16-17,24H,1-4,6,9,12,15,18-20H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. |
J Med Chem 30: 959-61 (1987)
BindingDB Entry DOI: 10.7270/Q2D50KZN |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50037126
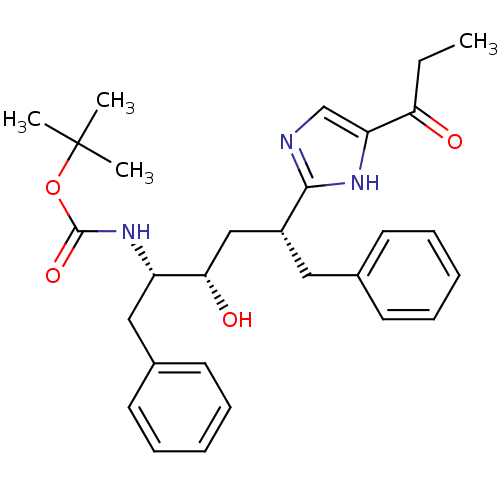
(CHEMBL80098 | [(1S,2S,4R)-1-Benzyl-2-hydroxy-5-phe...)Show SMILES CCC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C29H37N3O4/c1-5-25(33)24-19-30-27(31-24)22(16-20-12-8-6-9-13-20)18-26(34)23(17-21-14-10-7-11-15-21)32-28(35)36-29(2,3)4/h6-15,19,22-23,26,34H,5,16-18H2,1-4H3,(H,30,31)(H,32,35)/t22-,23+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50000439
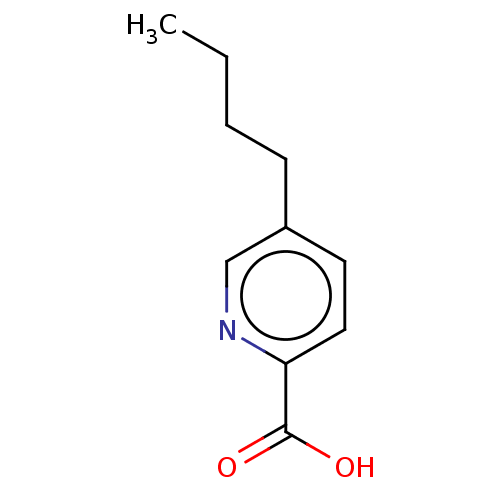
(5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...)Show InChI InChI=1S/C10H13NO2/c1-2-3-4-8-5-6-9(10(12)13)11-7-8/h5-7H,2-4H2,1H3,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity towards Dopamine beta hydroxylase using tyramine substrate at pH 4.5 in the absence of fumarate |
J Med Chem 29: 887-9 (1986)
BindingDB Entry DOI: 10.7270/Q2JH3K55 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50000439

(5-Butyl-pyridine-2-carboxylic acid | 5-Butyl-pyrid...)Show InChI InChI=1S/C10H13NO2/c1-2-3-4-8-5-6-9(10(12)13)11-7-8/h5-7H,2-4H2,1H3,(H,12,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 149 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 |
J Med Chem 29: 2465-72 (1987)
BindingDB Entry DOI: 10.7270/Q2BR8R5P |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403353

(CHEMBL309773)Show SMILES CC(C)[C@@H](NC(C)=O)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C31H42N4O3/c1-20(2)27-19-32-30(34-27)25(16-23-12-8-6-9-13-23)18-28(37)26(17-24-14-10-7-11-15-24)35-31(38)29(21(3)4)33-22(5)36/h6-15,19-21,25-26,28-29,37H,16-18H2,1-5H3,(H,32,34)(H,33,36)(H,35,38)/t25-,26+,28+,29-/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403362

(CHEMBL312709)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)C1CCCC1 Show InChI InChI=1S/C32H41N3O4/c1-32(2,3)39-31(38)35-26(19-23-14-8-5-9-15-23)28(36)20-25(18-22-12-6-4-7-13-22)30-33-21-27(34-30)29(37)24-16-10-11-17-24/h4-9,12-15,21,24-26,28,36H,10-11,16-20H2,1-3H3,(H,33,34)(H,35,38)/t25-,26+,28+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50021781

