 Found 1141 hits with Last Name = 'freeman' and Initial = 's'
Found 1141 hits with Last Name = 'freeman' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380758
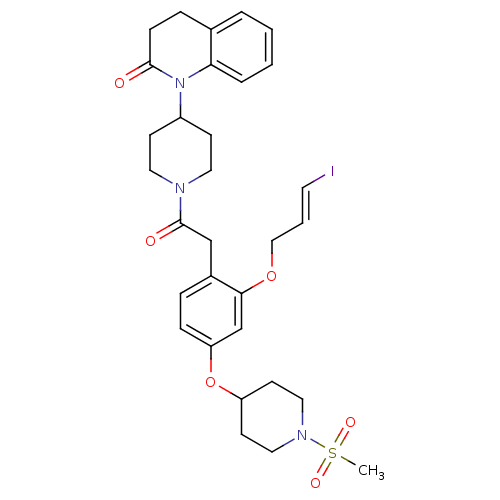
(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291312
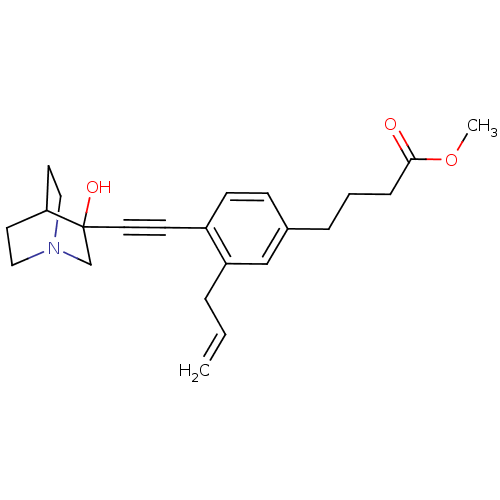
(4-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(20.76,-2.86,;19.42,-2.1,;18.08,-2.89,;18.1,-4.43,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(6-4-7-22(25)27-2)8-9-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,8-9,16,21,26H,1,4-7,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380759
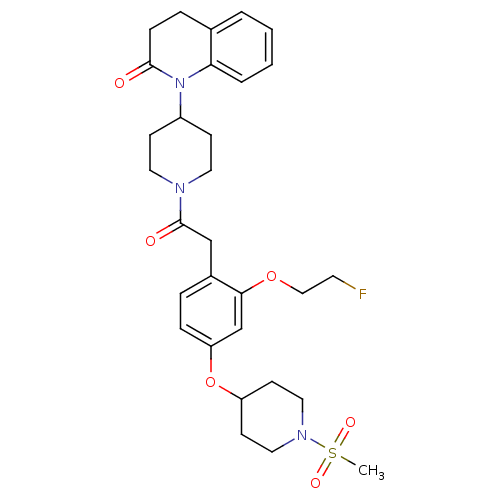
(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380758

(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380757
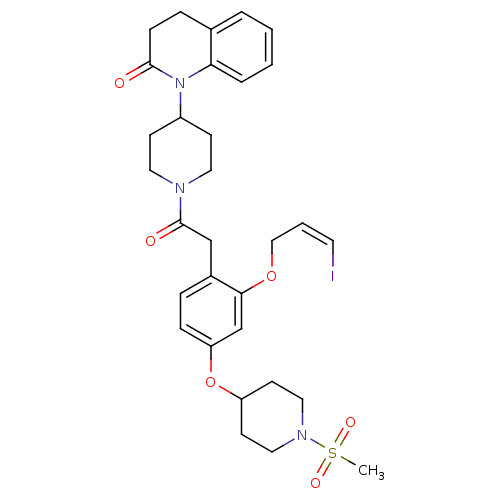
(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50435057

(CHEMBL2391300)Show SMILES CN[C@@H](CCS(C)(=O)=O)C(=O)N[C@H]1C[C@H]2CC[C@]1(CS(=O)(=O)N1CCN(CC1)c1ccccc1C)C2(C)C |r,TLB:11:12:35:16.15| Show InChI InChI=1S/C27H44N4O5S2/c1-20-8-6-7-9-23(20)30-13-15-31(16-14-30)38(35,36)19-27-12-10-21(26(27,2)3)18-24(27)29-25(32)22(28-4)11-17-37(5,33)34/h6-9,21-22,24,28H,10-19H2,1-5H3,(H,29,32)/t21-,22+,24+,27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to human OT receptor |
Bioorg Med Chem Lett 23: 902-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.116
BindingDB Entry DOI: 10.7270/Q29Z9690 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380757

(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50075719
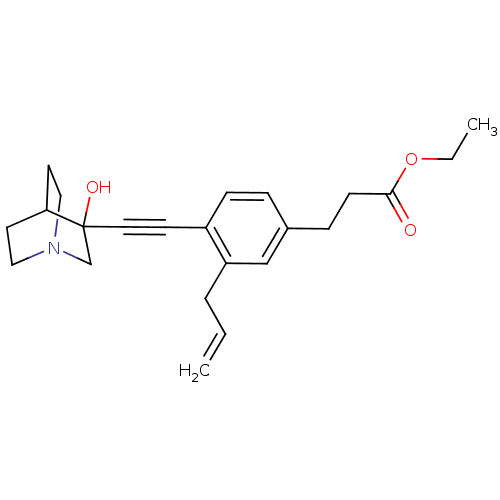
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291315

