 Found 2162 hits with Last Name = 'frie' and Initial = 'j'
Found 2162 hits with Last Name = 'frie' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000296

(CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...)Show SMILES CN([C@@H]1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 |r| Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17-,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50448583
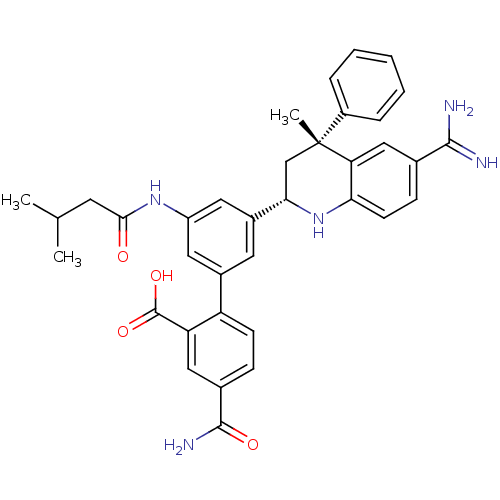
(CHEMBL3127491)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45)/t31-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032873
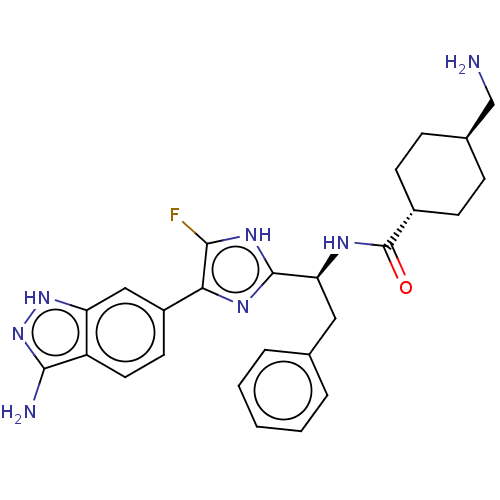
(CHEMBL3355684)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(38,-31.47,;39.32,-32.23,;39.32,-33.76,;40.64,-31.46,;41.96,-32.23,;40.64,-29.94,;41.95,-30.68,;15.71,-45.09,;16.47,-43.75,;18.01,-43.75,;18.79,-45.09,;20.33,-45.09,;21.09,-43.75,;20.33,-42.42,;18.79,-42.42,;22.63,-43.75,;23.41,-45.09,;23.41,-42.42,;24.95,-42.42,;25.71,-43.75,;27.25,-43.75,;28.03,-42.42,;29.57,-42.42,;30.33,-43.75,;29.57,-45.09,;28.03,-45.09,;25.71,-41.09,;25.09,-39.68,;26.23,-38.65,;27.56,-39.42,;28.97,-38.79,;27.24,-40.93,;26.07,-37.12,;27.32,-36.22,;27.16,-34.69,;25.75,-34.05,;25.27,-32.59,;26.18,-31.34,;23.73,-32.59,;23.25,-34.05,;24.5,-34.96,;24.66,-36.49,)| Show InChI InChI=1S/C26H30FN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032874
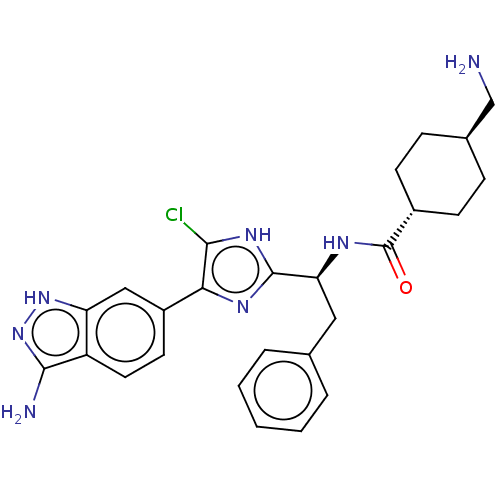
(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21015

((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...)Show SMILES C[C@@H](NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(=O)NCC(=O)N(C)[C@@H](Cc1ccccc1)C(=O)NCCO Show InChI InChI=1S/C26H35N5O6/c1-17(30-25(36)21(27)14-19-8-10-20(33)11-9-19)24(35)29-16-23(34)31(2)22(26(37)28-12-13-32)15-18-6-4-3-5-7-18/h3-11,17,21-22,32-33H,12-16,27H2,1-2H3,(H,28,37)(H,29,35)(H,30,36)/t17-,21+,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DAMGO from human mu opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032874

(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis at 37 degC by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPDPE from human delta opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50001465

((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)CNC(=O)CNC(=O)[C@@H](N)Cc1ccc(O)cc1)C(O)=O Show InChI InChI=1S/C28H37N5O7/c1-17(2)12-23(28(39)40)33-27(38)22(14-18-6-4-3-5-7-18)32-25(36)16-30-24(35)15-31-26(37)21(29)13-19-8-10-20(34)11-9-19/h3-11,17,21-23,34H,12-16,29H2,1-2H3,(H,30,35)(H,31,37)(H,32,36)(H,33,38)(H,39,40)/t21-,22-,23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-DPDPE from human delta opioid receptor expressed in CHO-K1 cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50448583

(CHEMBL3127491)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45)/t31-,36+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032873

