

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity measured after 24 hrs | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human PRB expressed in African green monkey CV1 cells | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human GR assessed as reduction in dexamethasone-induced transactivation | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human AR assessed as reduction in DHT-induced transactivation | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM18627 ((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Displacement of [1,2,6,7-3H]progesterone from recombinant human GST-tagged PGR LBD (678 to 933 residues) expressed in baculovirus-infected insect cel... | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50118690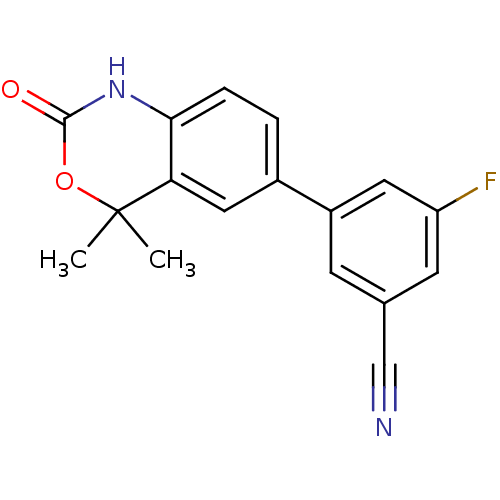 (3-(4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[d][1,3]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at human PRB expressed in African green monkey CV1 cells | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543404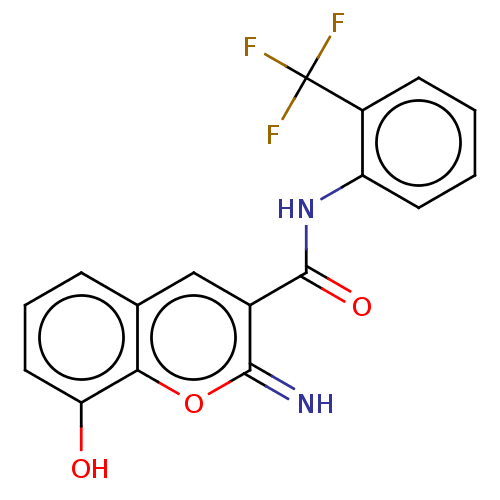 (CHEMBL4640154) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543398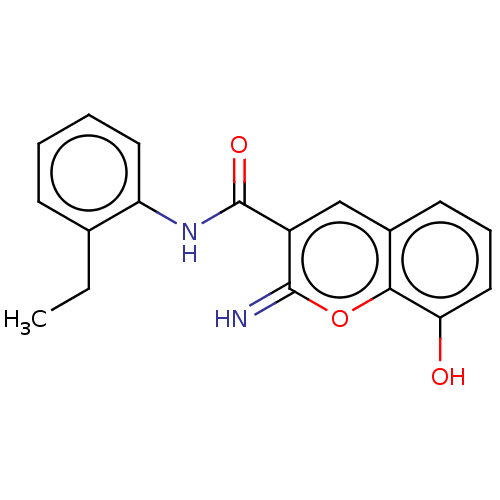 (CHEMBL4642187) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM444349 (N-(amino(((5,6-bis(4-chlorophenyl)pyridin-3-yl)met...