(5-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:13:14:19.18:21.22,15:14:19.18:21.22,(22.09,-2.1,;20.76,-2.86,;19.42,-2.1,;19.42,-.56,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C24H31NO3/c1-3-6-21-17-19(7-4-5-8-23(26)28-2)9-10-20(21)11-14-24(27)18-25-15-12-22(24)13-16-25/h3,9-10,17,22,27H,1,4-8,12-13,15-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380758

(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50326719
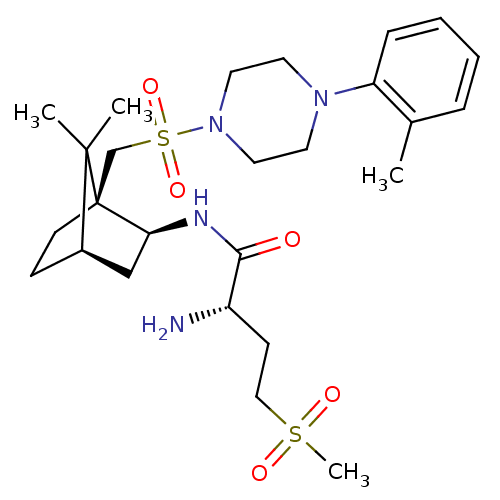
((S)-2-amino-N-((1S,2S,4R)-7,7-dimethyl-1-((4-o-tol...)Show SMILES Cc1ccccc1N1CCN(CC1)S(=O)(=O)C[C@]12CC[C@H](C[C@@H]1NC(=O)[C@@H](N)CCS(C)(=O)=O)C2(C)C |r,TLB:23:22:34:18.19| Show InChI InChI=1S/C26H42N4O5S2/c1-19-7-5-6-8-22(19)29-12-14-30(15-13-29)37(34,35)18-26-11-9-20(25(26,2)3)17-23(26)28-24(31)21(27)10-16-36(4,32)33/h5-8,20-21,23H,9-18,27H2,1-4H3,(H,28,31)/t20-,21+,23+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yerkes National Primate Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to human OT receptor |
Bioorg Med Chem Lett 23: 902-6 (2013)
Article DOI: 10.1016/j.bmcl.2012.10.116
BindingDB Entry DOI: 10.7270/Q29Z9690 |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50440392
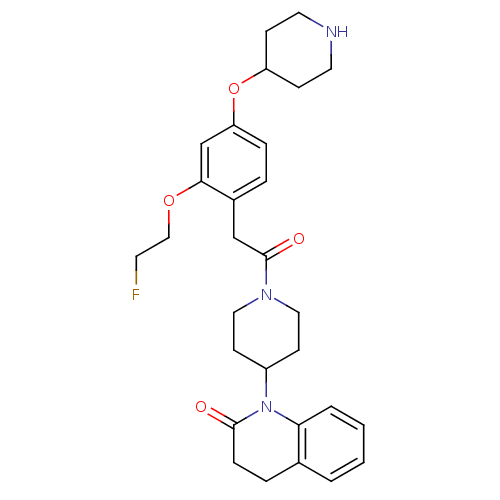
(CHEMBL2424668)Show SMILES FCCOc1cc(OC2CCNCC2)ccc1CC(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C29H36FN3O4/c30-13-18-36-27-20-25(37-24-9-14-31-15-10-24)7-5-22(27)19-29(35)32-16-11-23(12-17-32)33-26-4-2-1-3-21(26)6-8-28(33)34/h1-5,7,20,23-24,31H,6,8-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Binding affinity to human OTR by competitive binding assay |
Bioorg Med Chem Lett 23: 5415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.045
BindingDB Entry DOI: 10.7270/Q20R9QVH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Binding affinity to human OTR by competitive binding assay |
Bioorg Med Chem Lett 23: 5415-20 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.045
BindingDB Entry DOI: 10.7270/Q20R9QVH |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Oxytocin from human OXTR expressed in CHO cell membranes after 90 mins by liquid scintillation counting method |
Bioorg Med Chem 25: 305-315 (2017)
Article DOI: 10.1016/j.bmc.2016.10.035
BindingDB Entry DOI: 10.7270/Q24T6MNC |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380757

(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM50121753

(1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...)Show SMILES NC1=[N+](Cc2[nH]c(=O)[nH]c(=O)c2Cl)CCC1 |c:1| Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against human thymidine phosphorylase |
Bioorg Med Chem Lett 13: 3705-9 (2003)
BindingDB Entry DOI: 10.7270/Q2SQ90ZZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50233853

