

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031895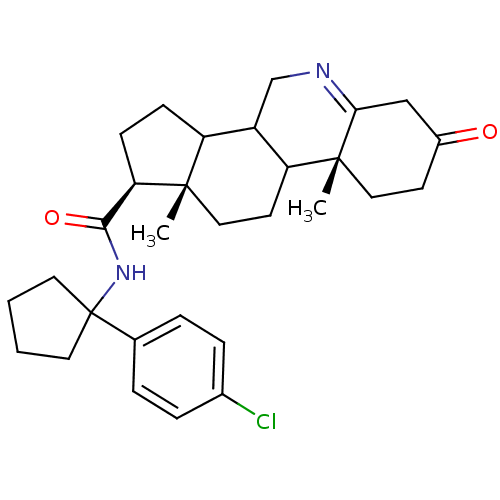 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity (in vitro) | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031877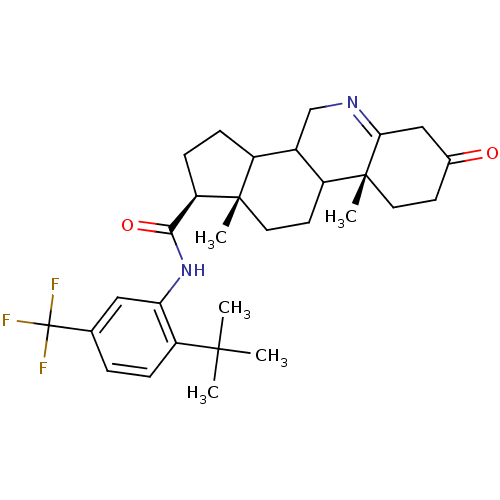 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031895 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031874 ((4aR,6aS,7S)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Binding affinity to recombinant human Steroid 5-alpha-reductase type I was evaluated | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031877 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM497267 ((2,6-dimethylpyridin-4- yl)(4-(7-((1R,4S)-4- hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description ATP competitive inhibition of MERTK (unknown origin) | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113534 BindingDB Entry DOI: 10.7270/Q2M90DGJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50407405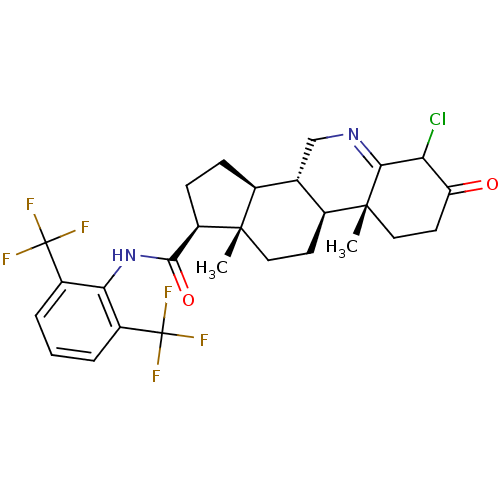 (CHEMBL2115222) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50407405 (CHEMBL2115222) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031883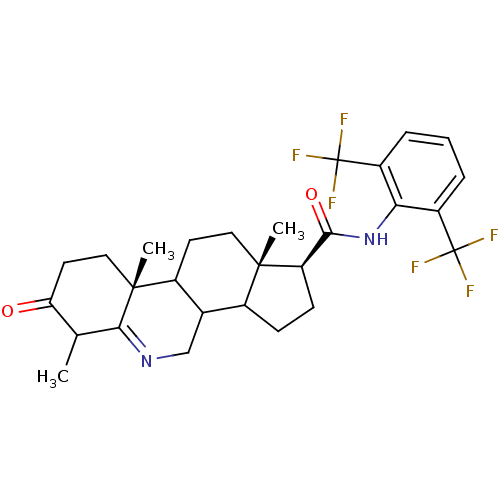 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Rattus