(CHEMBL3355684)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(38,-31.47,;39.32,-32.23,;39.32,-33.76,;40.64,-31.46,;41.96,-32.23,;40.64,-29.94,;41.95,-30.68,;15.71,-45.09,;16.47,-43.75,;18.01,-43.75,;18.79,-45.09,;20.33,-45.09,;21.09,-43.75,;20.33,-42.42,;18.79,-42.42,;22.63,-43.75,;23.41,-45.09,;23.41,-42.42,;24.95,-42.42,;25.71,-43.75,;27.25,-43.75,;28.03,-42.42,;29.57,-42.42,;30.33,-43.75,;29.57,-45.09,;28.03,-45.09,;25.71,-41.09,;25.09,-39.68,;26.23,-38.65,;27.56,-39.42,;28.97,-38.79,;27.24,-40.93,;26.07,-37.12,;27.32,-36.22,;27.16,-34.69,;25.75,-34.05,;25.27,-32.59,;26.18,-31.34,;23.73,-32.59,;23.25,-34.05,;24.5,-34.96,;24.66,-36.49,)| Show InChI InChI=1S/C26H30FN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032875

(CHEMBL3355682)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(39.15,-34.6,;40.47,-35.36,;40.47,-36.89,;41.79,-34.6,;43.11,-35.36,;41.79,-33.07,;43.11,-33.82,;26.66,-34.02,;27.43,-35.36,;26.66,-36.68,;25.12,-36.68,;24.35,-38.02,;25.12,-39.35,;26.66,-39.35,;27.43,-38.02,;24.35,-40.69,;22.81,-40.69,;25.12,-42.02,;24.35,-43.36,;22.81,-43.36,;22.04,-44.69,;22.81,-46.02,;22.04,-47.35,;20.5,-47.35,;19.73,-46.02,;20.5,-44.69,;25.12,-44.69,;26.65,-44.85,;26.97,-46.36,;25.64,-47.13,;24.49,-46.1,;28.38,-46.98,;28.54,-48.51,;29.95,-49.15,;31.19,-48.24,;32.7,-48.56,;33.32,-49.96,;33.47,-47.22,;32.44,-46.08,;31.03,-46.71,;29.63,-46.08,)| Show InChI InChI=1S/C26H31N7O.C2HF3O2/c27-14-17-6-8-18(9-7-17)26(34)31-22(12-16-4-2-1-3-5-16)25-29-15-23(30-25)19-10-11-20-21(13-19)32-33-24(20)28;3-2(4,5)1(6)7/h1-5,10-11,13,15,17-18,22H,6-9,12,14,27H2,(H,29,30)(H,31,34)(H3,28,32,33);(H,6,7)/t17-,18-,22-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032876

(CHEMBL3355681)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30FN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103208

(CHEMBL3393381)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:5.8,wD:2.1,11.11,(26.66,-34.02,;27.43,-35.36,;26.66,-36.68,;25.12,-36.68,;24.35,-38.02,;25.12,-39.35,;26.66,-39.35,;27.43,-38.02,;24.35,-40.69,;22.81,-40.69,;25.12,-42.02,;24.35,-43.36,;22.81,-43.36,;22.04,-44.69,;22.81,-46.02,;22.04,-47.35,;20.5,-47.35,;19.73,-46.02,;20.5,-44.69,;25.12,-44.69,;26.65,-44.85,;26.97,-46.36,;25.64,-47.13,;24.49,-46.1,;28.38,-46.98,;28.54,-48.51,;29.95,-49.15,;31.19,-48.24,;32.7,-48.56,;33.32,-49.96,;33.47,-47.22,;32.44,-46.08,;31.03,-46.71,;29.63,-46.08,)| Show InChI InChI=1S/C26H29N7O/c27-14-17-6-8-18(9-7-17)26(34)31-22(12-16-4-2-1-3-5-16)25-29-15-23(30-25)19-10-11-20-21(13-19)32-33-24(20)28/h1-5,10-11,13,15,17-18,22H,6-9,12,14,27-28H2,(H,31,34)/b23-19-/t17-,18-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103201
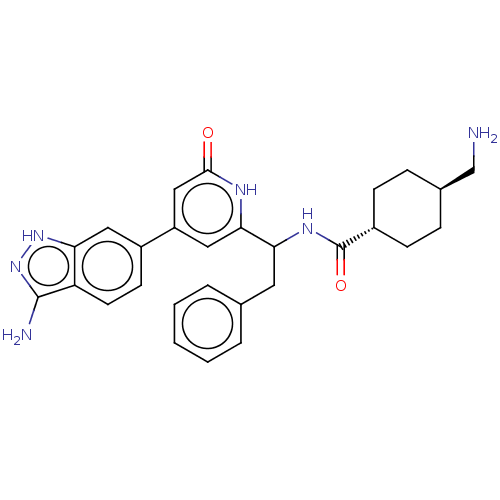
(CHEMBL3393388)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)NC(Cc1ccccc1)c1cc(cc(=O)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-3.72,3.07,;-3.71,1.53,;-5.05,.76,;-6.38,1.53,;-7.45,.91,;-6.38,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032877
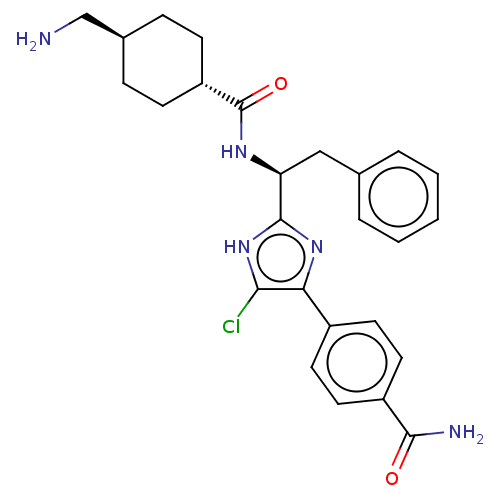
(CHEMBL3355680)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30ClN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24198
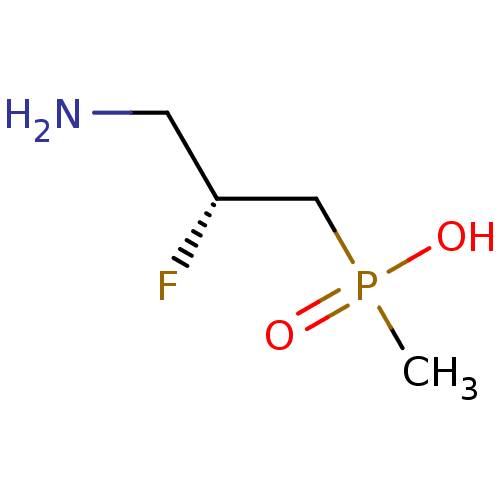
(3-aminopropylphosphinic derivative, (R)-8 | [(2R)-...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.30 | -47.3 | n/a | n/a | 14 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032874