(CHEMBL3392901)Show SMILES Cc1ccccc1N1CCN(CC1)S(=O)(=O)CC12CCC(CC1NC(=O)C(N)CCS(C)(=O)=O)C2(C)C |TLB:23:22:34:18.19| Show InChI InChI=1S/C26H42N4O5S2/c1-19-7-5-6-8-22(19)29-12-14-30(15-13-29)37(34,35)18-26-11-9-20(25(26,2)3)17-23(26)28-24(31)21(27)10-16-36(4,32)33/h5-8,20-21,23H,9-18,27H2,1-4H3,(H,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]Oxytocin from human OXTR expressed in CHO cell membranes after 90 mins by liquid scintillation counting method |
Bioorg Med Chem 25: 305-315 (2017)
Article DOI: 10.1016/j.bmc.2016.10.035
BindingDB Entry DOI: 10.7270/Q24T6MNC |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Microtus ochrogaster) | BDBM50380760
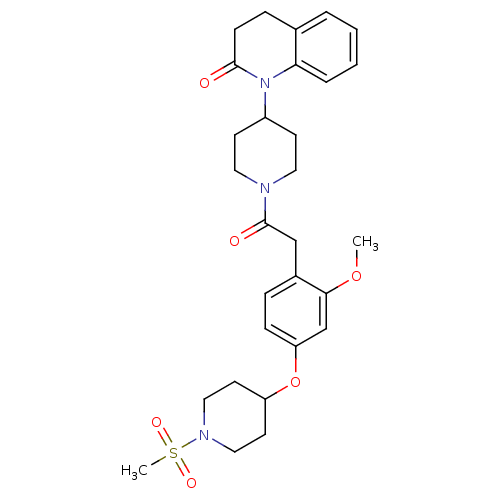
(CHEMBL2017979)Show SMILES COc1cc(OC2CCN(CC2)S(C)(=O)=O)ccc1CC(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C29H37N3O6S/c1-37-27-20-25(38-24-13-17-31(18-14-24)39(2,35)36)9-7-22(27)19-29(34)30-15-11-23(12-16-30)32-26-6-4-3-5-21(26)8-10-28(32)33/h3-7,9,20,23-24H,8,10-19H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-OVTA from prairie vole Oxytocin receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079
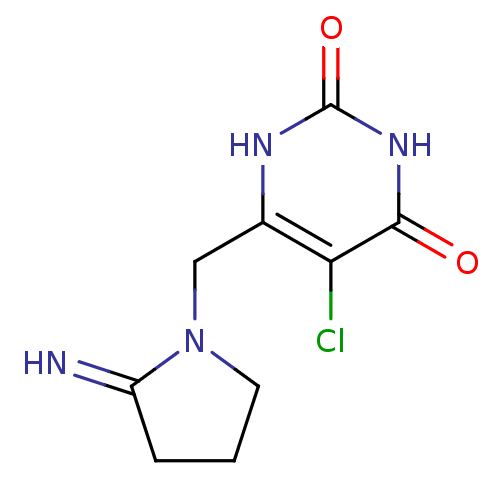
(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidine phosphorylase
(Homo sapiens (Human)) | BDBM20079

(5-chloro-6-[(2-iminopyrrolidin-1-yl)methyl]-1,2,3,...)Show InChI InChI=1S/C9H11ClN4O2/c10-7-5(12-9(16)13-8(7)15)4-14-3-1-2-6(14)11/h11H,1-4H2,(H2,12,13,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant thymidine phosphorylase by [3H]thymidine incorporation assay |
Eur J Med Chem 43: 1248-60 (2008)
Article DOI: 10.1016/j.ejmech.2007.07.015
BindingDB Entry DOI: 10.7270/Q2Z31ZDP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Squalene synthase
(Rattus norvegicus) | BDBM50291311

(6-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCCCCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:14:15:20.19:22.23,16:15:20.19:22.23,(23.43,-2.86,;22.09,-2.1,;20.76,-2.86,;20.76,-4.4,;19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C25H33NO3/c1-3-7-22-18-20(8-5-4-6-9-24(27)29-2)10-11-21(22)12-15-25(28)19-26-16-13-23(25)14-17-26/h3,10-11,18,23,28H,1,4-9,13-14,16-17,19H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291316
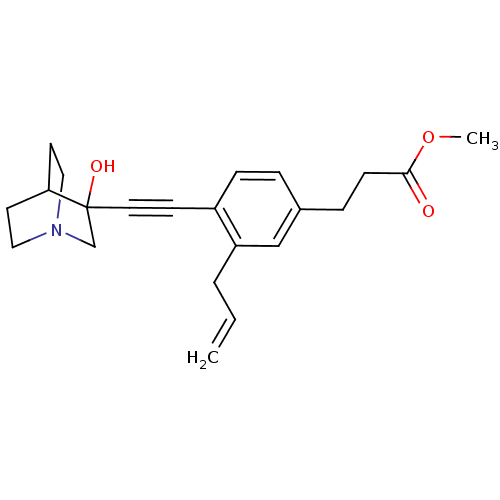
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES COC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H27NO3/c1-3-4-19-15-17(6-8-21(24)26-2)5-7-18(19)9-12-22(25)16-23-13-10-20(22)11-14-23/h3,5,7,15,20,25H,1,4,6,8,10-11,13-14,16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380760