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. US Patent | Assay Description Full-length cDNA of human MALT1 gene (GenBank accession No: AB026118.1) amplified by PCR was inserted in flame to a SalI site located downstream of G... | US Patent US10662156 (2020) BindingDB Entry DOI: 10.7270/Q2W380B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198670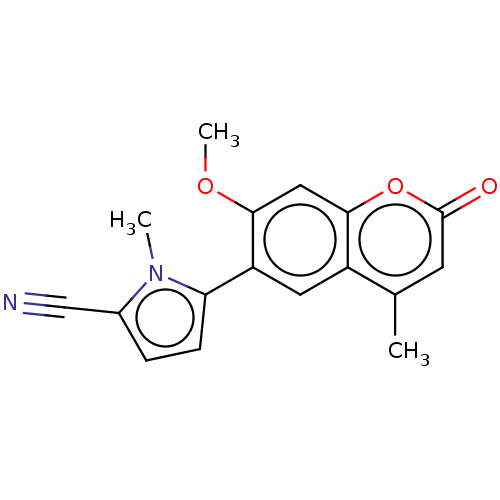 (CHEMBL3960590) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543409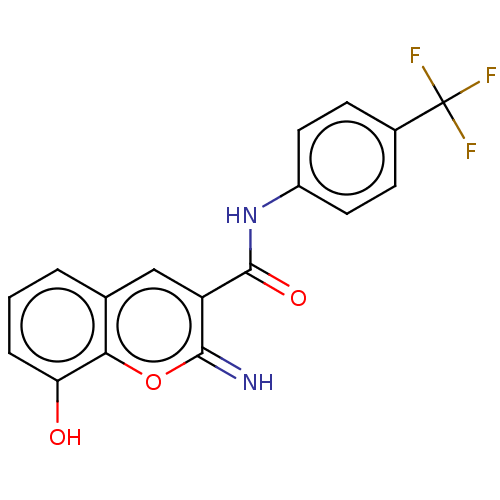 (CHEMBL4642852) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM444338 (N-(amino(((6-(4-chlorophenyl)-5-(4-methoxyphenyl)p...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. US Patent | Assay Description Full-length cDNA of human MALT1 gene (GenBank accession No: AB026118.1) amplified by PCR was inserted in flame to a SalI site located downstream of G... | US Patent US10662156 (2020) BindingDB Entry DOI: 10.7270/Q2W380B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062743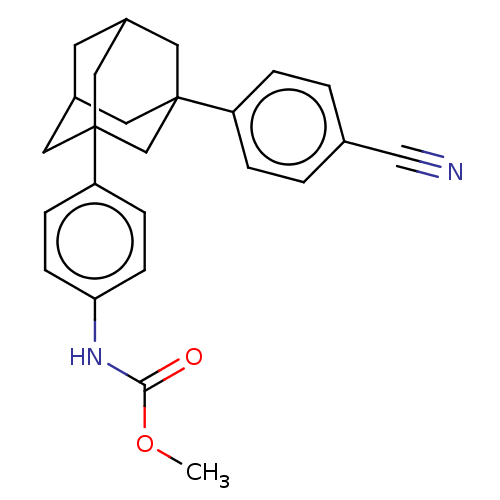 (CHEMBL3397787) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-inducted alkaline phosphatase expression after 24 hrs by plate r... | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543401 (CHEMBL4644092) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062741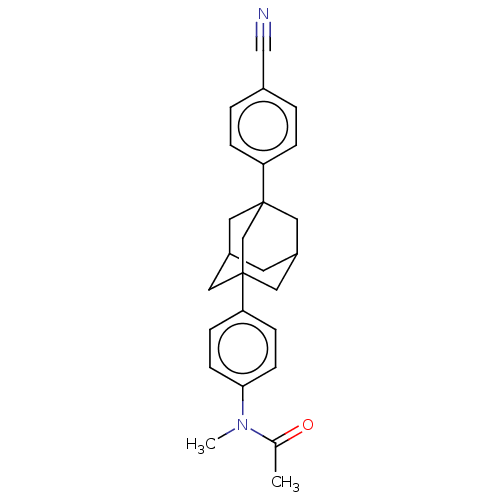 (CHEMBL3397781) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-inducted alkaline phosphatase expression after 24 hrs by plate r... | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50367097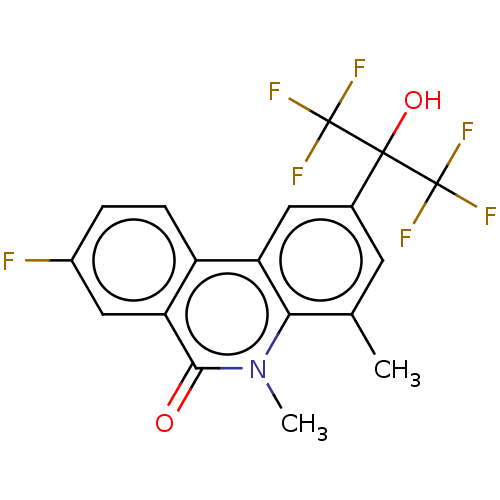 (CHEMBL4173799) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity measured after 24 hrs | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543399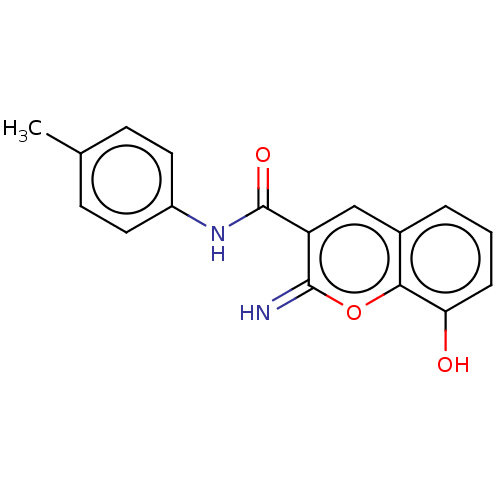 (CHEMBL4648793) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543402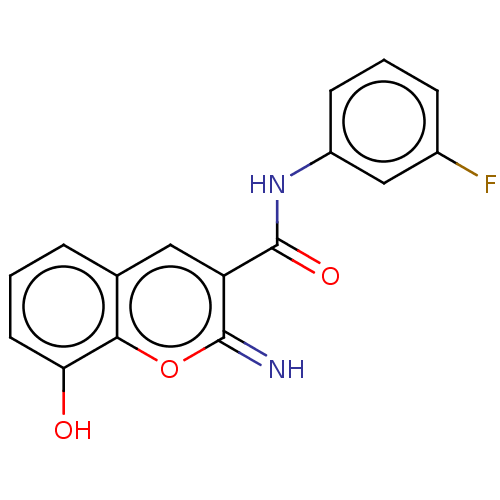 (CHEMBL4634960) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543407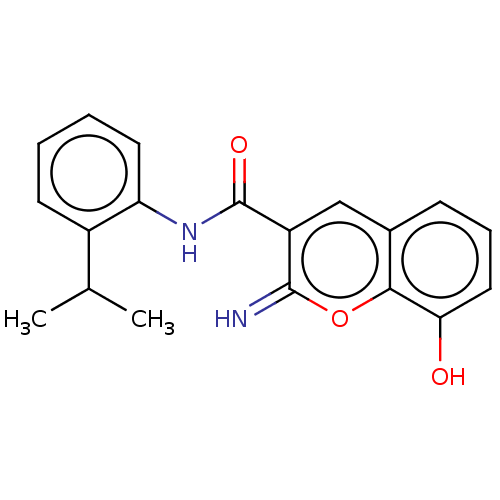 (CHEMBL4641899) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198661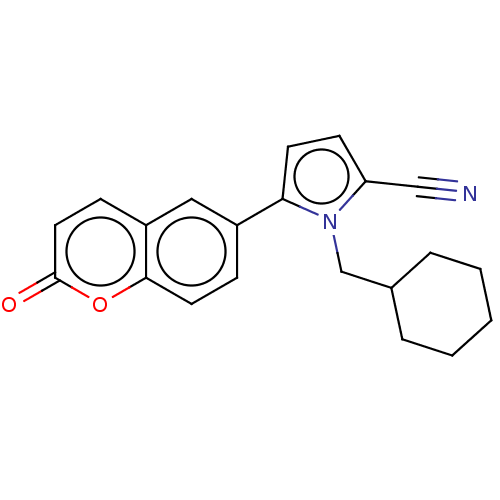 (CHEMBL3955849) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50203153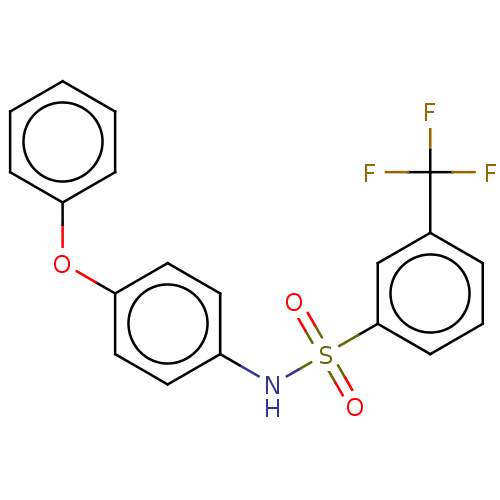 (CHEMBL3927163) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at progesterone receptor in human T47D cells incubated for 24 hrs in presence of P4 by alkaline phosphatase assay | ACS Med Chem Lett 7: 1028-1033 (2016) Article DOI: 10.1021/acsmedchemlett.6b00184 BindingDB Entry