norvegicus) | BDBM50031896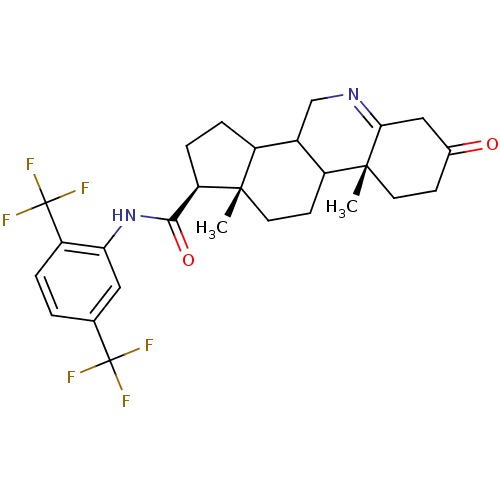 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant Steroid 5-alpha-reductase type I was evaluated as binding affinity of the compound | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031889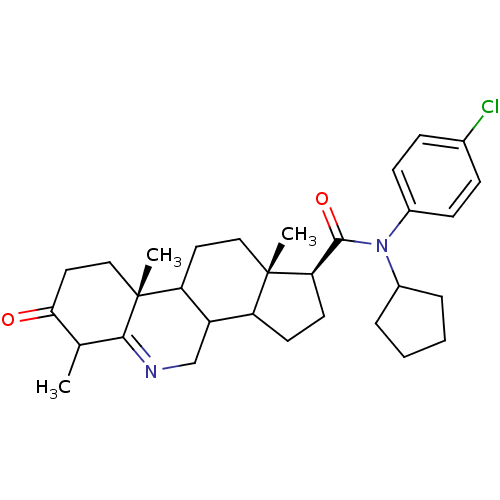 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185696 (UNC10108017 | US9156822, 40) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50349866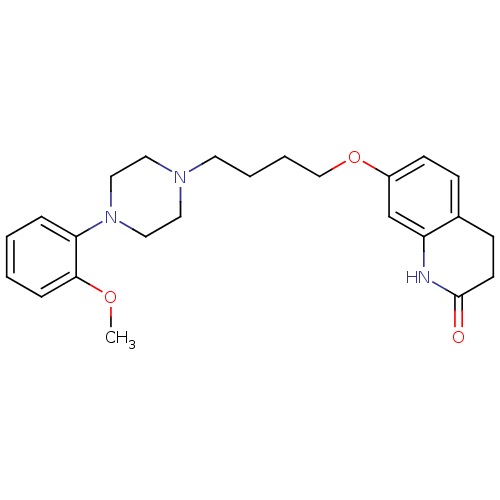 (CHEMBL160296 | CHEMBL1813590 | UNC10108016 | US915...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in CHO cells after 1.5 hrs by microbeta counting method | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50349866 (CHEMBL160296 | CHEMBL1813590 | UNC10108016 | US915...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50055499 (CHEMBL3326002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Inhibition of Mer (unknown origin) by Off-chip Mobility Shift Assay | J Med Chem 57: 7031-41 (2014) Article DOI: 10.1021/jm500749d BindingDB Entry DOI: 10.7270/Q2K075XQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185698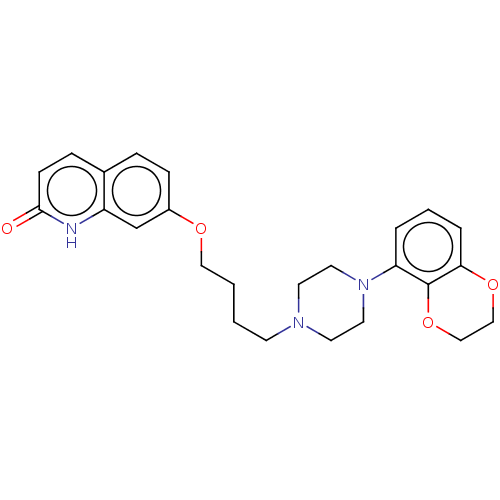 (UNC10108019 | US9156822, 42) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50368883 (CHEMBL1159458) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50469353 (CHEMBL4283353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