(CHEMBL2017979)Show SMILES COc1cc(OC2CCN(CC2)S(C)(=O)=O)ccc1CC(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C29H37N3O6S/c1-37-27-20-25(38-24-13-17-31(18-14-24)39(2,35)36)9-7-22(27)19-29(34)30-15-11-23(12-16-30)32-26-6-4-3-5-21(26)8-10-28(32)33/h3-7,9,20,23-24H,8,10-19H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50034993
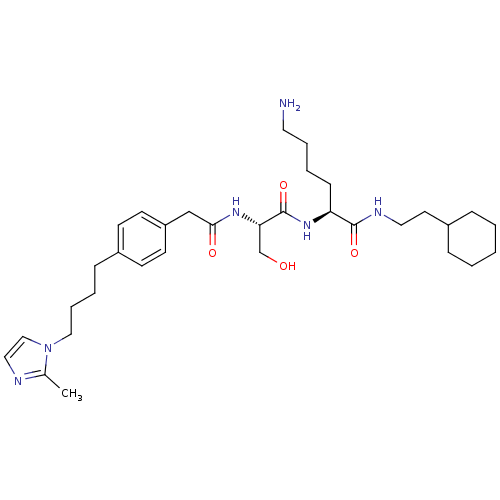
((S)-6-Amino-2-[(S)-3-hydroxy-2-(2-{4-[4-(2-methyl-...)Show SMILES Cc1nccn1CCCCc1ccc(CC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)NCCC2CCCCC2)cc1 Show InChI InChI=1S/C33H52N6O4/c1-25-35-20-22-39(25)21-8-6-11-27-13-15-28(16-14-27)23-31(41)37-30(24-40)33(43)38-29(12-5-7-18-34)32(42)36-19-17-26-9-3-2-4-10-26/h13-16,20,22,26,29-30,40H,2-12,17-19,21,23-24,34H2,1H3,(H,36,42)(H,37,41)(H,38,43)/t29-,30-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Co.
Curated by ChEMBL
| Assay Description
Apparent binding affinity against Candida albicans N-Myristoyltransferase (NMT). |
J Med Chem 38: 1837-40 (1995)
Article DOI: 10.1021/jm00011a001
BindingDB Entry DOI: 10.7270/Q23J3GPZ |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase
(Candida albicans (Yeast)) | BDBM50034993

((S)-6-Amino-2-[(S)-3-hydroxy-2-(2-{4-[4-(2-methyl-...)Show SMILES Cc1nccn1CCCCc1ccc(CC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)NCCC2CCCCC2)cc1 Show InChI InChI=1S/C33H52N6O4/c1-25-35-20-22-39(25)21-8-6-11-27-13-15-28(16-14-27)23-31(41)37-30(24-40)33(43)38-29(12-5-7-18-34)32(42)36-19-17-26-9-3-2-4-10-26/h13-16,20,22,26,29-30,40H,2-12,17-19,21,23-24,34H2,1H3,(H,36,42)(H,37,41)(H,38,43)/t29-,30-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G. D. Searle and Company
Curated by ChEMBL
| Assay Description
The Compound was tested for the inhibition potency against C. albicans N-myristoyltransferase (NMT) and reported as apparent Ki |
J Med Chem 41: 996-1000 (1998)
Article DOI: 10.1021/jm980001q
BindingDB Entry DOI: 10.7270/Q27080KF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Squalene synthase
(Homo sapiens (Human)) | BDBM50075719

(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCOC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:12:13:18.17:20.21,14:13:18.17:20.21,(10.63,-1.41,;9.54,-2.5,;9.94,-3.98,;8.86,-5.08,;9.25,-6.57,;7.36,-4.68,;6.27,-5.77,;4.78,-5.38,;4.38,-3.89,;2.9,-3.49,;1.81,-4.59,;.33,-4.19,;-1.17,-3.79,;-2.66,-3.4,;-2.26,-1.9,;-3.89,-4.07,;-4.88,-2.49,;-6.75,-2.53,;-5.51,-1.8,;-3.62,-1.88,;-3.15,-.9,;-4.25,-1.27,;2.22,-6.06,;1.13,-7.15,;-.37,-6.75,;-1.45,-7.84,;3.7,-6.46,)| Show InChI InChI=1S/C23H29NO3/c1-3-5-20-16-18(7-9-22(25)27-4-2)6-8-19(20)10-13-23(26)17-24-14-11-21(23)12-15-24/h3,6,8,16,21,26H,1,4-5,7,9,11-12,14-15,17H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against human microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50034988
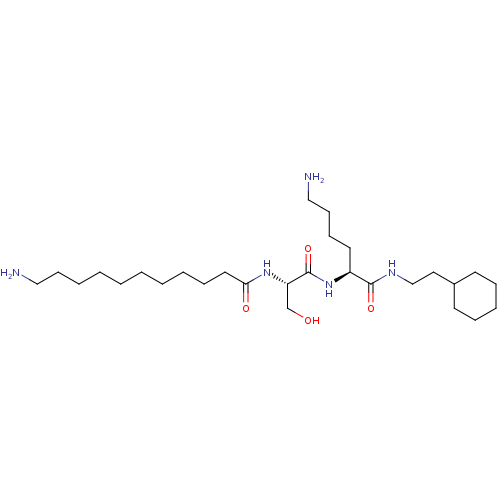
(11-Amino-undecanoic acid {(S)-1-[(S)-5-amino-1-(2-...)Show SMILES NCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)NCCC1CCCCC1 Show InChI InChI=1S/C28H55N5O4/c29-19-12-6-4-2-1-3-5-10-17-26(35)32-25(22-34)28(37)33-24(16-11-13-20-30)27(36)31-21-18-23-14-8-7-9-15-23/h23-25,34H,1-22,29-30H2,(H,31,36)(H,32,35)(H,33,37)/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Co.
Curated by ChEMBL
| Assay Description
Apparent binding affinity against Candida albicans N-Myristoyltransferase (NMT). |
J Med Chem 38: 1837-40 (1995)
Article DOI: 10.1021/jm00011a001
BindingDB Entry DOI: 10.7270/Q23J3GPZ |
More data for this
Ligand-Target Pair | |
Oxytocin receptor
(Homo sapiens (Human)) | BDBM50380761