(3-(2-Carboxy-ethylsulfanyl)-2-hydroxy-3-[2-(8-phen...)Show SMILES O[C@@H]([C@@H](SCCC(O)=O)c1ccccc1CCCCCCCCc1ccccc1)C(O)=O Show InChI InChI=1S/C26H34O5S/c27-23(28)18-19-32-25(24(29)26(30)31)22-17-11-10-16-21(22)15-9-4-2-1-3-6-12-20-13-7-5-8-14-20/h5,7-8,10-11,13-14,16-17,24-25,29H,1-4,6,9,12,15,18-19H2,(H,27,28)(H,30,31)/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. |
J Med Chem 30: 959-61 (1987)
BindingDB Entry DOI: 10.7270/Q2D50KZN |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403356

(CHEMBL430437)Show SMILES CC(C)[C@H](NC(=O)OC(C)(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(C)C Show InChI InChI=1S/C34H48N4O4/c1-22(2)28-21-35-31(36-28)26(18-24-14-10-8-11-15-24)20-29(39)27(19-25-16-12-9-13-17-25)37-32(40)30(23(3)4)38-33(41)42-34(5,6)7/h8-17,21-23,26-27,29-30,39H,18-20H2,1-7H3,(H,35,36)(H,37,40)(H,38,41)/t26-,27+,29+,30+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50014968

