

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK2 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK3 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK3 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK3 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572486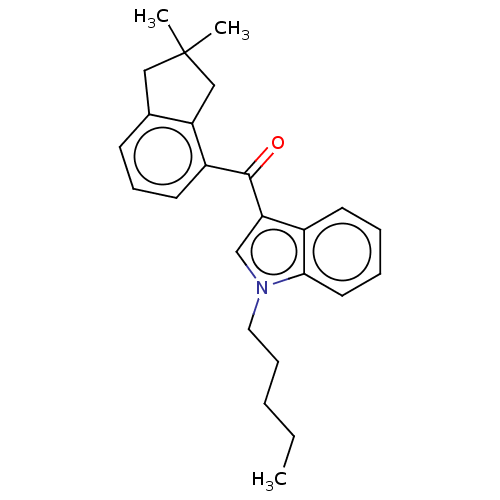 (CHEMBL4860950) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572486 (CHEMBL4860950) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572487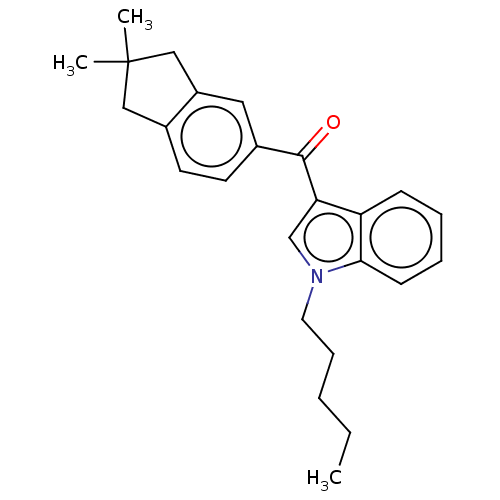 (CHEMBL4856192) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50353747 (CHEMBL561013 | JWH-018) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572485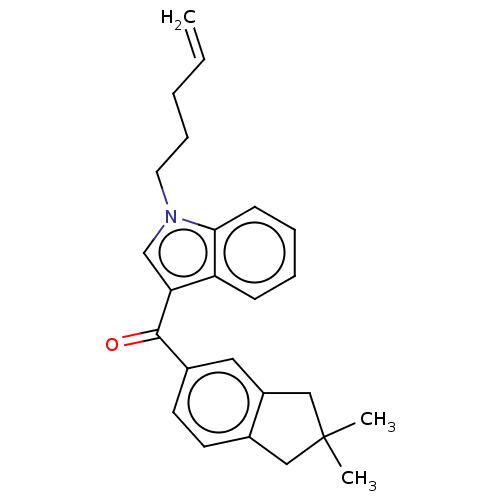 (CHEMBL4855206) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of ALK | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572489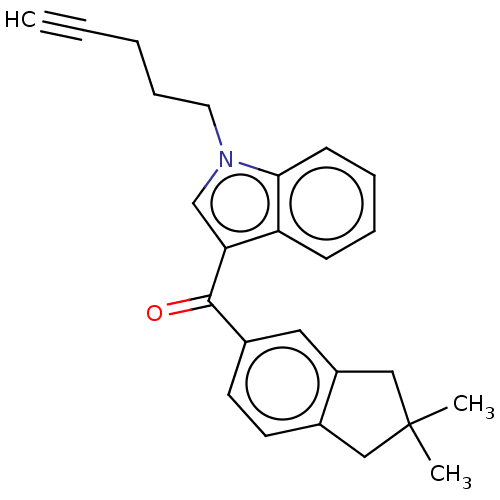 (CHEMBL4877815) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572487 (CHEMBL4856192) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572488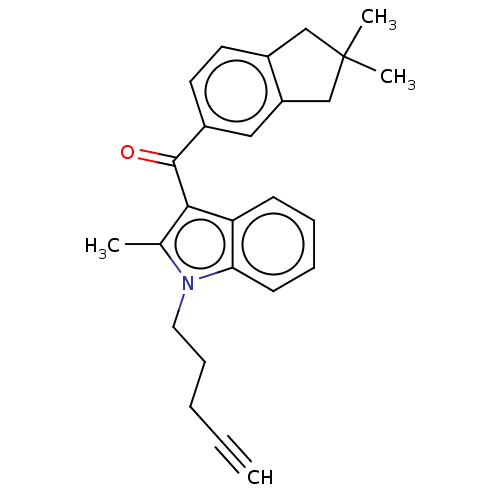 (CHEMBL4866238) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50004704 ((+)-cis-Diltiazem | (2S,3S)-5-(2-(dimethylamino)et...