(CHEMBL2017980)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(O)c1 Show InChI InChI=1S/C28H35N3O6S/c1-38(35,36)30-16-12-23(13-17-30)37-24-8-6-21(26(32)19-24)18-28(34)29-14-10-22(11-15-29)31-25-5-3-2-4-20(25)7-9-27(31)33/h2-6,8,19,22-23,32H,7,9-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]oxytocin from human oxytocin receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Microtus ochrogaster) | BDBM50380760

(CHEMBL2017979)Show SMILES COc1cc(OC2CCN(CC2)S(C)(=O)=O)ccc1CC(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C29H37N3O6S/c1-37-27-20-25(38-24-13-17-31(18-14-24)39(2,35)36)9-7-22(27)19-29(34)30-15-11-23(12-16-30)32-26-6-4-3-5-21(26)8-10-28(32)33/h3-7,9,20,23-24H,8,10-19H2,1-2H3 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [125I]-LVA from prairie vole vasopressin V1A receptor after 72 hrs |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50034988

(11-Amino-undecanoic acid {(S)-1-[(S)-5-amino-1-(2-...)Show SMILES NCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)NCCC1CCCCC1 Show InChI InChI=1S/C28H55N5O4/c29-19-12-6-4-2-1-3-5-10-17-26(35)32-25(22-34)28(37)33-24(16-11-13-20-30)27(36)31-21-18-23-14-8-7-9-15-23/h23-25,34H,1-22,29-30H2,(H,31,36)(H,32,35)(H,33,37)/t24-,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Co.
Curated by ChEMBL
| Assay Description
Apparent binding affinity against Human NMT. |
J Med Chem 38: 1837-40 (1995)
Article DOI: 10.1021/jm00011a001
BindingDB Entry DOI: 10.7270/Q23J3GPZ |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291317
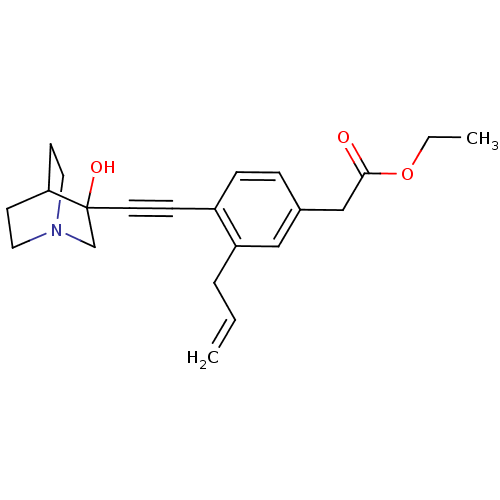
(CHEMBL154472 | [3-Allyl-4-(3-hydroxy-1-aza-bicyclo...)Show SMILES CCOC(=O)Cc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;15.43,-2.9,;15.43,-4.44,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H27NO3/c1-3-5-19-14-17(15-21(24)26-4-2)6-7-18(19)8-11-22(25)16-23-12-9-20(22)10-13-23/h3,6-7,14,20,25H,1,4-5,9-10,12-13,15-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50470170
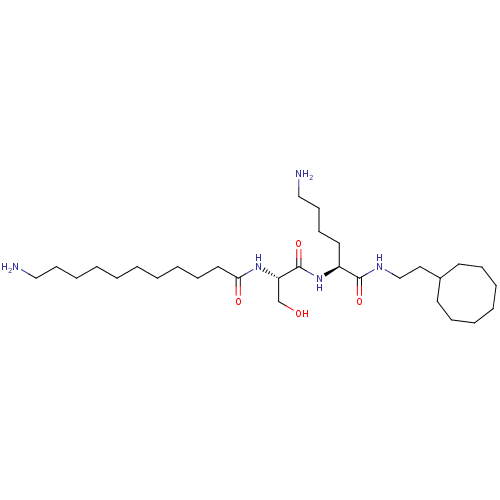
(CHEMBL54883)Show SMILES NCCCCCCCCCCC(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)NCCC1CCCCCCC1 Show InChI InChI=1S/C30H59N5O4/c31-21-14-9-4-2-1-3-8-12-19-28(37)34-27(24-36)30(39)35-26(18-13-15-22-32)29(38)33-23-20-25-16-10-6-5-7-11-17-25/h25-27,36H,1-24,31-32H2,(H,33,38)(H,34,37)(H,35,39)/t26-,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Co.
Curated by ChEMBL
| Assay Description
Apparent binding affinity against Candida albicans N-Myristoyltransferase (NMT). |
J Med Chem 38: 1837-40 (1995)
Article DOI: 10.1021/jm00011a001
BindingDB Entry DOI: 10.7270/Q23J3GPZ |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291314
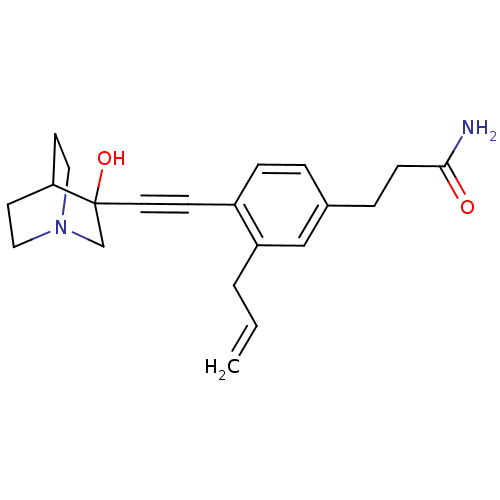