DOI: 10.7270/Q22Z17HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198660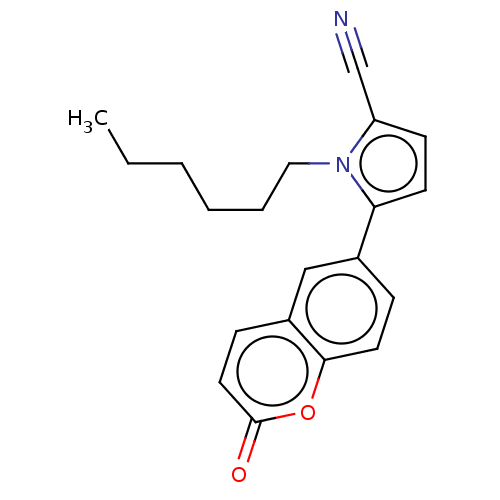 (CHEMBL3928231) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonyl reductase [NADPH] 1 (Homo sapiens (Human)) | BDBM50543400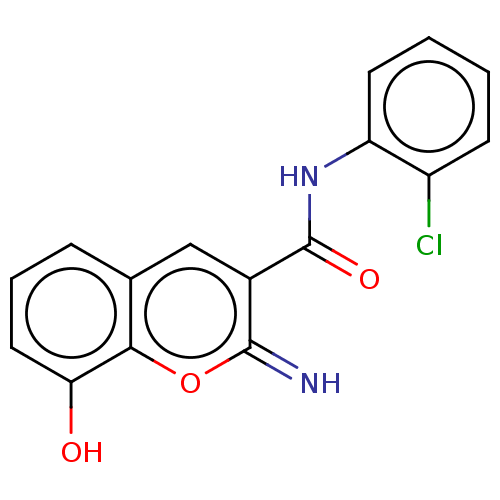 (CHEMBL4640248) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of CBR1 (unknown origin) | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543397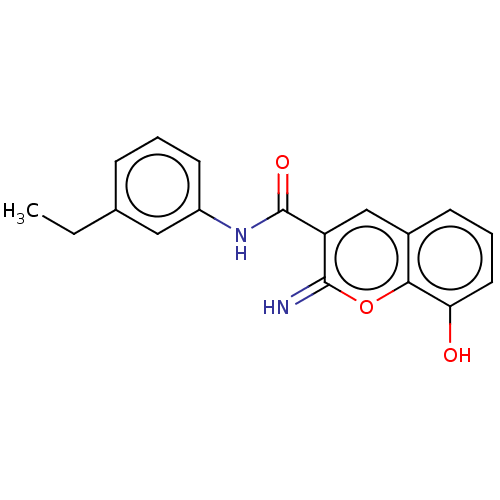 (CHEMBL4646593) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543416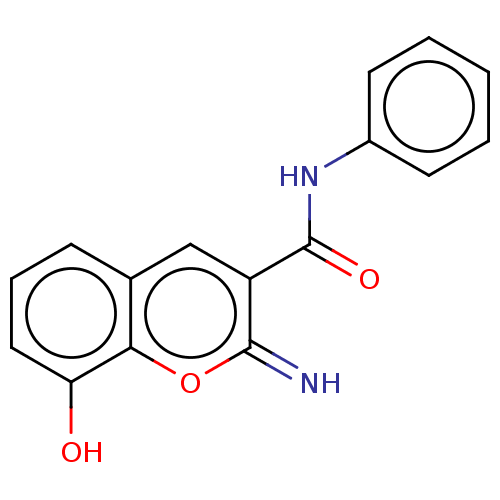 (CHEMBL4639909) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062751 (CHEMBL3397788) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-inducted alkaline phosphatase expression after 24 hrs by plate r... | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mucosa-associated lymphoid tissue lymphoma translocation protein 1 (Homo sapiens (Human)) | BDBM444360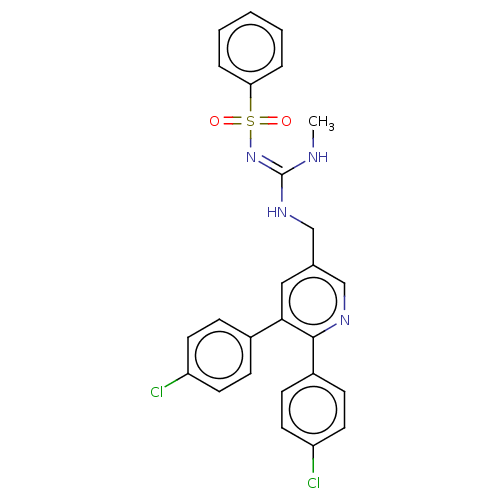 (N-((((5,6-bis(4-chlorophenyl)pyridin-3-yl)methyl)a...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Toray Industries, Inc. US Patent | Assay Description Full-length cDNA of human MALT1 gene (GenBank accession No: AB026118.1) amplified by PCR was inserted in flame to a SalI