UNC Eshelman School of Pharmacy Curated by ChEMBL | Assay Description Inhibition of MERTK (unknown origin) using 5'-FAM-EFPIYDFLPAKKK-CONH2 as substrate after 180 mins by MCE assay | J Med Chem 61: 10242-10254 (2018) Article DOI: 10.1021/acs.jmedchem.8b01229 BindingDB Entry DOI: 10.7270/Q2K076ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185697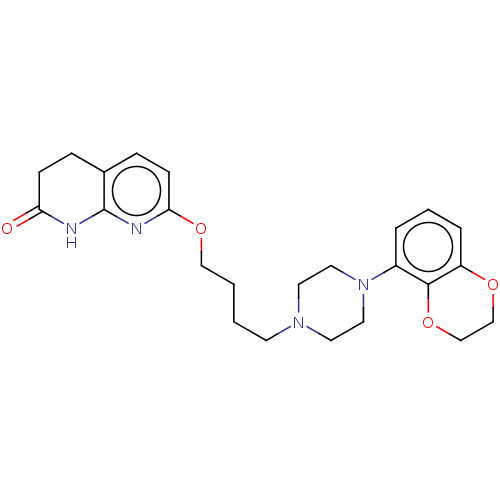 (UNC10108018 | US9156822, 41) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185695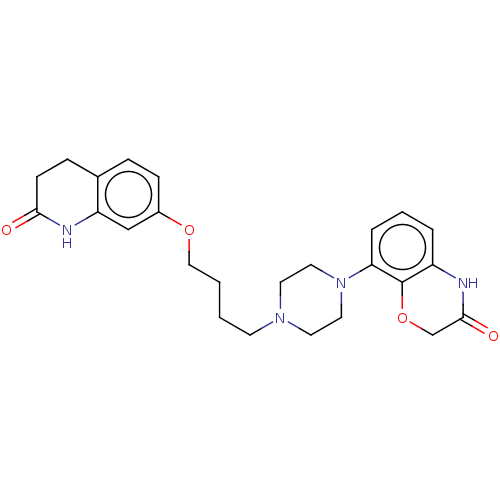 (UNC10108010 | US9156822, 38) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031878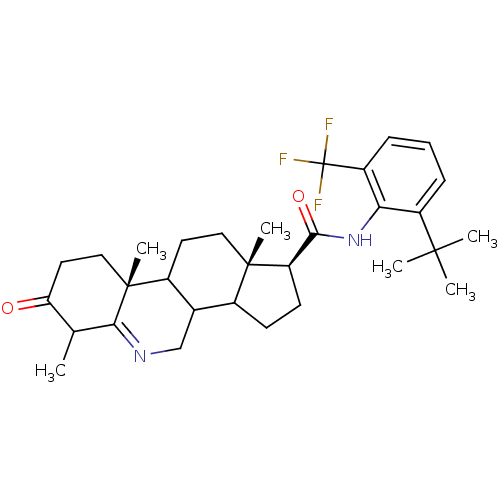 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384584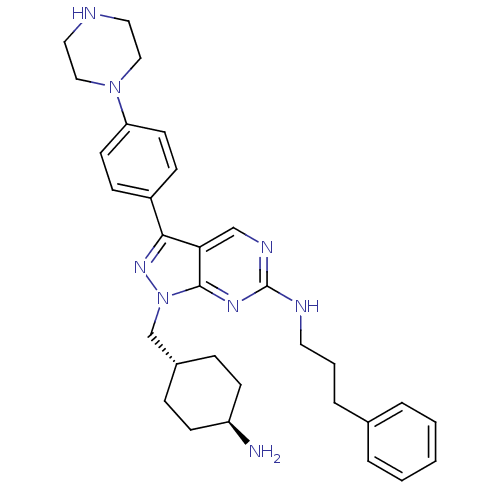 (CHEMBL2036807 | US9744172, Compound UNC607A) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50395587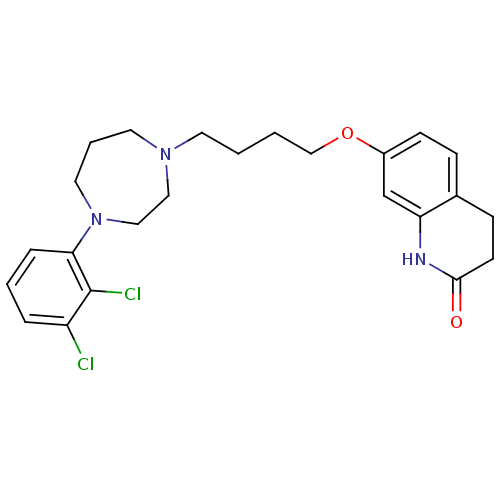 (CHEMBL2165126 | UNC10000006 | US9156822, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT2B receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50395578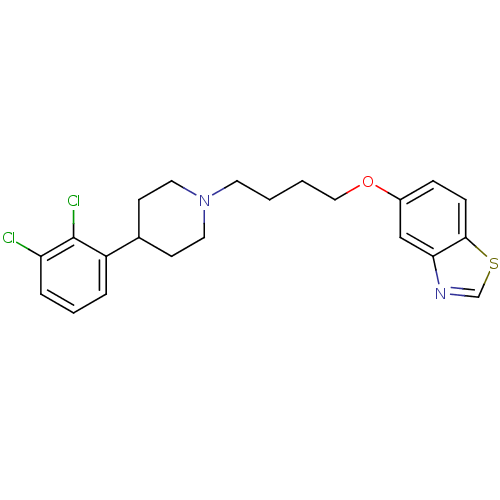 (CHEMBL2165137 | UNC10099993 | US9156822, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT2B receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50395587 (CHEMBL2165126 | UNC10000006 | US9156822, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT2C receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Homo sapiens (Human)) | BDBM50395578 (CHEMBL2165137 | UNC10099993 | US9156822, 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT2C receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50395587 (CHEMBL2165126 | UNC10000006 | US9156822, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT1A receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50395578 (CHEMBL2165137 | UNC10099993 | US9156822, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50395587 (CHEMBL2165126 | UNC10000006 | US9156822, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031897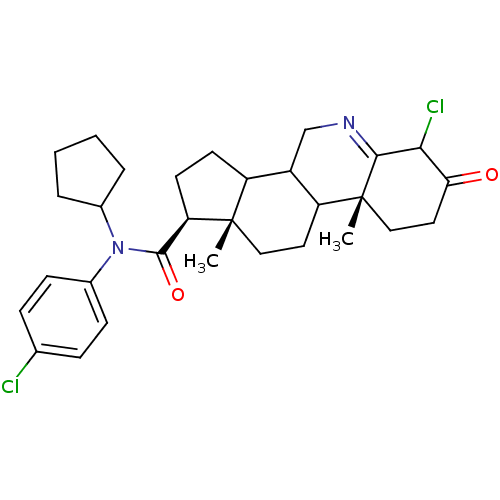 ((1S,9aR,11aS)-6-Chloro-9a,11a-dimethyl-7-oxo-2,3,3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50395587 (CHEMBL2165126 | UNC10000006 | US9156822, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT2A receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50395578 (CHEMBL2165137 | UNC10099993 | US9156822, 21) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Binding affinity to 5-HT1A receptor | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384583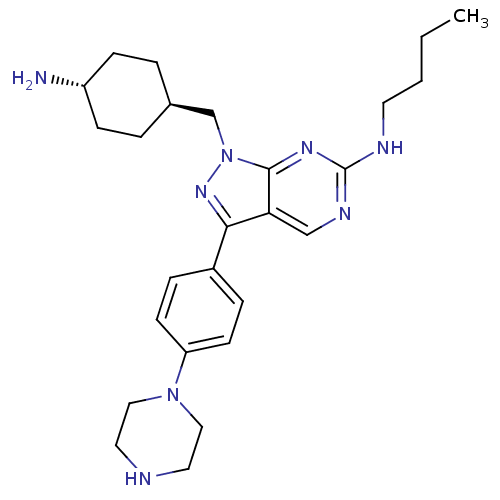 (CHEMBL2036806) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185686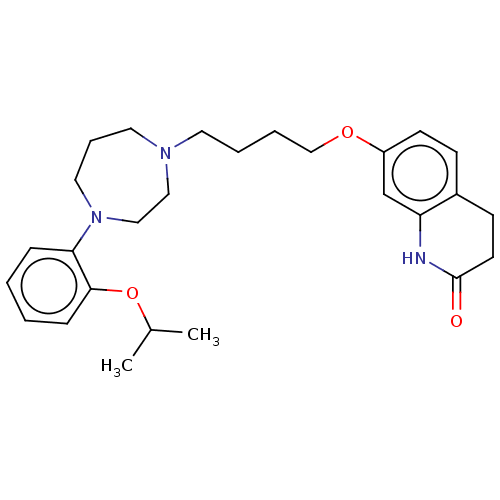 (UNC10107967 | US9156822, 16) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185688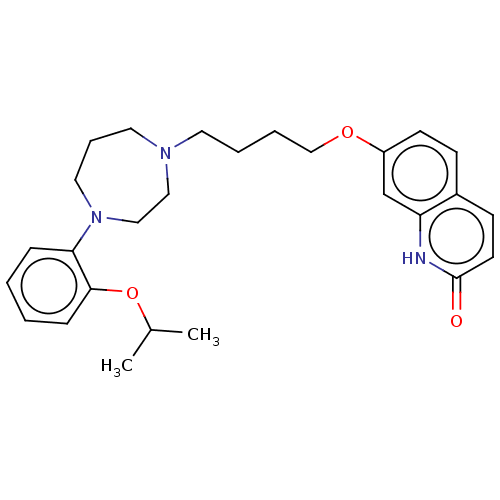 (UNC10107969 | US9156822, 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.930 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039276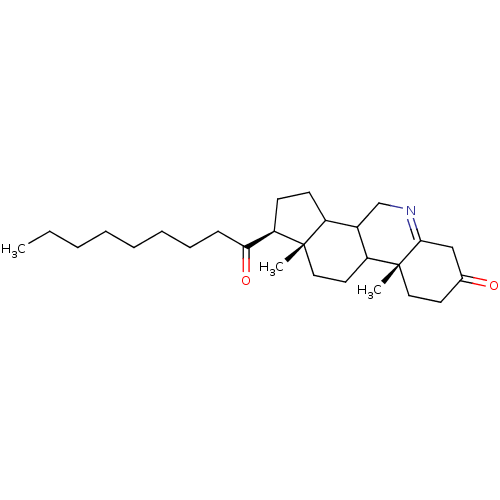 ((1S,9aR,11aS)-9a,11a-Dimethyl-1-nonanoyl-1,2,3,3a,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50039277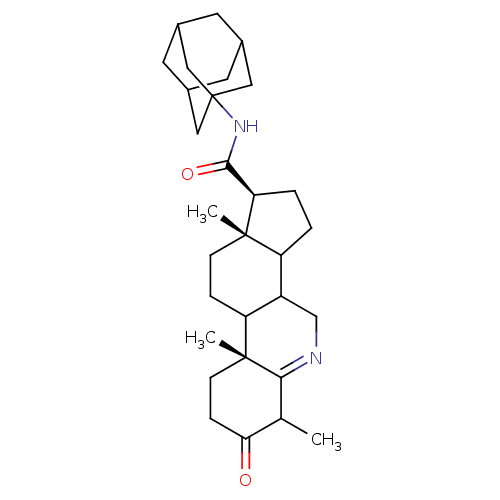 ((1S,9aR,11aS)-6,9a,11a-Trimethyl-7-oxo-2,3,3a,3b,4...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against human type 1 5-alpha reductase | J Med Chem 37: 2352-60 (1994) BindingDB Entry DOI: 10.7270/Q228088W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50395569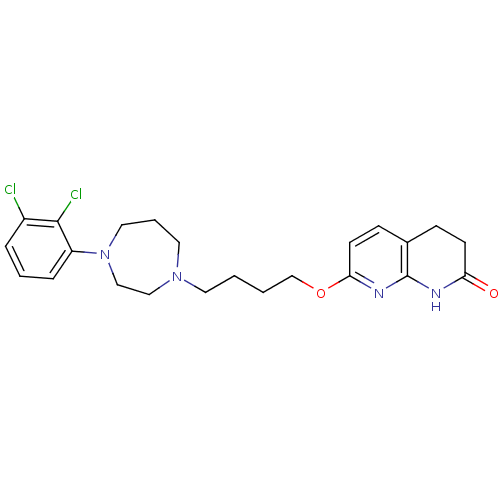 (CHEMBL2165119 | UNC10099975 | US9156822, 3 or UNC9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 2 (Rattus norvegicus) | BDBM50031896 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibitory activity was measured on rat Steroid 5-alpha-reductase type 2 | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395569 (CHEMBL2165119 | UNC10099975 | US9156822, 3 or UNC9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of