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES NC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:10:11:16.15:18.19,12:11:16.15:18.19,(18.09,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.24,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.64,;3.26,-8.24,;3.14,-6.74,;4.77,-6.15,;4.96,-4.38,;5.36,-5.61,;10.09,-2.93,;8.76,-2.17,;7.43,-2.94,;6.1,-2.18,;11.43,-2.15,)| Show InChI InChI=1S/C21H26N2O2/c1-2-3-18-14-16(5-7-20(22)24)4-6-17(18)8-11-21(25)15-23-12-9-19(21)10-13-23/h2,4,6,14,19,25H,1,3,5,7,9-10,12-13,15H2,(H2,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291318
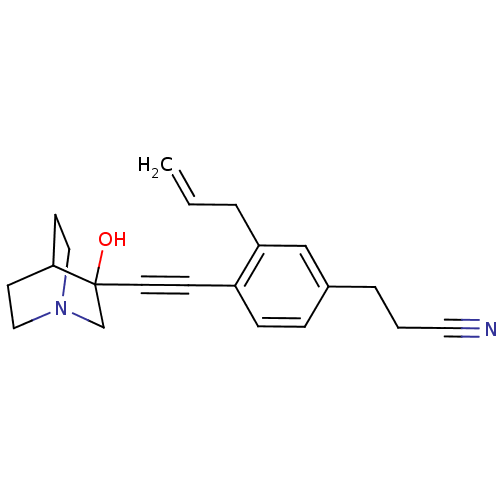
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES OC1(CN2CCC1CC2)C#Cc1ccc(CCC#N)cc1CC=C |THB:9:1:5.4:7.8,0:1:5.4:7.8,(7.67,-7.29,;6.19,-6.88,;6.4,-8.42,;4.96,-7.64,;3.26,-8.25,;3.14,-6.75,;4.78,-6.15,;4.96,-4.38,;5.37,-5.61,;7.45,-6.01,;8.78,-5.24,;10.12,-4.46,;11.45,-5.23,;12.77,-4.46,;12.77,-2.91,;14.1,-2.13,;15.44,-2.91,;16.76,-2.12,;18.1,-1.35,;11.43,-2.15,;10.09,-2.93,;8.77,-2.17,;7.44,-2.94,;6.1,-2.18,)| Show InChI InChI=1S/C21H24N2O/c1-2-4-19-15-17(5-3-12-22)6-7-18(19)8-11-21(24)16-23-13-9-20(21)10-14-23/h2,6-7,15,20,24H,1,3-5,9-10,13-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291319
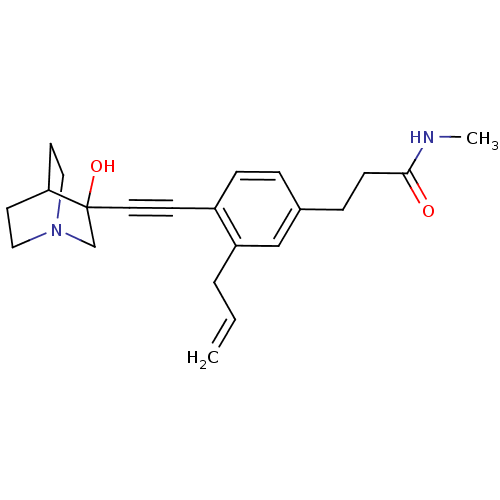
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CNC(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:11:12:17.16:19.20,13:12:17.16:19.20,(19.42,-2.1,;18.08,-2.89,;16.75,-2.12,;16.75,-.58,;15.43,-2.9,;14.09,-2.13,;12.76,-2.91,;12.76,-4.45,;11.44,-5.23,;10.11,-4.45,;8.77,-5.23,;7.45,-6.01,;6.19,-6.88,;7.66,-7.28,;6.4,-8.41,;4.96,-7.63,;3.26,-8.24,;3.14,-6.74,;4.77,-6.14,;4.96,-4.38,;5.36,-5.6,;10.08,-2.93,;8.76,-2.17,;7.43,-2.94,;6.09,-2.18,;11.42,-2.15,)| Show InChI InChI=1S/C22H28N2O2/c1-3-4-19-15-17(6-8-21(25)23-2)5-7-18(19)9-12-22(26)16-24-13-10-20(22)11-14-24/h3,5,7,15,20,26H,1,4,6,8,10-11,13-14,16H2,2H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Squalene synthase
(Rattus norvegicus) | BDBM50291313
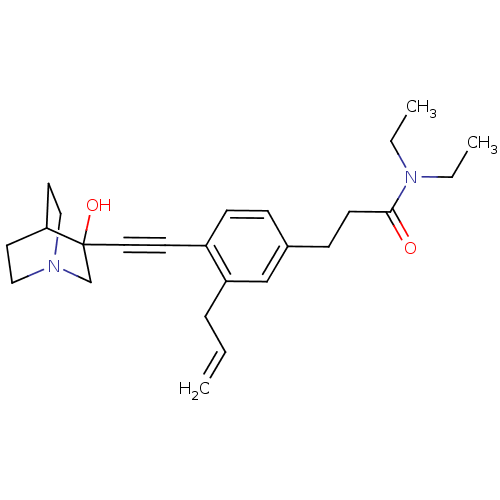
(3-[3-Allyl-4-(3-hydroxy-1-aza-bicyclo[2.2.2]oct-3-...)Show SMILES CCN(CC)C(=O)CCc1ccc(C#CC2(O)CN3CCC2CC3)c(CC=C)c1 |THB:14:15:20.19:22.23,16:15:20.19:22.23,(19.46,-5.19,;18.12,-4.43,;18.1,-2.89,;19.44,-2.11,;20.78,-2.86,;16.76,-2.12,;16.76,-.58,;15.44,-2.91,;14.1,-2.13,;12.77,-2.91,;12.77,-4.46,;11.45,-5.23,;10.12,-4.46,;8.78,-5.24,;7.45,-6.01,;6.19,-6.88,;7.67,-7.29,;6.4,-8.42,;4.96,-7.64,;3.26,-8.25,;3.14,-6.75,;4.78,-6.15,;4.96,-4.38,;5.37,-5.61,;10.09,-2.93,;8.77,-2.17,;7.44,-2.94,;6.1,-2.18,;11.43,-2.15,)| Show InChI InChI=1S/C25H34N2O2/c1-4-7-22-18-20(9-11-24(28)27(5-2)6-3)8-10-21(22)12-15-25(29)19-26-16-13-23(25)14-17-26/h4,8,10,18,23,29H,1,5-7,9,11,13-14,16-17,19H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| >250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its inhibitory activity against rat microsomal quinuclidine squalene synthase (SQS) |
Bioorg Med Chem Lett 7: 597-600 (1997)
Article DOI: 10.1016/S0960-894X(97)00053-X
BindingDB Entry DOI: 10.7270/Q2C24WFT |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50380757