(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24195
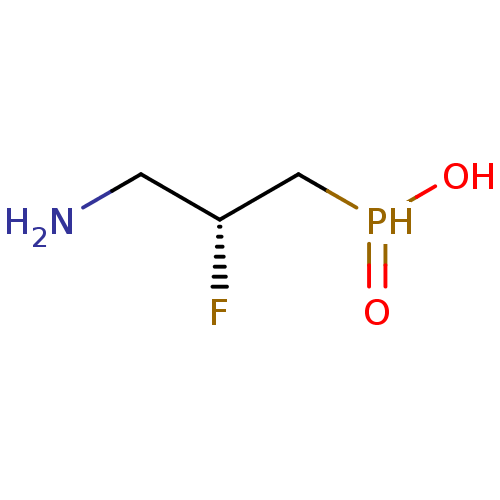
(3-aminopropylphosphinic derivative, (R)-7 | AZD335...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.10 | -46.9 | n/a | n/a | 8.64 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032876

(CHEMBL3355681)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30FN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103200
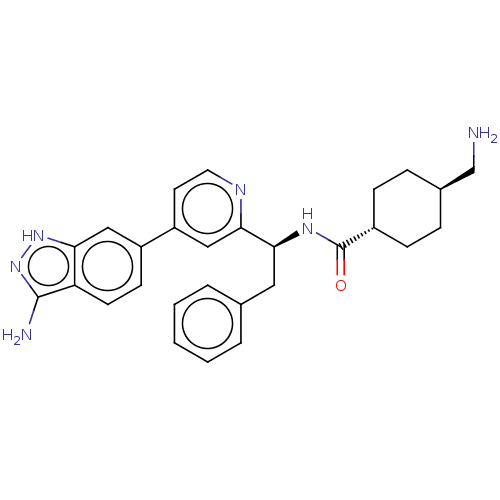
(CHEMBL3393386)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1cc(ccn1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,11.12,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-3.72,3.07,;-3.71,1.53,;-5.05,.76,;-6.38,1.53,;-6.38,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032878
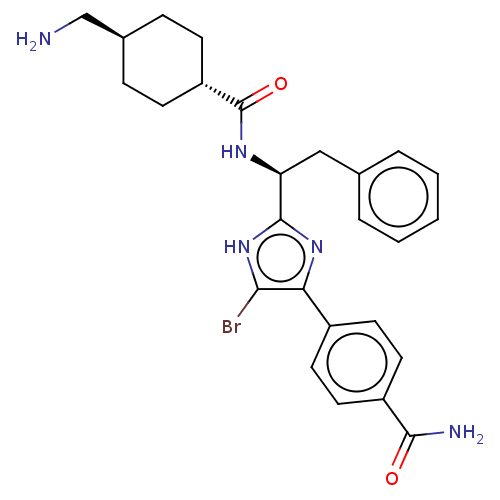
(CHEMBL3355679)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Br)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.78,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.36,-24.67,;17.7,-25.45,;19.04,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.07,;27.03,-18.52,;25.69,-17.75,;24.36,-18.53,;24.36,-23.14,;25.77,-22.51,;26.8,-23.65,;26.03,-24.99,;26.66,-26.41,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.39,-23.18,;30.76,-24.58,;29.23,-24.74,;32.92,-23.01,;33.83,-24.25,;33.55,-21.6,)| Show InChI InChI=1S/C26H30BrN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24193
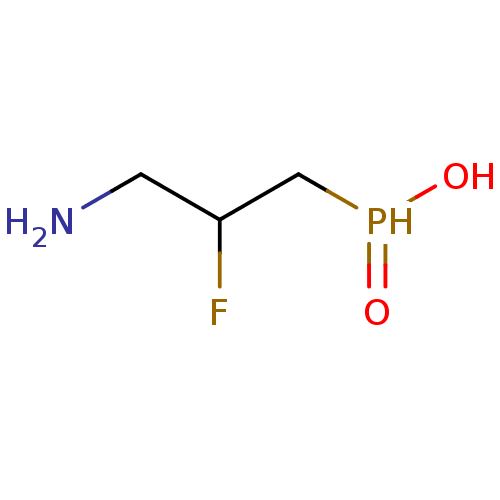
((3-amino-2-fluoropropyl)phosphinic acid | 3-aminop...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | -45.2 | n/a | n/a | 15 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103216