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes by liquid scintillation counting | J Med Chem 52: 2352-62 (2009) Article DOI: 10.1021/jm801351u BindingDB Entry DOI: 10.7270/Q2MP562C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50572490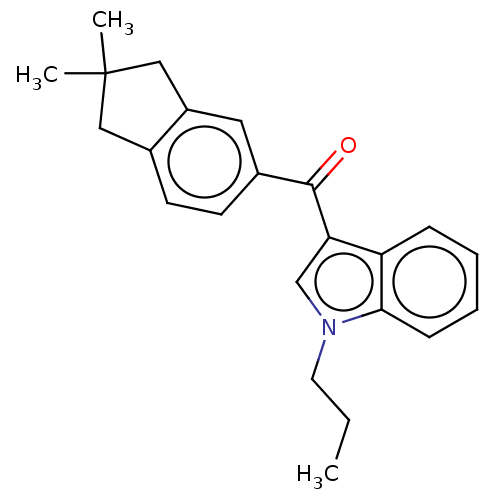 (CHEMBL4872576) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB2 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572485 (CHEMBL4855206) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 152 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of cKit | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50479923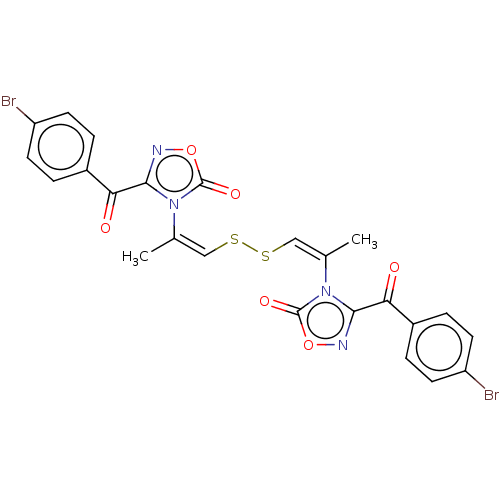 (CHEMBL521744) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes by liquid scintillation counting | J Med Chem 52: 2352-62 (2009) Article DOI: 10.1021/jm801351u BindingDB Entry DOI: 10.7270/Q2MP562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50300198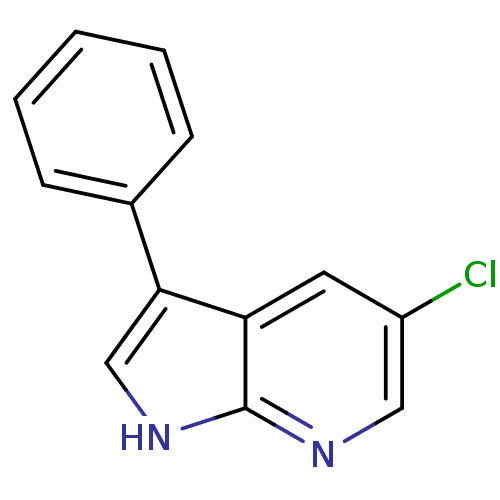 (5-chloro-3-phenyl-1H-pyrrolo[2,3-b]pyridine | CHEM...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK2 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50479922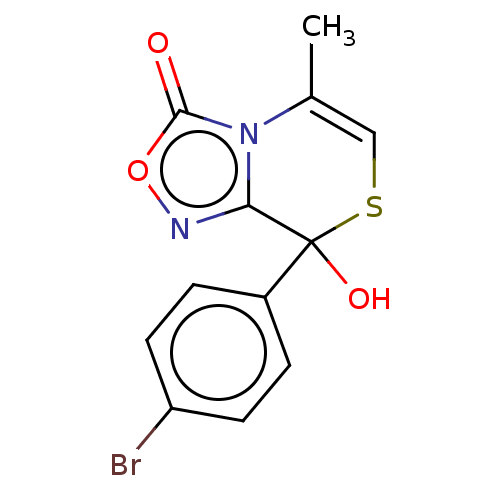 (CHEMBL489002) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes by liquid scintillation counting | J Med Chem 52: 2352-62 (2009) Article DOI: 10.1021/jm801351u BindingDB