(1-(4-Hydroxy-benzyl)-1,3-dihydro-imidazole-2-thion...)Show InChI InChI=1S/C10H10N2OS/c13-9-3-1-8(2-4-9)7-12-6-5-11-10(12)14/h1-6,13H,7H2,(H,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 344 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 |
J Med Chem 29: 2465-72 (1987)
BindingDB Entry DOI: 10.7270/Q2BR8R5P |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403347
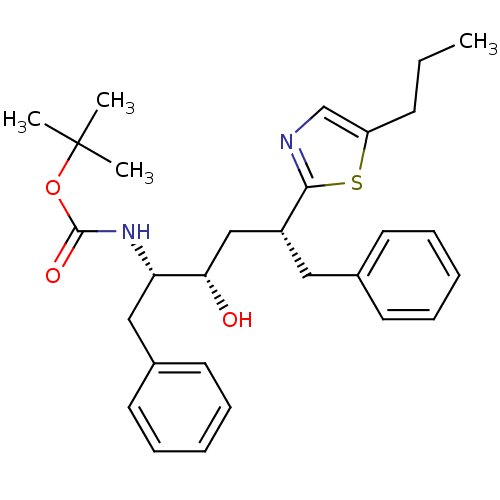
(CHEMBL2115564)Show SMILES CCCc1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 |r| Show InChI InChI=1S/C29H38N2O3S/c1-5-12-24-20-30-27(35-24)23(17-21-13-8-6-9-14-21)19-26(32)25(18-22-15-10-7-11-16-22)31-28(33)34-29(2,3)4/h6-11,13-16,20,23,25-26,32H,5,12,17-19H2,1-4H3,(H,31,33)/t23-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403355
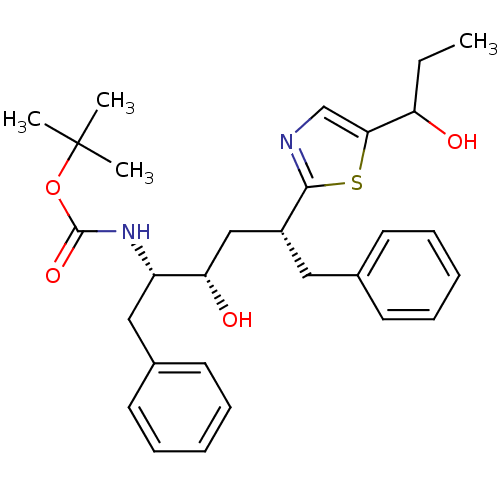
(CHEMBL315930)Show SMILES CCC(O)c1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C29H38N2O4S/c1-5-24(32)26-19-30-27(36-26)22(16-20-12-8-6-9-13-20)18-25(33)23(17-21-14-10-7-11-15-21)31-28(34)35-29(2,3)4/h6-15,19,22-25,32-33H,5,16-18H2,1-4H3,(H,31,34)/t22-,23+,24?,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Cysteinyl leukotriene receptor 1
(GUINEA PIG) | BDBM50006812
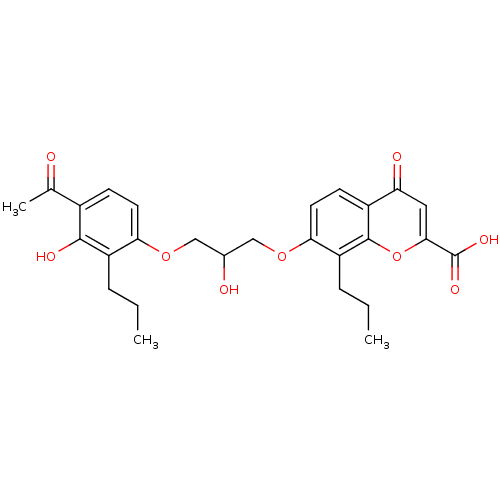
(7-[3-(4-Acetyl-3-hydroxy-2-propyl-phenoxy)-2-hydro...)Show SMILES CCCc1c(OCC(O)COc2ccc3c(oc(cc3=O)C(O)=O)c2CCC)ccc(C(C)=O)c1O Show InChI InChI=1S/C27H30O9/c1-4-6-19-22(10-8-17(15(3)28)25(19)31)34-13-16(29)14-35-23-11-9-18-21(30)12-24(27(32)33)36-26(18)20(23)7-5-2/h8-12,16,29,31H,4-7,13-14H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against Cysteinyl leukotriene D4 receptor in guinea pig lung membranes using [3H]-LTD4 as the radioligand. |
J Med Chem 30: 959-61 (1987)
BindingDB Entry DOI: 10.7270/Q2D50KZN |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403342
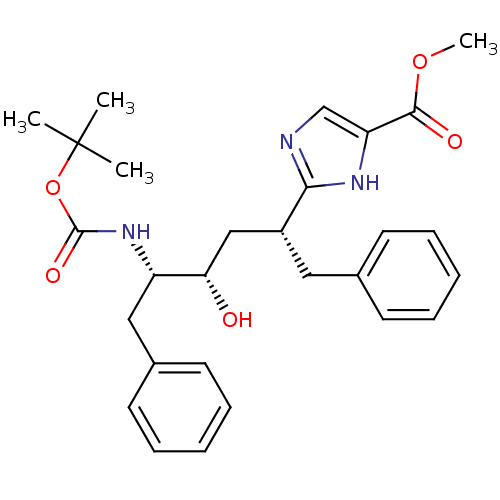
(CHEMBL79719)Show SMILES COC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C28H35N3O5/c1-28(2,3)36-27(34)31-22(16-20-13-9-6-10-14-20)24(32)17-21(15-19-11-7-5-8-12-19)25-29-18-23(30-25)26(33)35-4/h5-14,18,21-22,24,32H,15-17H2,1-4H3,(H,29,30)(H,31,34)/t21-,22+,24+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403344

(CHEMBL83384)Show SMILES CCc1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C28H36N2O3S/c1-5-23-19-29-26(34-23)22(16-20-12-8-6-9-13-20)18-25(31)24(17-21-14-10-7-11-15-21)30-27(32)33-28(2,3)4/h6-15,19,22,24-25,31H,5,16-18H2,1-4H3,(H,30,32)/t22-,24+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403359