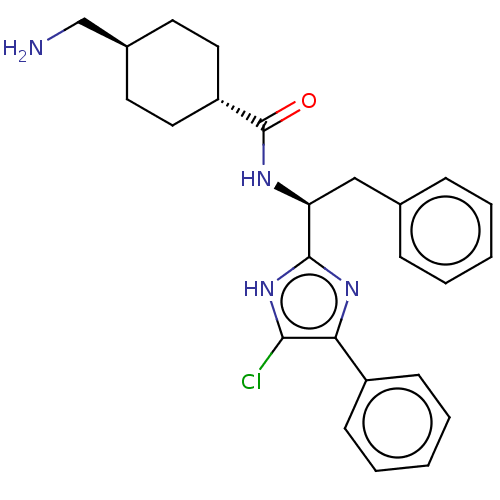
(CHEMBL3393373)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccccc1 |r,wU:5.8,11.12,wD:2.1,(3.74,.92,;2.67,1.54,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;-2.67,-1.54,;-3.73,-.93,;-2.66,-3.08,;-4,-3.86,;-3.99,-5.4,;-5.33,-6.17,;-5.33,-7.71,;-6.66,-8.48,;-8,-7.71,;-8,-6.17,;-6.66,-5.4,;-5.33,-3.09,;-5.47,-1.57,;-6.98,-1.25,;-7.75,-2.58,;-8.98,-2.71,;-6.72,-3.73,;-7.61,.16,;-9.12,.43,;-9.64,1.88,;-8.64,3.06,;-7.13,2.78,;-6.61,1.33,)| Show InChI InChI=1S/C25H29ClN4O/c26-23-22(19-9-5-2-6-10-19)29-24(30-23)21(15-17-7-3-1-4-8-17)28-25(31)20-13-11-18(16-27)12-14-20/h1-10,18,20-21H,11-16,27H2,(H,28,31)(H,29,30)/t18-,20-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103204
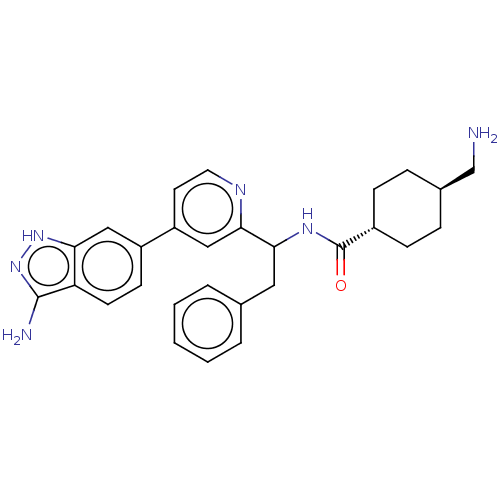
(CHEMBL3393385)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)NC(Cc1ccccc1)c1cc(ccn1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-3.72,3.07,;-3.71,1.53,;-5.05,.76,;-6.38,1.53,;-6.38,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24196
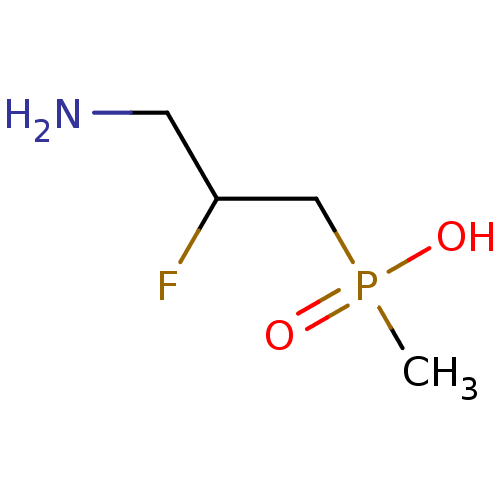
((3-amino-2-fluoropropyl)(methyl)phosphinic acid | ...)Show InChI InChI=1S/C4H11FNO2P/c1-9(7,8)3-4(5)2-6/h4H,2-3,6H2,1H3,(H,7,8) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | -44.4 | n/a | n/a | 23 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24184
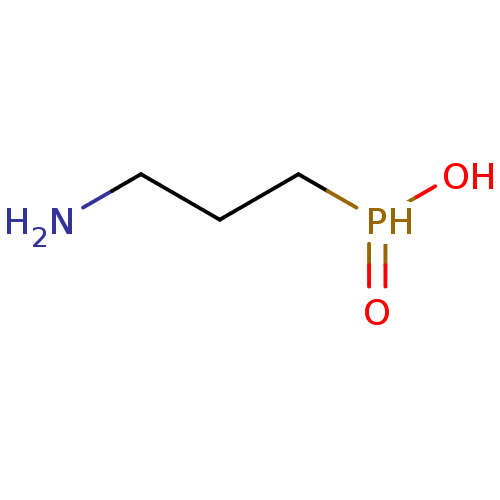
((3-aminopropyl)phosphinic acid | 3-aminopropylphos...)Show InChI InChI=1S/C3H10NO2P/c4-2-1-3-7(5)6/h7H,1-4H2,(H,5,6) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 15 | -44.2 | n/a | n/a | 19 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032877