Entry DOI: 10.7270/Q2MP562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572489 (CHEMBL4877815) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 325 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase kinase kinase kinase 2 (Homo sapiens (Human)) | BDBM50300196 (10-(4-hydroxyphenyl)-8,13,15-triazatetracyclo[9.6....) | MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of GCK | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50479455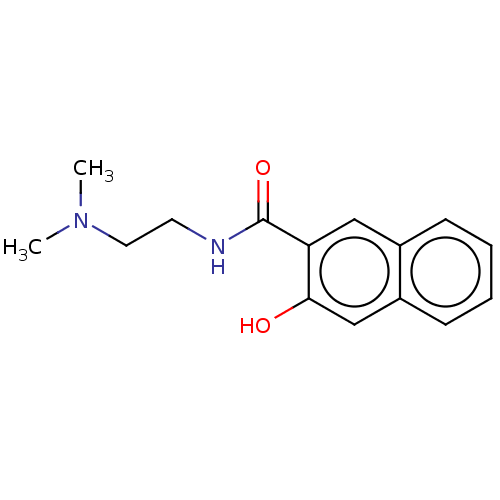 (CHEMBL492668) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes | J Med Chem 51: 5552-65 (2008) Article DOI: 10.1021/jm800151n BindingDB Entry DOI: 10.7270/Q24T6N5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase JAK2 (Homo sapiens (Human)) | BDBM50300197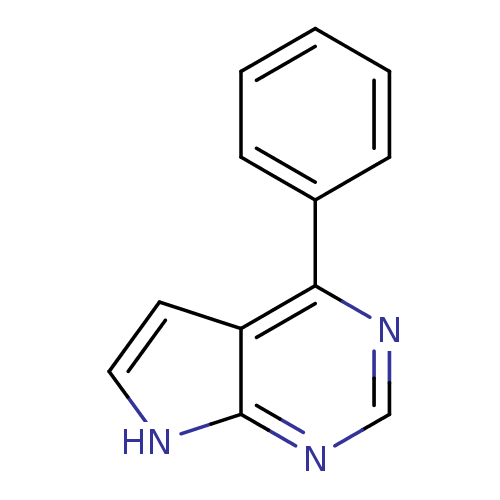 (4-phenyl-7H-pyrrolo[2,3-d]pyrimidine | CHEMBL57897...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant JAK2 | J Med Chem 52: 7938-41 (2009) Checked by Author Article DOI: 10.1021/jm901383u BindingDB Entry DOI: 10.7270/Q2GF0TK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50479921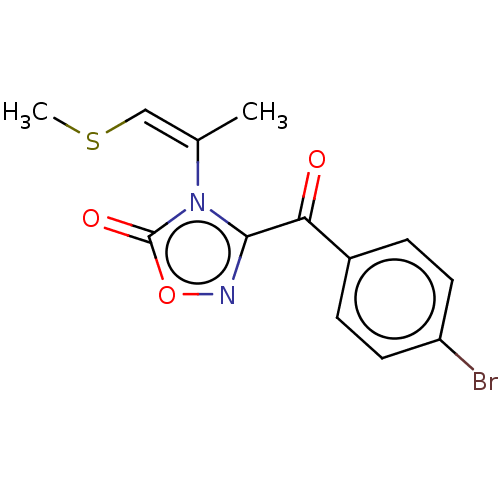 (CHEMBL508166) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes by liquid scintillation counting | J Med Chem 52: 2352-62 (2009) Article