(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50380757

(CHEMBL2017868)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C/I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50470169
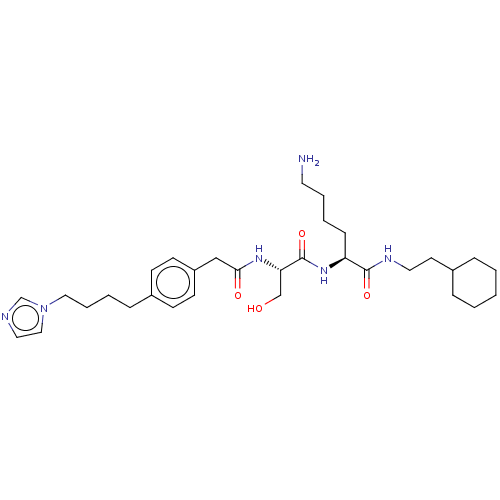
(CHEMBL294596)Show SMILES NCCCC[C@H](NC(=O)[C@H](CO)NC(=O)Cc1ccc(CCCCn2ccnc2)cc1)C(=O)NCCC1CCCCC1 Show InChI InChI=1S/C32H50N6O4/c33-17-6-4-11-28(31(41)35-18-16-25-8-2-1-3-9-25)37-32(42)29(23-39)36-30(40)22-27-14-12-26(13-15-27)10-5-7-20-38-21-19-34-24-38/h12-15,19,21,24-25,28-29,39H,1-11,16-18,20,22-23,33H2,(H,35,41)(H,36,40)(H,37,42)/t28-,29-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Co.
Curated by ChEMBL
| Assay Description
Apparent binding affinity against Candida albicans N-Myristoyltransferase (NMT). |
J Med Chem 38: 1837-40 (1995)
Article DOI: 10.1021/jm00011a001
BindingDB Entry DOI: 10.7270/Q23J3GPZ |
More data for this
Ligand-Target Pair | |
Glycylpeptide N-tetradecanoyltransferase 1/2
(Homo sapiens (Human)) | BDBM50470171