(CHEMBL3355680)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.83,-22.98,;31.17,-23.75,;31.17,-25.29,;32.5,-22.98,;33.84,-23.75,;33.83,-22.19,;32.5,-21.43,;13.75,-29.96,;15.08,-30.74,;16.42,-29.97,;17.76,-30.75,;19.1,-29.98,;19.09,-28.44,;17.76,-27.67,;16.42,-28.43,;20.42,-27.67,;20.42,-26.13,;21.76,-28.44,;23.09,-27.67,;23.09,-26.13,;24.43,-25.36,;25.76,-26.13,;27.09,-25.36,;27.1,-23.82,;25.75,-23.05,;24.42,-23.82,;24.43,-28.44,;25.83,-27.81,;26.86,-28.95,;26.09,-30.29,;26.72,-31.7,;24.58,-29.97,;28.39,-28.8,;29.01,-27.39,;30.53,-27.23,;31.45,-28.47,;30.82,-29.88,;29.29,-30.04,;32.98,-28.31,;33.89,-29.55,;33.61,-26.9,)| Show InChI InChI=1S/C26H30ClN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103206
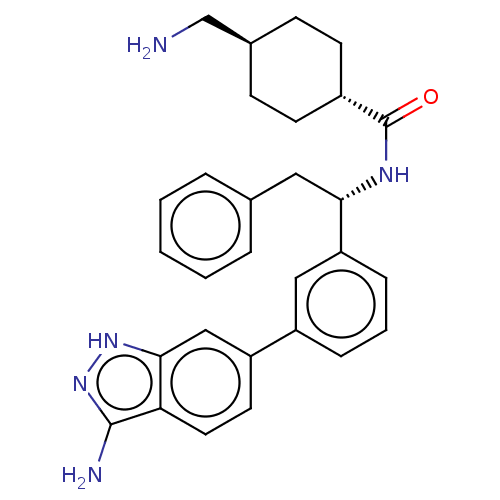
(CHEMBL3393383)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1cccc(c1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,11.12,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-6.38,3.07,;-6.38,1.53,;-5.05,.76,;-3.71,1.53,;-3.72,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50032874

(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation trypsin using N-benzoyl-Ile-Glu-(OH, OMe)-Gly-Arg-pNA as substrate by spectrophotometric analysis |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50103200

(CHEMBL3393386)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1cc(ccn1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,11.12,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-3.72,3.07,;-3.71,1.53,;-5.05,.76,;-6.38,1.53,;-6.38,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032858
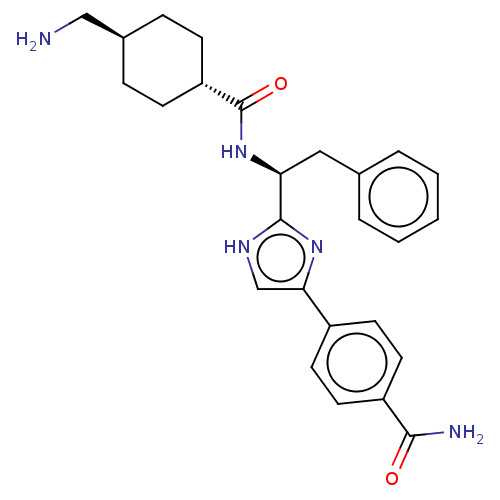
(CHEMBL3355670)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(24.17,-9.75,;25.51,-10.52,;25.51,-12.06,;26.84,-9.75,;28.17,-10.52,;28.17,-8.96,;26.84,-8.21,;8.49,-17.32,;9.81,-18.09,;11.15,-17.33,;12.49,-18.1,;13.83,-17.34,;13.83,-15.79,;12.5,-15.03,;11.16,-15.79,;15.16,-15.02,;15.16,-13.48,;16.49,-15.79,;17.83,-15.02,;17.83,-13.48,;19.16,-12.71,;20.49,-13.49,;21.83,-12.72,;21.83,-11.18,;20.49,-10.41,;19.16,-11.18,;19.16,-15.79,;20.56,-15.17,;21.59,-16.31,;20.82,-17.65,;19.32,-17.32,;23.12,-16.15,;23.74,-14.75,;25.27,-14.59,;26.18,-15.83,;25.55,-17.24,;24.02,-17.4,;27.71,-15.67,;28.62,-16.91,;28.34,-14.26,)| Show InChI InChI=1S/C26H31N5O2.C2HF3O2/c27-15-18-6-8-21(9-7-18)26(33)31-22(14-17-4-2-1-3-5-17)25-29-16-23(30-25)19-10-12-20(13-11-19)24(28)32;3-2(4,5)1(6)7/h1-5,10-13,16,18,21-22H,6-9,14-15,27H2,(H2,28,32)(H,29,30)(H,31,33);(H,6,7)/t18-,21-,22-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24185
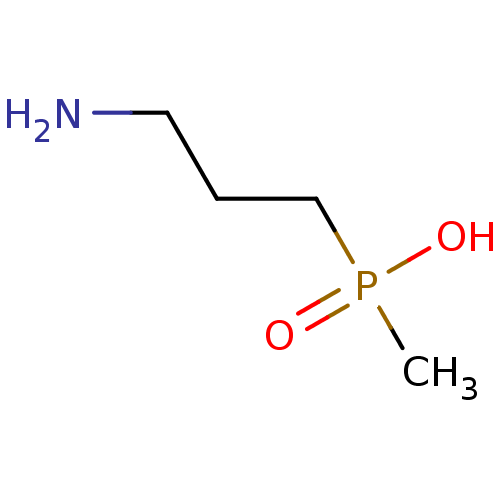
((3-aminopropyl)(methyl)phosphinic acid | 3-Apmpa |...)Show InChI InChI=1S/C4H12NO2P/c1-8(6,7)4-2-3-5/h2-5H2,1H3,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 33 | -42.3 | n/a | n/a | 41 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB2 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM60994