DOI: 10.1021/jm801351u BindingDB Entry DOI: 10.7270/Q2MP562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM81464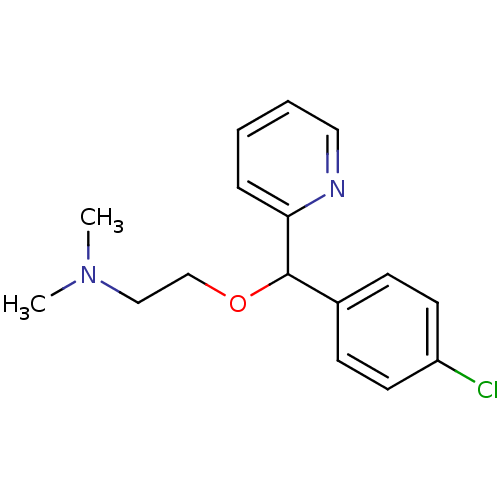 (2-[(4-chlorophenyl)-(2-pyridyl)methoxy]ethyl-dimet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes | J Med Chem 51: 5552-65 (2008) Article DOI: 10.1021/jm800151n BindingDB Entry DOI: 10.7270/Q24T6N5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572488 (CHEMBL4866238) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50572490 (CHEMBL4872576) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-CP55940 from human CB1 receptor expressed in CHO cells incubated for 90 mins by liquid scintillation spectrophotometry relative ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00442 BindingDB Entry DOI: 10.7270/Q2B56PHQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1C (RAT) | BDBM50475854 (CHEMBL386435) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia Curated by ChEMBL | Assay Description Inhibition of [3H]diltiazem binding to Sprague-Dawley rat cardiomyocytes | J Med Chem 49: 5206-16 (2006) Article DOI: 10.1021/jm0604373 BindingDB Entry DOI: 10.7270/Q2KP84W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM60973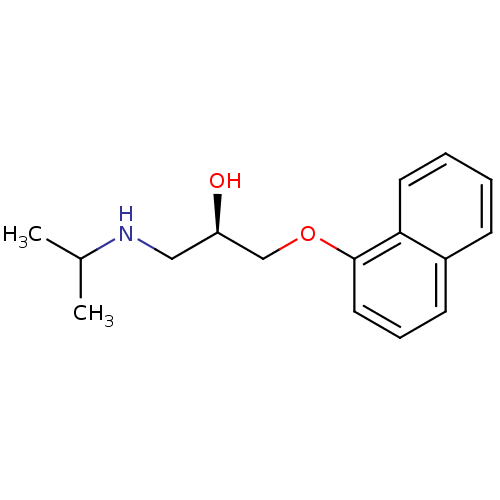 ((2R)-1-(1-naphthalenyloxy)-3-(propan-2-ylamino)-2-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Perugia Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes | J Med Chem 51: 5552-65 (2008) Article DOI: 10.1021/jm800151n BindingDB Entry DOI: 10.7270/Q24T6N5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Voltage-dependent L-type calcium channel subunit alpha-1S (Rattus norvegicus) | BDBM50479920 (CHEMBL523271) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universita degli Studi di Bologna Curated by ChEMBL | Assay Description Displacement of [3H]diltiazem from L-type calcium channel in Sprague-Dawley rat cardiac myocytes by liquid scintillation counting | J Med Chem 52: 2352-62 (2009) Article DOI: 10.1021/jm801351u BindingDB Entry DOI: 10.7270/Q2MP562C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50061096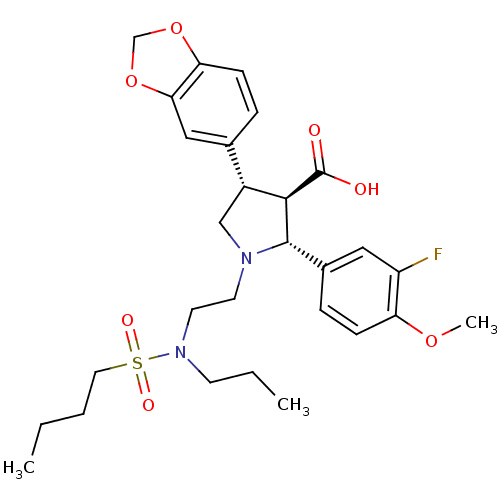 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-{2-[(butane-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50146613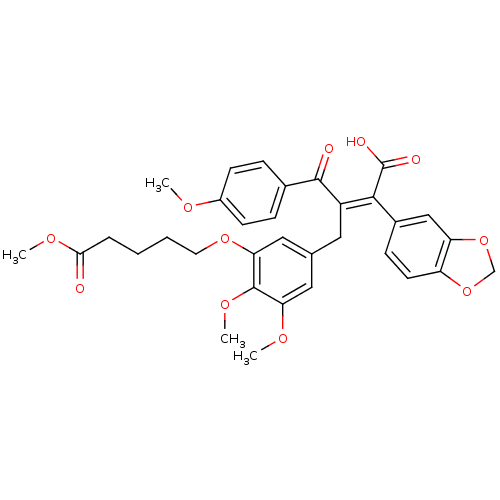 (5-{5-[(Z)-3-Benzo[1,3]dioxol-5-yl-3-carboxy-2-(4-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145527 ((S)-5-{4-[3-(5-Fluoro-1,4,5,6-tetrahydro-pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0550 