(CHEMBL312136)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1nccs1 Show InChI InChI=1S/C26H32N2O3S/c1-26(2,3)31-25(30)28-22(17-20-12-8-5-9-13-20)23(29)18-21(24-27-14-15-32-24)16-19-10-6-4-7-11-19/h4-15,21-23,29H,16-18H2,1-3H3,(H,28,30)/t21-,22+,23+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403343
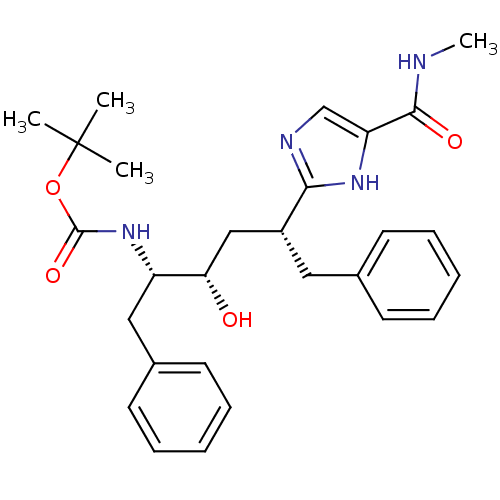
(CHEMBL309195)Show SMILES CNC(=O)c1cnc([nH]1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C28H36N4O4/c1-28(2,3)36-27(35)32-22(16-20-13-9-6-10-14-20)24(33)17-21(15-19-11-7-5-8-12-19)25-30-18-23(31-25)26(34)29-4/h5-14,18,21-22,24,33H,15-17H2,1-4H3,(H,29,34)(H,30,31)(H,32,35)/t21-,22+,24+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
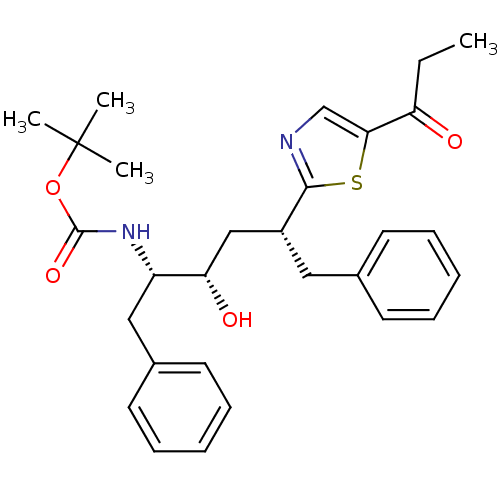
(Human immunodeficiency virus type 1) | BDBM50403346
(CHEMBL81775)Show SMILES CCC(=O)c1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C29H36N2O4S/c1-5-24(32)26-19-30-27(36-26)22(16-20-12-8-6-9-13-20)18-25(33)23(17-21-14-10-7-11-15-21)31-28(34)35-29(2,3)4/h6-15,19,22-23,25,33H,5,16-18H2,1-4H3,(H,31,34)/t22-,23+,25+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403347

(CHEMBL2115564)Show SMILES CCCc1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 |r| Show InChI InChI=1S/C29H38N2O3S/c1-5-12-24-20-30-27(35-24)23(17-21-13-8-6-9-14-21)19-26(32)25(18-22-15-10-7-11-16-22)31-28(33)34-29(2,3)4/h6-11,13-16,20,23,25-26,32H,5,12,17-19H2,1-4H3,(H,31,33)/t23-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403347