((10R,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tet...)Show SMILES CCCCCc1cc(O)c2[C@@H]3C=C(C)CC[C@H]3C(C)(C)Oc2c1 |r,t:11| Show InChI InChI=1S/C21H30O2/c1-5-6-7-8-15-12-18(22)20-16-11-14(2)9-10-17(16)21(3,4)23-19(20)13-15/h11-13,16-17,22H,5-10H2,1-4H3/t16-,17-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]CP-55,940 from human CB1 receptor expressed in CHO cells incubated for 1 hr by liquid scintillation spectrometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00724
BindingDB Entry DOI: 10.7270/Q2697778 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103207
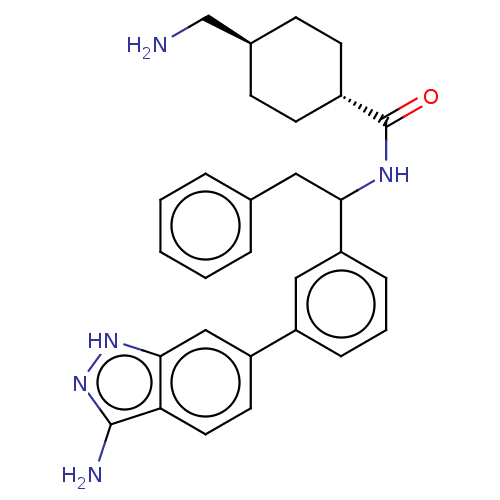
(CHEMBL3393382)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)NC(Cc1ccccc1)c1cccc(c1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-6.38,3.07,;-6.38,1.53,;-5.05,.76,;-3.71,1.53,;-3.72,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032878

(CHEMBL3355679)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Br)[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(29.77,-17.68,;31.11,-18.45,;31.11,-19.99,;32.44,-17.68,;33.78,-18.45,;33.77,-16.89,;32.44,-16.14,;13.69,-24.66,;15.02,-25.44,;16.36,-24.67,;17.7,-25.45,;19.04,-24.68,;19.03,-23.14,;17.7,-22.37,;16.36,-23.13,;20.36,-22.37,;20.36,-20.83,;21.69,-23.14,;23.03,-22.37,;23.03,-20.83,;24.37,-20.06,;25.7,-20.83,;27.03,-20.07,;27.03,-18.52,;25.69,-17.75,;24.36,-18.53,;24.36,-23.14,;25.77,-22.51,;26.8,-23.65,;26.03,-24.99,;26.66,-26.41,;24.52,-24.67,;28.32,-23.5,;28.94,-22.09,;30.47,-21.93,;31.39,-23.18,;30.76,-24.58,;29.23,-24.74,;32.92,-23.01,;33.83,-24.25,;33.55,-21.6,)| Show InChI InChI=1S/C26H30BrN5O2.C2HF3O2/c27-23-22(18-10-12-19(13-11-18)24(29)33)31-25(32-23)21(14-16-4-2-1-3-5-16)30-26(34)20-8-6-17(15-28)7-9-20;3-2(4,5)1(6)7/h1-5,10-13,17,20-21H,6-9,14-15,28H2,(H2,29,33)(H,30,34)(H,31,32);(H,6,7)/t17-,20-,21-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24191

((3-amino-2-oxopropyl)phosphinic acid | (3-amino-2-...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h8H,1-2,4H2,(H,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 48 | -41.4 | n/a | n/a | 81 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186
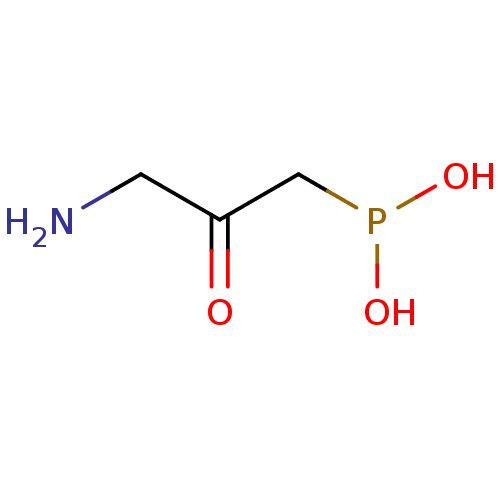
((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | -41.3 | n/a | n/a | 130 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032875

(CHEMBL3355682)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(39.15,-34.6,;40.47,-35.36,;40.47,-36.89,;41.79,-34.6,;43.11,-35.36,;41.79,-33.07,;43.11,-33.82,;26.66,-34.02,;27.43,-35.36,;26.66,-36.68,;25.12,-36.68,;24.35,-38.02,;25.12,-39.35,;26.66,-39.35,;27.43,-38.02,;24.35,-40.69,;22.81,-40.69,;25.12,-42.02,;24.35,-43.36,;22.81,-43.36,;22.04,-44.69,;22.81,-46.02,;22.04,-47.35,;20.5,-47.35,;19.73,-46.02,;20.5,-44.69,;25.12,-44.69,;26.65,-44.85,;26.97,-46.36,;25.64,-47.13,;24.49,-46.1,;28.38,-46.98,;28.54,-48.51,;29.95,-49.15,;31.19,-48.24,;32.7,-48.56,;33.32,-49.96,;33.47,-47.22,;32.44,-46.08,;31.03,-46.71,;29.63,-46.08,)| Show InChI InChI=1S/C26H31N7O.C2HF3O2/c27-14-17-6-8-18(9-7-17)26(34)31-22(12-16-4-2-1-3-5-16)25-29-15-23(30-25)19-10-11-20-21(13-19)32-33-24(20)28;3-2(4,5)1(6)7/h1-5,10-11,13,15,17-18,22H,6-9,12,14,27H2,(H,29,30)(H,31,34)(H3,28,32,33);(H,6,7)/t17-,18-,22-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Plasma kallikrein
(Homo sapiens (Human)) | BDBM50032858