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145533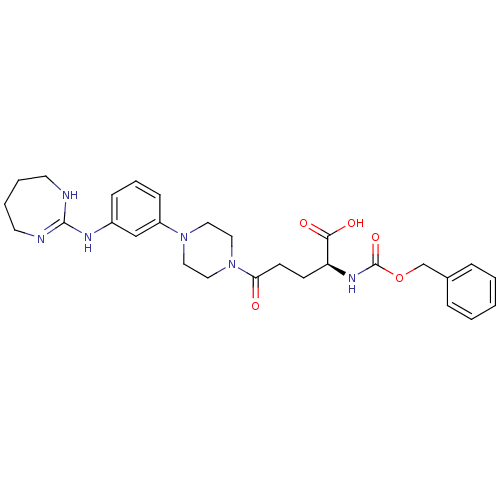 ((S)-2-Benzyloxycarbonylamino-5-oxo-5-{4-[3-(4,5,6,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0560 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of binding to human alphaV-beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145528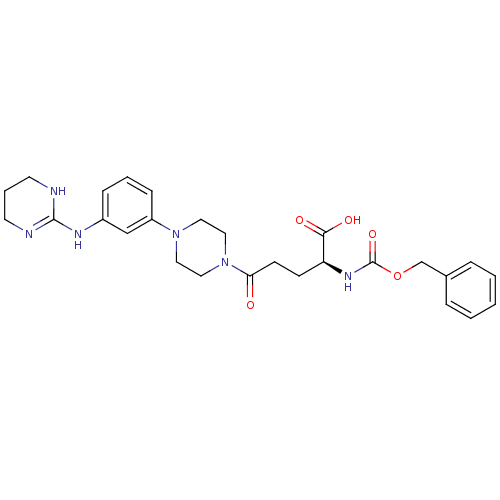 ((S)-2-Benzyloxycarbonylamino-5-oxo-5-{4-[3-(1,4,5,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0580 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145530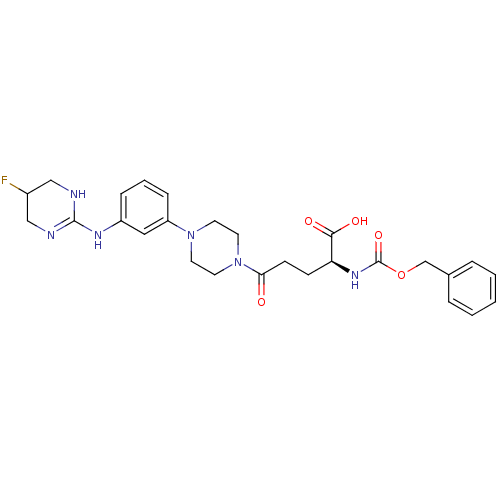 ((S)-2-Benzyloxycarbonylamino-5-{4-[3-(5-fluoro-1,4...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0630 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50146606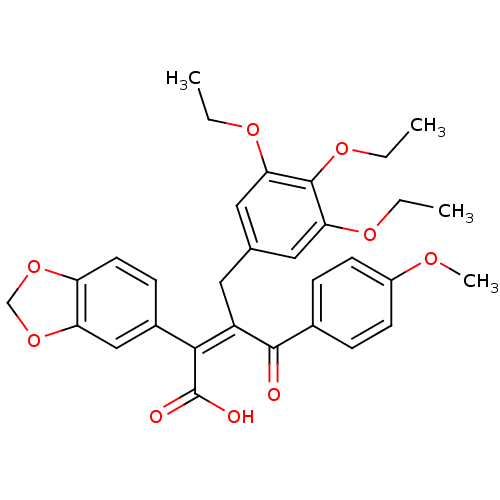 ((Z)-2-Benzo[1,3]dioxol-5-yl-4-(4-methoxy-phenyl)-4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145526 ((S)-2-Benzyloxycarbonylamino-5-{4-[3-(5-hydroxy-1,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145525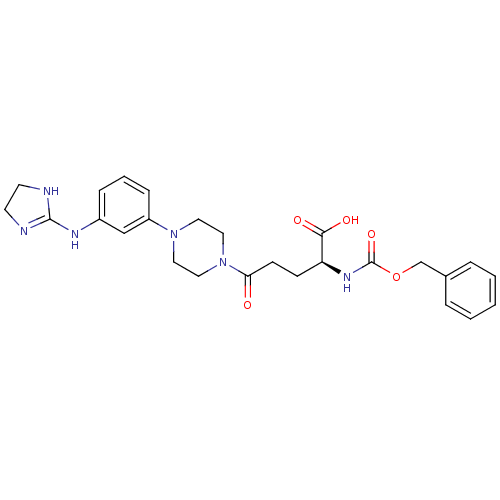 (2-Benzyloxycarbonylamino-5-{4-[3-(4,5-dihydro-1H-i...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of binding to human alphaV-beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50112678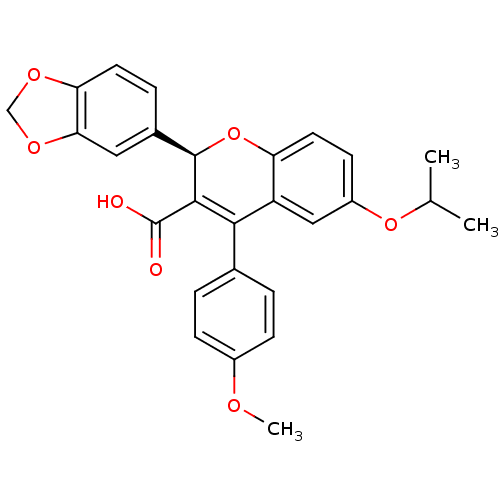 ((R)-2-Benzo[1,3]dioxol-5-yl-6-isopropoxy-4-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145523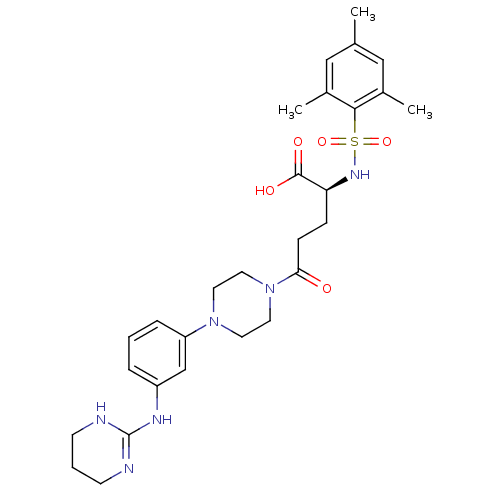 ((S)-5-Oxo-5-{4-[3-(1,4,5,6-tetrahydro-pyrimidin-2-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50146619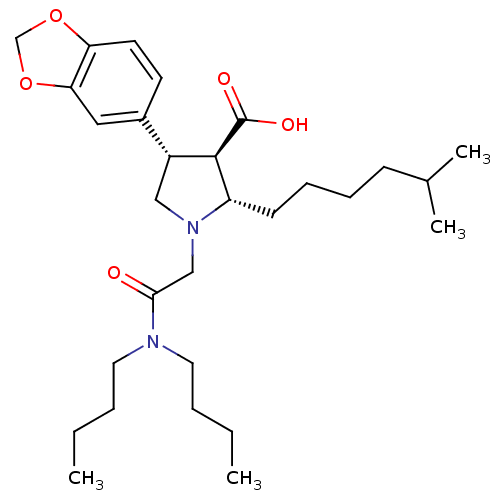 ((2S,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neprilysin (Homo sapiens (Human)) | BDBM153128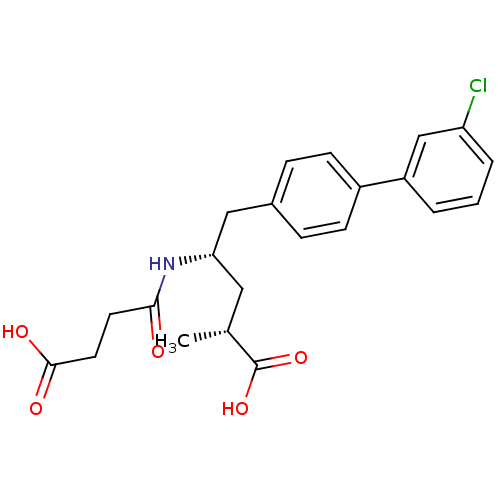 (US8993631, 36) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human NEP expressed in insect cells preincubated for 1 hr using Cys(PT14)-Arg-Arg-Leu-Trp-OH as substrate and measured afte... | ACS Med Chem Lett 11: 188-194 (2020) Article DOI: 10.1021/acsmedchemlett.9b00578 BindingDB Entry DOI: 10.7270/Q2C82DK4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145522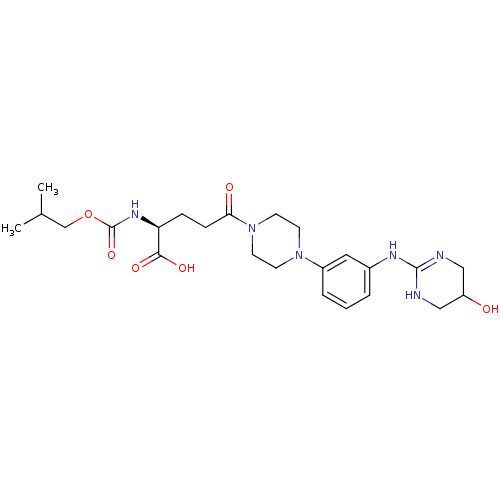 ((S)-5-{4-[3-(5-Hydroxy-1,4,5,6-tetrahydro-pyrimidi...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Homo sapiens (Human)) | BDBM50444550 (CHEMBL3099704) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 11B1, mitochondrial (Rattus norvegicus) | BDBM50444550 (CHEMBL3099704) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of rat recombinant CYP11B1 using 11-deoxycortisol as substrate by cell-based assay | ACS Med Chem Lett 4: 