(CHEMBL2115564)Show SMILES CCCc1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 |r| Show InChI InChI=1S/C29H38N2O3S/c1-5-12-24-20-30-27(35-24)23(17-21-13-8-6-9-14-21)19-26(32)25(18-22-15-10-7-11-16-22)31-28(33)34-29(2,3)4/h6-11,13-16,20,23,25-26,32H,5,12,17-19H2,1-4H3,(H,31,33)/t23-,25+,26+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403354
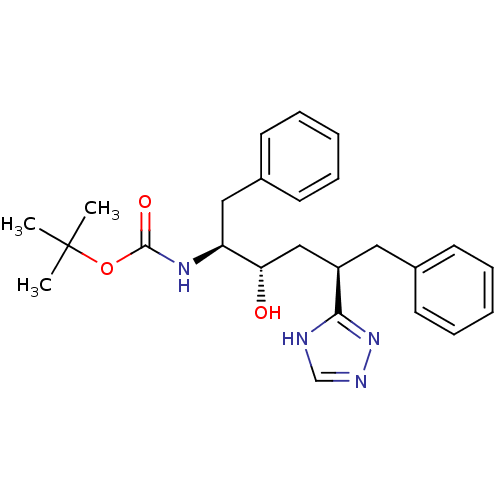
(CHEMBL79570)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1nnc[nH]1 Show InChI InChI=1S/C25H32N4O3/c1-25(2,3)32-24(31)28-21(15-19-12-8-5-9-13-19)22(30)16-20(23-26-17-27-29-23)14-18-10-6-4-7-11-18/h4-13,17,20-22,30H,14-16H2,1-3H3,(H,28,31)(H,26,27,29)/t20-,21+,22+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403361

(CHEMBL432179)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C[C@@H](Cc1ccccc1)c1ncc([nH]1)C(=O)c1ccccc1 Show InChI InChI=1S/C33H37N3O4/c1-33(2,3)40-32(39)36-27(20-24-15-9-5-10-16-24)29(37)21-26(19-23-13-7-4-8-14-23)31-34-22-28(35-31)30(38)25-17-11-6-12-18-25/h4-18,22,26-27,29,37H,19-21H2,1-3H3,(H,34,35)(H,36,39)/t26-,27+,29+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50403363

(CHEMBL78534)Show SMILES CCCCc1cnc(s1)[C@@H](C[C@H](O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C)Cc1ccccc1 Show InChI InChI=1S/C30H40N2O3S/c1-5-6-17-25-21-31-28(36-25)24(18-22-13-9-7-10-14-22)20-27(33)26(19-23-15-11-8-12-16-23)32-29(34)35-30(2,3)4/h7-16,21,24,26-27,33H,5-6,17-20H2,1-4H3,(H,32,34)/t24-,26+,27+/m1/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against HIV-1 protease |
Bioorg Med Chem Lett 4: 2441-2446 (1994)
Article DOI: 10.1016/S0960-894X(01)80406-6
BindingDB Entry DOI: 10.7270/Q2X63P48 |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50241361
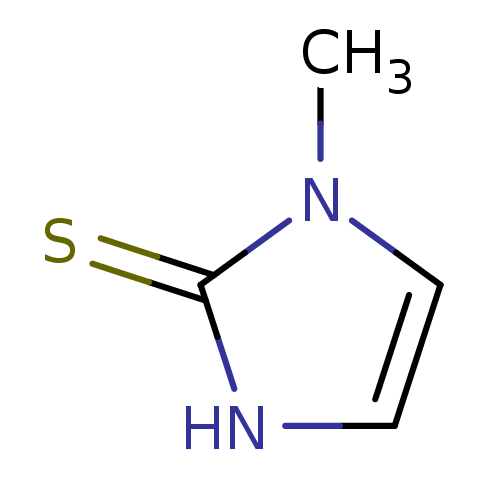
(CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...)Show InChI InChI=1S/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 4.5 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 6.6 |
J Med Chem 29: 2465-72 (1987)
BindingDB Entry DOI: 10.7270/Q2BR8R5P |
More data for this
Ligand-Target Pair | |
Dopamine beta-hydroxylase
(Bos taurus) | BDBM50241361

(CHEMBL1515 | METHIMAZOLE | US9138393, Methimazole ...)Show InChI InChI=1S/C4H6N2S/c1-6-3-2-5-4(6)7/h2-3H,1H3,(H,5,7) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| 7.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 6.6 | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Dopamine beta hydroxylase at pH 4.5 |
J Med Chem 29: 2465-72 (1987)
BindingDB Entry DOI: 10.7270/Q2BR8R5P |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304973

((S)-4-((2-bromobenzyl)(1-(methylsulfonyl)pyrrolidi...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1Br)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19BrClN3O2S/c1-27(25,26)23-9-8-17(13-23)24(12-15-4-2-3-5-18(15)20)16-7-6-14(11-22)19(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Sgk1
(Homo sapiens (Human)) | BDBM50296010