North Carolina at Chapel Hill Curated by ChEMBL | Assay Description Displacement of [3H]N-methylspiperone from human D2L receptor expressed in CHO cells after 1.5 hrs by microbeta counting method | J Med Chem 55: 7141-53 (2012) Article DOI: 10.1021/jm300603y BindingDB Entry DOI: 10.7270/Q2JD4XXH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395569 (CHEMBL2165119 | UNC10099975 | US9156822, 3 or UNC9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185687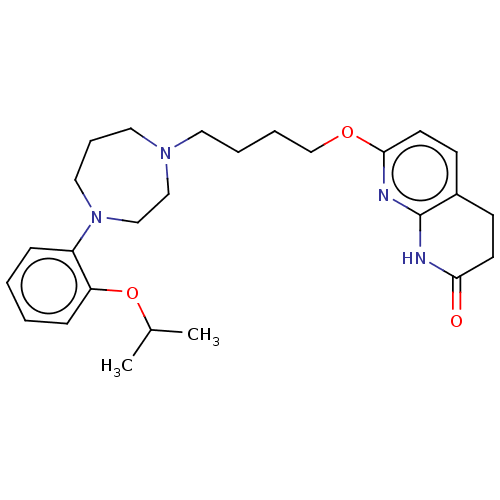 (UNC10107968 | US9156822, 17) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.29 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031903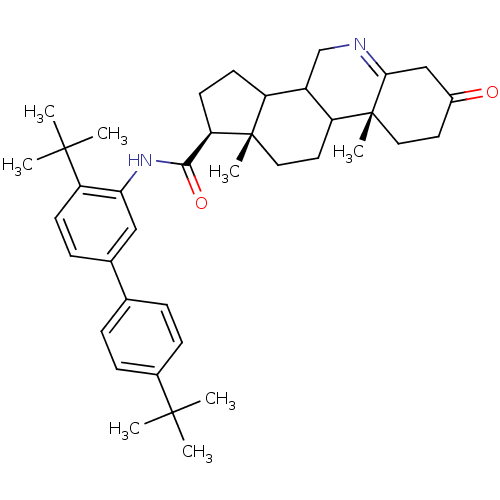 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2B (Homo sapiens (Human)) | BDBM50130293 (7-{4-[4-(2,3-dichlorophenyl)piperazin-1-yl]butoxy}...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384582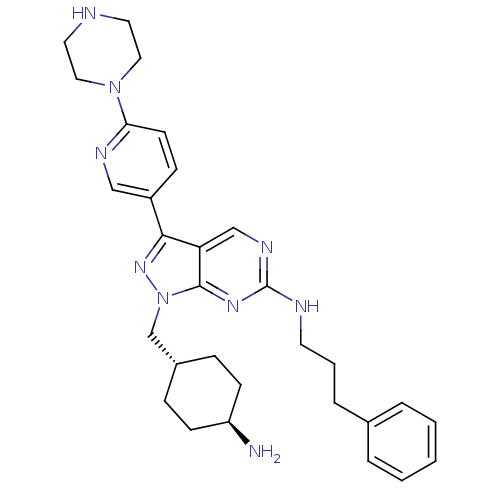 (CHEMBL2036805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-oxo-5-alpha-steroid 4-dehydrogenase 1 (Homo sapiens (Human)) | BDBM50031879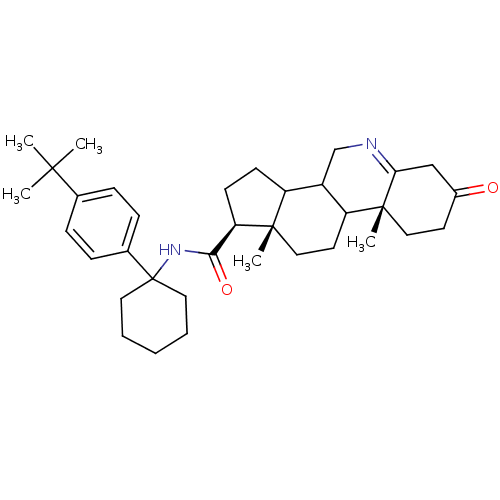 ((1S,9aR,11aS)-9a,11a-Dimethyl-7-oxo-2,3,3a,3b,4,5,...