1203-7 (2013) Article DOI: 10.1021/ml400324c BindingDB Entry DOI: 10.7270/Q2NZ8938 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (RAT) | BDBM50051007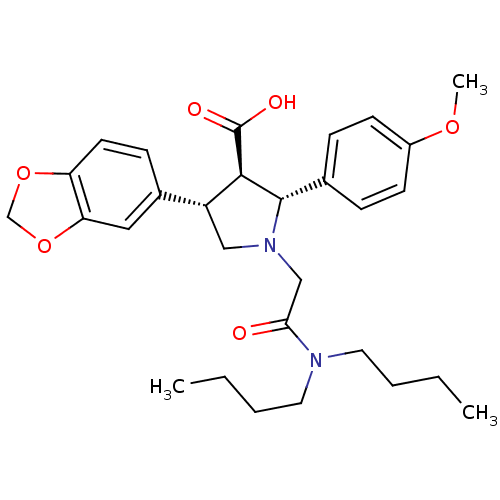 ((2R,3R,4S)-4-Benzo[1,3]dioxol-5-yl-1-dibutylcarbam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Ability to displace [125I]-ET-1 from the rat endothelin A receptor expressed in rat aorta smooth muscle cells. | J Med Chem 47: 2750-60 (2004) Article DOI: 10.1021/jm031041j BindingDB Entry DOI: 10.7270/Q26T0M3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145529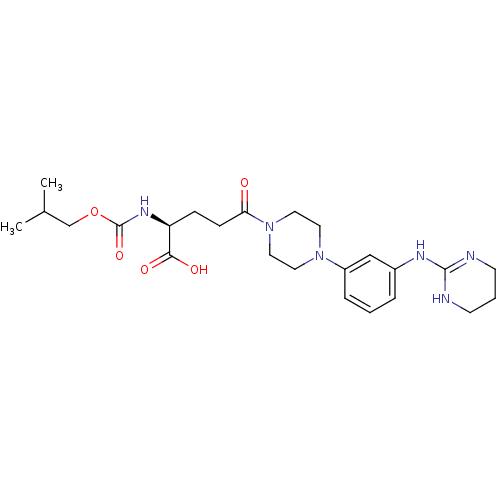 ((S)-2-Isobutoxycarbonylamino-5-oxo-5-{4-[3-(1,4,5,...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human alpha IIb beta3 integrin | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-V/beta-3 (Homo sapiens (Human)) | BDBM50145532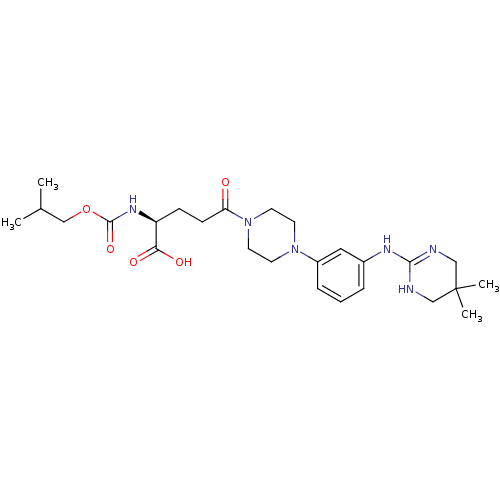 ((S)-5-{4-[3-(5,5-Dimethyl-1,4,5,6-tetrahydro-pyrim...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibitory activity was determined against human vitronectin receptor (alpha V beta 3) | Bioorg Med Chem Lett 14: 2567-70 (2004) Article DOI: 10.1016/j.bmcl.2004.02.075 BindingDB Entry DOI: 10.7270/Q2445KWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 462 total ) | Next | Last >> |