(2-isobutyl-4-(5-phenyl-1H-pyrrolo[2,3-b]pyridin-3-...)Show SMILES CC(C)Cc1cc(ccc1C(O)=O)-c1c[nH]c2ncc(cc12)-c1ccccc1 Show InChI InChI=1S/C24H22N2O2/c1-15(2)10-18-11-17(8-9-20(18)24(27)28)22-14-26-23-21(22)12-19(13-25-23)16-6-4-3-5-7-16/h3-9,11-15H,10H2,1-2H3,(H,25,26)(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of SGK1 by fluorescence polarization assay |
Bioorg Med Chem Lett 19: 4441-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.051
BindingDB Entry DOI: 10.7270/Q2WQ03VC |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304972

((S)-2-chloro-4-((2-chlorobenzyl)(1-(methylsulfonyl...)Show SMILES CS(=O)(=O)N1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C19H19Cl2N3O2S/c1-27(25,26)23-9-8-17(13-23)24(12-15-4-2-3-5-18(15)20)16-7-6-14(11-22)19(21)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304987
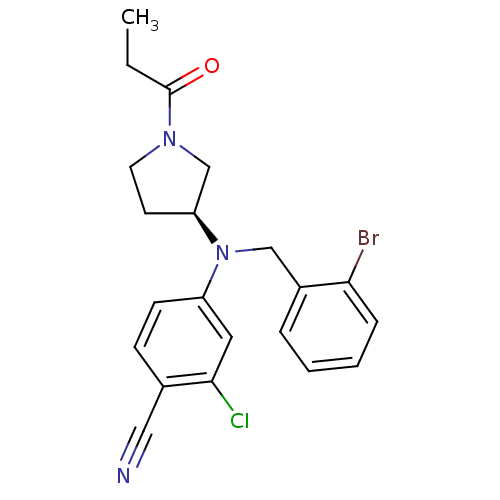
((S)-4-((2-bromobenzyl)(1-propionylpyrrolidin-3-yl)...)Show SMILES CCC(=O)N1CC[C@@H](C1)N(Cc1ccccc1Br)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H21BrClN3O/c1-2-21(27)25-10-9-18(14-25)26(13-16-5-3-4-6-19(16)22)17-8-7-15(12-24)20(23)11-17/h3-8,11,18H,2,9-10,13-14H2,1H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304978

((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-chloroben...)Show SMILES COC(=O)N1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H19Cl2N3O2/c1-27-20(26)24-9-8-17(13-24)25(12-15-4-2-3-5-18(15)21)16-7-6-14(11-23)19(22)10-16/h2-7,10,17H,8-9,12-13H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298032

((S)-2-chloro-4-((2-chlorobenzyl)(1-(cyanomethyl)py...)Show SMILES Clc1ccccc1CN([C@H]1CCN(CC#N)C1)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl2N4/c21-19-4-2-1-3-16(19)13-26(18-7-9-25(14-18)10-8-23)17-6-5-15(12-24)20(22)11-17/h1-6,11,18H,7,9-10,13-14H2/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to PR by fluorescence polarization based competition binding assay |
Bioorg Med Chem Lett 19: 4777-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.055
BindingDB Entry DOI: 10.7270/Q2TB16XG |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298033