(CHEMBL3355670)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccc(cc1)C(N)=O |r,wU:18.18,12.14,wD:9.7,(24.17,-9.75,;25.51,-10.52,;25.51,-12.06,;26.84,-9.75,;28.17,-10.52,;28.17,-8.96,;26.84,-8.21,;8.49,-17.32,;9.81,-18.09,;11.15,-17.33,;12.49,-18.1,;13.83,-17.34,;13.83,-15.79,;12.5,-15.03,;11.16,-15.79,;15.16,-15.02,;15.16,-13.48,;16.49,-15.79,;17.83,-15.02,;17.83,-13.48,;19.16,-12.71,;20.49,-13.49,;21.83,-12.72,;21.83,-11.18,;20.49,-10.41,;19.16,-11.18,;19.16,-15.79,;20.56,-15.17,;21.59,-16.31,;20.82,-17.65,;19.32,-17.32,;23.12,-16.15,;23.74,-14.75,;25.27,-14.59,;26.18,-15.83,;25.55,-17.24,;24.02,-17.4,;27.71,-15.67,;28.62,-16.91,;28.34,-14.26,)| Show InChI InChI=1S/C26H31N5O2.C2HF3O2/c27-15-18-6-8-21(9-7-18)26(33)31-22(14-17-4-2-1-3-5-17)25-29-16-23(30-25)19-10-12-20(13-11-19)24(28)32;3-2(4,5)1(6)7/h1-5,10-13,16,18,21-22H,6-9,14-15,27H2,(H2,28,32)(H,29,30)(H,31,33);(H,6,7)/t18-,21-,22-;/m0./s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human plasma kallikrein using H-D-Pro-Phe-Arg-pNA as substrate |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Trypsin
(Homo sapiens (Human)) | BDBM50103200

(CHEMBL3393386)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1cc(ccn1)-c1ccc2c(N)n[nH]c2c1 |r,wU:2.1,wD:5.8,11.12,(2.94,9.71,;2.94,8.48,;1.61,7.7,;1.62,6.16,;.29,5.39,;-1.05,6.16,;-1.05,7.7,;.28,8.47,;-2.38,5.38,;-2.38,4.15,;-3.72,6.15,;-5.05,5.38,;-6.39,6.15,;-7.72,5.38,;-9.06,6.15,;-10.39,5.37,;-10.39,3.83,;-9.05,3.07,;-7.72,3.84,;-5.05,3.84,;-3.72,3.07,;-3.71,1.53,;-5.05,.76,;-6.38,1.53,;-6.38,3.07,;-2.38,.77,;-2.38,-.77,;-1.03,-1.55,;.3,-.77,;1.76,-1.24,;2.15,-2.41,;2.66,.02,;1.76,1.24,;.3,.77,;-1.03,1.55,)| | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human trypsin |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24194

(3-aminopropylphosphinic derivative, (S)-7 | [(2S)-...)Show InChI InChI=1S/C3H9FNO2P/c4-3(1-5)2-8(6)7/h3,8H,1-2,5H2,(H,6,7)/t3-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 70 | -40.4 | n/a | n/a | 250 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50292340
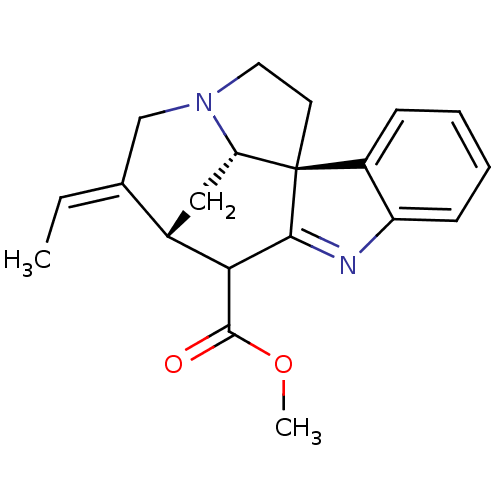
(AKUAMMICINE | CHEMBL508955)Show SMILES COC(=O)C1[C@H]2C[C@@H]3N(CC[C@]33C1=Nc1ccccc31)C\C2=C\C |r,t:14| Show InChI InChI=1S/C20H22N2O2/c1-3-12-11-22-9-8-20-14-6-4-5-7-15(14)21-18(20)17(19(23)24-2)13(12)10-16(20)22/h3-7,13,16-17H,8-11H2,1-2H3/b12-3-/t13-,16-,17?,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50292340

(AKUAMMICINE | CHEMBL508955)Show SMILES COC(=O)C1[C@H]2C[C@@H]3N(CC[C@]33C1=Nc1ccccc31)C\C2=C\C |r,t:14| Show InChI InChI=1S/C20H22N2O2/c1-3-12-11-22-9-8-20-14-6-4-5-7-15(14)21-18(20)17(19(23)24-2)13(12)10-16(20)22/h3-7,13,16-17H,8-11H2,1-2H3/b12-3-/t13-,16-,17?,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-U69593 from human kappa opioid receptor expressed in U2OS cells by radioligand binding assay |
Citation and Details
Article DOI: 10.1021/acs.jnatprod.0c01036
BindingDB Entry DOI: 10.7270/Q2057KP0 |
More data for this
Ligand-Target Pair | |
Gamma-aminobutyric acid (GABA) B receptor 1/type B receptor subunit 2 [41-940]
(Rattus norvegicus (rat)) | BDBM24186