(CHEMBL299249)Show SMILES NCCCC[C@H](NC(=O)[C@H](CO)NC(=O)CCCCCCCCCCn1ccnc1)C(=O)NCCC1CCCCC1 Show InChI InChI=1S/C31H56N6O4/c32-19-12-11-16-27(30(40)34-20-18-26-14-8-7-9-15-26)36-31(41)28(24-38)35-29(39)17-10-5-3-1-2-4-6-13-22-37-23-21-33-25-37/h21,23,25-28,38H,1-20,22,24,32H2,(H,34,40)(H,35,39)(H,36,41)/t27-,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
G.D. Searle and Co.
Curated by ChEMBL
| Assay Description
Apparent binding affinity against Candida albicans N-Myristoyltransferase (NMT). |
J Med Chem 38: 1837-40 (1995)
Article DOI: 10.1021/jm00011a001
BindingDB Entry DOI: 10.7270/Q23J3GPZ |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50380758

(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Thymidine phosphorylase
(Escherichia coli) | BDBM50122770
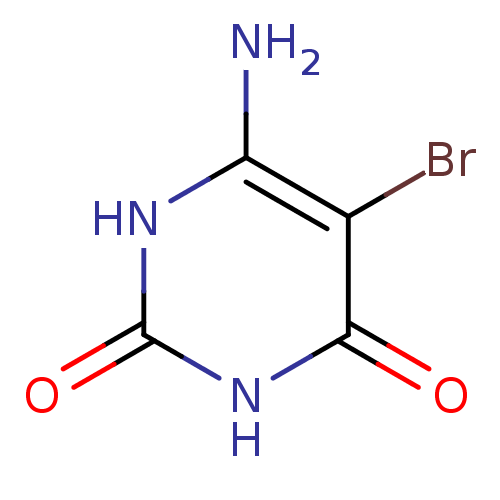
(6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...)Show InChI InChI=1S/C4H4BrN3O2/c5-1-2(6)7-4(10)8-3(1)9/h(H4,6,7,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibitory activity against Escherichia coli thymidine phosphorylase |
J Med Chem 48: 392-402 (2005)
Article DOI: 10.1021/jm049494r
BindingDB Entry DOI: 10.7270/Q2445N8K |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50380760

(CHEMBL2017979)Show SMILES COc1cc(OC2CCN(CC2)S(C)(=O)=O)ccc1CC(=O)N1CCC(CC1)N1C(=O)CCc2ccccc12 Show InChI InChI=1S/C29H37N3O6S/c1-37-27-20-25(38-24-13-17-31(18-14-24)39(2,35)36)9-7-22(27)19-29(34)30-15-11-23(12-16-30)32-26-6-4-3-5-21(26)8-10-28(32)33/h3-7,9,20,23-24H,8,10-19H2,1-2H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50380761

(CHEMBL2017980)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(O)c1 Show InChI InChI=1S/C28H35N3O6S/c1-38(35,36)30-16-12-23(13-17-30)37-24-8-6-21(26(32)19-24)18-28(34)29-14-10-22(11-15-29)31-25-5-3-2-4-20(25)7-9-27(31)33/h2-6,8,19,22-23,32H,7,9-18H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50380758

(CHEMBL2017867)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OC\C=C\I)c1 Show InChI InChI=1S/C31H38IN3O6S/c1-42(38,39)34-18-13-26(14-19-34)41-27-9-7-24(29(22-27)40-20-4-15-32)21-31(37)33-16-11-25(12-17-33)35-28-6-3-2-5-23(28)8-10-30(35)36/h2-7,9,15,22,25-26H,8,10-14,16-21H2,1H3/b15-4+ | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V2 receptor
(Homo sapiens (Human)) | BDBM50380761

(CHEMBL2017980)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(O)c1 Show InChI InChI=1S/C28H35N3O6S/c1-38(35,36)30-16-12-23(13-17-30)37-24-8-6-21(26(32)19-24)18-28(34)29-14-10-22(11-15-29)31-25-5-3-2-4-20(25)7-9-27(31)33/h2-6,8,19,22-23,32H,7,9-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human vasopressin V2 receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V1a receptor
(Homo sapiens (Human)) | BDBM50380761

(CHEMBL2017980)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(O)c1 Show InChI InChI=1S/C28H35N3O6S/c1-38(35,36)30-16-12-23(13-17-30)37-24-8-6-21(26(32)19-24)18-28(34)29-14-10-22(11-15-29)31-25-5-3-2-4-20(25)7-9-27(31)33/h2-6,8,19,22-23,32H,7,9-18H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1A receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |
Vasopressin V1b receptor
(Homo sapiens (Human)) | BDBM50380759

(CHEMBL2017869)Show SMILES CS(=O)(=O)N1CCC(CC1)Oc1ccc(CC(=O)N2CCC(CC2)N2C(=O)CCc3ccccc23)c(OCCF)c1 Show InChI InChI=1S/C30H38FN3O6S/c1-41(37,38)33-17-12-25(13-18-33)40-26-8-6-23(28(21-26)39-19-14-31)20-30(36)32-15-10-24(11-16-32)34-27-5-3-2-4-22(27)7-9-29(34)35/h2-6,8,21,24-25H,7,9-20H2,1H3 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Displacement of [3H]-AVP from human V1b receptor after 1.5 hrs by liquid scintillation counting |
Bioorg Med Chem 20: 2721-38 (2012)
Article DOI: 10.1016/j.bmc.2012.02.019
BindingDB Entry DOI: 10.7270/Q29K4C7G |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data