((S)-ethyl 2-(3-((3-chloro-4-cyanophenyl)(2-chlorob...)Show SMILES CCOC(=O)CN1CC[C@@H](C1)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C22H23Cl2N3O2/c1-2-29-22(28)15-26-10-9-19(14-26)27(13-17-5-3-4-6-20(17)23)18-8-7-16(12-25)21(24)11-18/h3-8,11,19H,2,9-10,13-15H2,1H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to PR by fluorescence polarization based competition binding assay |
Bioorg Med Chem Lett 19: 4777-80 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.055
BindingDB Entry DOI: 10.7270/Q2TB16XG |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298207
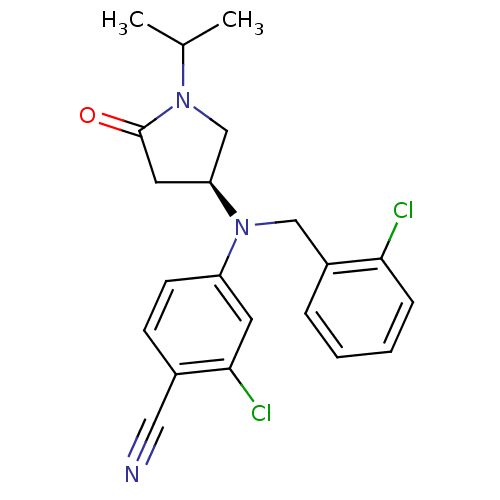
((S)-2-chloro-4-((2-chlorobenzyl)(1-isopropyl-5-oxo...)Show SMILES CC(C)N1C[C@H](CC1=O)N(Cc1ccccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H21Cl2N3O/c1-14(2)25-13-18(10-21(25)27)26(12-16-5-3-4-6-19(16)22)17-8-7-15(11-24)20(23)9-17/h3-9,14,18H,10,12-13H2,1-2H3/t18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 19: 4664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.081
BindingDB Entry DOI: 10.7270/Q25H7G9T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298210
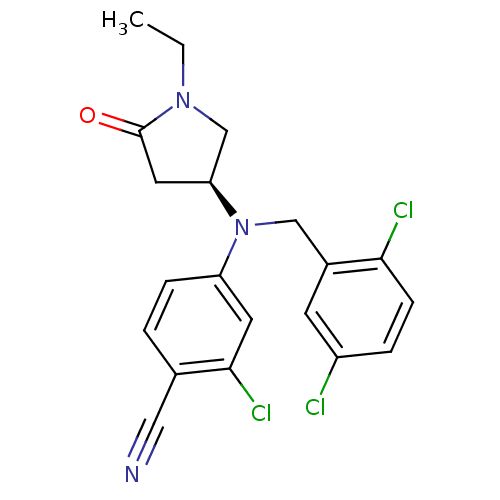
((S)-2-chloro-4-((2,5-dichlorobenzyl)(1-ethyl-5-oxo...)Show SMILES CCN1C[C@H](CC1=O)N(Cc1cc(Cl)ccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl3N3O/c1-2-25-12-17(9-20(25)27)26(11-14-7-15(21)4-6-18(14)22)16-5-3-13(10-24)19(23)8-16/h3-8,17H,2,9,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 19: 4664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.081
BindingDB Entry DOI: 10.7270/Q25H7G9T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50298208

((S)-2-chloro-4-((2-chloro-5-fluorobenzyl)(1-ethyl-...)Show SMILES CCN1C[C@H](CC1=O)N(Cc1cc(F)ccc1Cl)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C20H18Cl2FN3O/c1-2-25-12-17(9-20(25)27)26(11-14-7-15(23)4-6-18(14)21)16-5-3-13(10-24)19(22)8-16/h3-8,17H,2,9,11-12H2,1H3/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to progesterone receptor |
Bioorg Med Chem Lett 19: 4664-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.081
BindingDB Entry DOI: 10.7270/Q25H7G9T |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50304977

((S)-methyl 3-((3-chloro-4-cyanophenyl)(2-methylben...)Show SMILES COC(=O)N1CC[C@@H](C1)N(Cc1ccccc1C)c1ccc(C#N)c(Cl)c1 |r| Show InChI InChI=1S/C21H22ClN3O2/c1-15-5-3-4-6-17(15)13-25(18-8-7-16(12-23)20(22)11-18)19-9-10-24(14-19)21(26)27-2/h3-8,11,19H,9-10,13-14H2,1-2H3/t19-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of PR |
Bioorg Med Chem Lett 20: 371-4 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.092
BindingDB Entry DOI: 10.7270/Q2057G17 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data