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Glaxo Inc. Research Institute Curated by ChEMBL | Assay Description Inhibition of recombinant steroid 5-alpha-reductase type I | J Med Chem 38: 2621-7 (1995) BindingDB Entry DOI: 10.7270/Q2C829XC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185694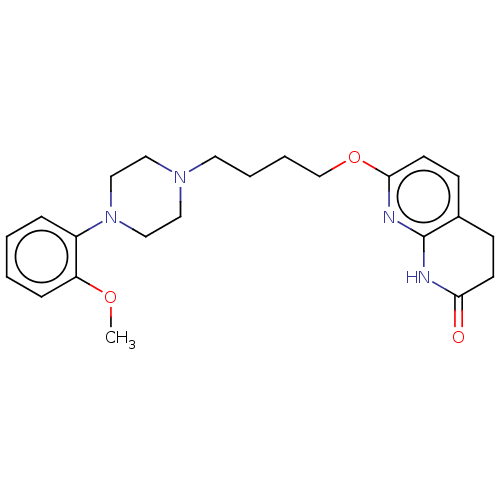 (UNC10107957 | US9156822, 37) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase Mer (Homo sapiens (Human)) | BDBM50384585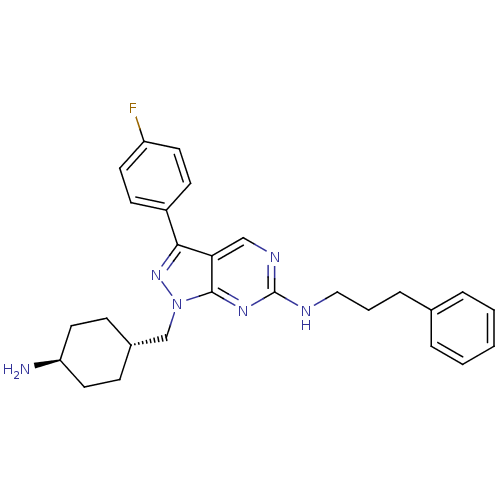 (CHEMBL2036809) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of Mer using EFPIYDFLPAKKK-CONH2 as substrate by Michaelis-Menton equation | ACS Med Chem Lett 3: 129-134 (2012) Article DOI: 10.1021/ml200239k BindingDB Entry DOI: 10.7270/Q2F76DMC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM185680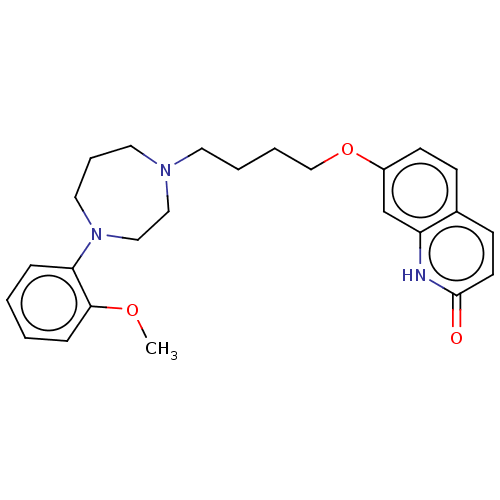 (UNC10107954 | US9156822, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50395569 (CHEMBL2165119 | UNC10099975 | US9156822, 3 or UNC9...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
The University of North Carolina at Chapel Hill US Patent | Assay Description Membranes prepared as above were resuspended to 1 ug protein/ul (measured by Bradford assay using BSA as standard), and 50 ul were added to each well... | US Patent US9156822 (2015) BindingDB Entry DOI: 10.7270/Q2H41Q6D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5610 total ) | Next | Last >> |