((3-amino-2-hydroxypropyl)phosphinic acid | 3-amino...)Show InChI InChI=1S/C3H8NO3P/c4-1-3(5)2-8(6)7/h6-7H,1-2,4H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 94 | -39.7 | n/a | n/a | 220 | n/a | n/a | 7.4 | 22 |
AstraZeneca
| Assay Description
Inhibition of [3H]GABA binding at GABAB receptor sites in rat brain synaptic membranes by test compounds was measured using a filtration binding assa... |
J Med Chem 51: 4315-20 (2008)
Article DOI: 10.1021/jm701425k
BindingDB Entry DOI: 10.7270/Q2M32T2T |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103211

(CHEMBL3393378)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)NC(Cc1ccccc1)c1cc(cc(O)n1)-c1ccccc1 |r,wU:5.8,wD:2.1,(3.74,.92,;2.67,1.54,;1.33,.77,;,1.54,;-1.33,.77,;-1.33,-.77,;,-1.54,;1.33,-.77,;-2.67,-1.54,;-3.73,-.93,;-2.66,-3.08,;-4,-3.86,;-3.99,-5.4,;-5.33,-6.17,;-5.33,-7.71,;-6.66,-8.48,;-8,-7.71,;-8,-6.17,;-6.66,-5.4,;-5.33,-3.09,;-5.33,-1.55,;-6.67,-.78,;-8,-1.55,;-8,-3.09,;-9.07,-3.71,;-6.67,-3.86,;-6.67,.76,;-8,1.53,;-8,3.07,;-6.66,3.84,;-5.33,3.07,;-5.33,1.53,)| Show InChI InChI=1S/C27H31N3O2/c28-18-20-11-13-22(14-12-20)27(32)30-24(15-19-7-3-1-4-8-19)25-16-23(17-26(31)29-25)21-9-5-2-6-10-21/h1-10,16-17,20,22,24H,11-15,18,28H2,(H,29,31)(H,30,32)/t20-,22-,24? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 113 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032866
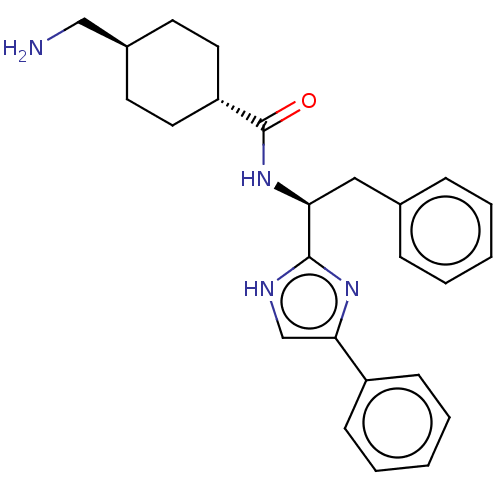
(CHEMBL3355664)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccccc1 |r,wU:18.18,12.14,wD:9.7,(25.97,-5.14,;27.3,-5.91,;27.3,-7.45,;28.64,-5.14,;29.97,-5.91,;29.96,-4.35,;28.64,-3.6,;10.28,-12.71,;11.61,-13.48,;12.94,-12.72,;14.28,-13.49,;15.62,-12.73,;15.62,-11.18,;14.29,-10.41,;12.95,-11.18,;16.95,-10.41,;16.95,-8.87,;18.28,-11.18,;19.62,-10.41,;19.62,-8.87,;20.96,-8.1,;22.29,-8.88,;23.62,-8.11,;23.62,-6.57,;22.28,-5.8,;20.95,-6.57,;20.95,-11.18,;22.36,-10.56,;23.39,-11.7,;22.62,-13.04,;21.11,-12.71,;24.92,-11.54,;25.54,-10.13,;27.06,-9.97,;27.98,-11.22,;27.35,-12.63,;25.82,-12.79,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50103366
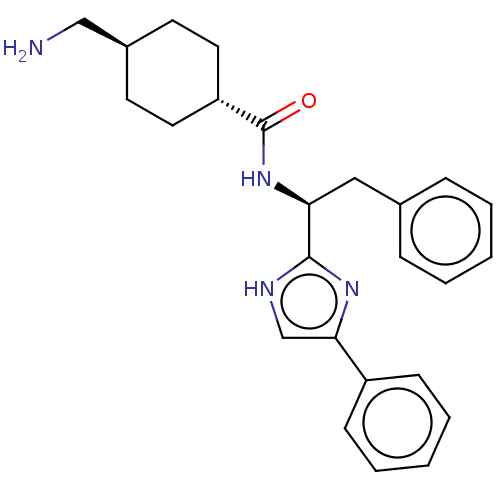
(CHEMBL3393362)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c[nH]1)-c1ccccc1 |r,wU:11.12,5.8,wD:2.1,(10.28,-12.71,;11.61,-13.48,;12.94,-12.72,;14.28,-13.49,;15.62,-12.73,;15.62,-11.18,;14.29,-10.41,;12.95,-11.18,;16.95,-10.41,;16.95,-8.87,;18.28,-11.18,;19.62,-10.41,;19.62,-8.87,;20.96,-8.1,;22.29,-8.88,;23.62,-8.11,;23.62,-6.57,;22.28,-5.8,;20.95,-6.57,;20.95,-11.18,;22.36,-10.56,;23.39,-11.7,;22.62,-13.04,;21.11,-12.71,;24.92,-11.54,;25.54,-10.13,;27.06,-9.97,;27.98,-11.22,;27.35,-12.63,;25.82,-12.79,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a after 10 to 120 mins |
Bioorg Med Chem Lett 25: 925-30 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.050
BindingDB Entry DOI: 10.7270/Q2CF9RWP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data