site located downstream of G... | US Patent US10662156 (2020) BindingDB Entry DOI: 10.7270/Q2W380B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198677 (CHEMBL3929427) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062747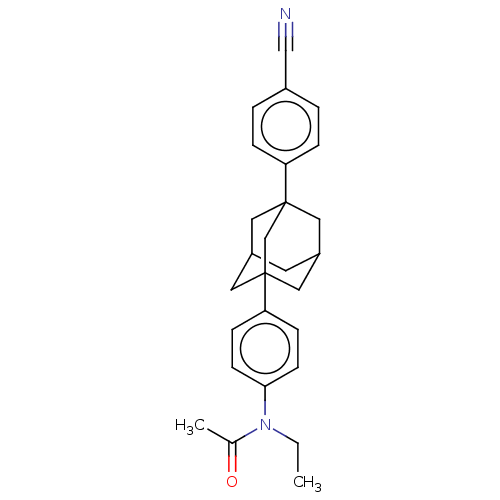 (CHEMBL3397782) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-inducted alkaline phosphatase expression after 24 hrs by plate r... | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50203153 (CHEMBL3927163) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Displacement of [1,2,6,7-3H]-PG from recombinant human GST-tagged PR-LBD (657 to 933 residues) expressed in baculovirus infected insect cells measure... | ACS Med Chem Lett 7: 1028-1033 (2016) Article DOI: 10.1021/acsmedchemlett.6b00184 BindingDB Entry DOI: 10.7270/Q22Z17HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543396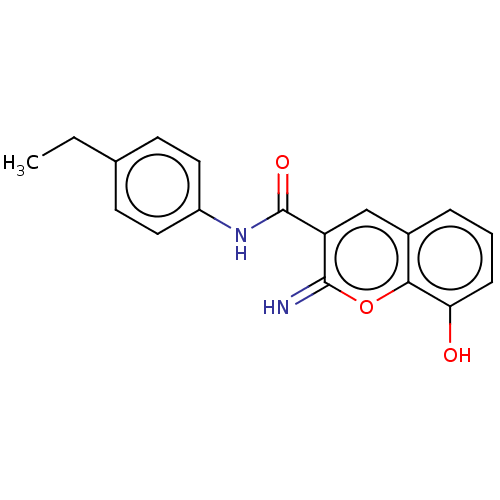 (CHEMBL4641012) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50367121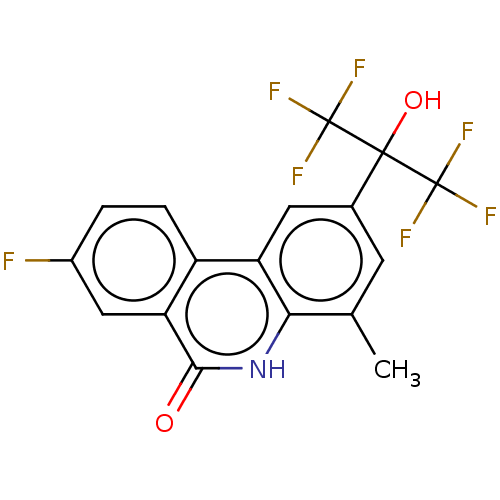 (CHEMBL4175249) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
The University of Tokyo Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity measured after 24 hrs | ACS Med Chem Lett 9: 641-645 (2018) Article DOI: 10.1021/acsmedchemlett.8b00058 BindingDB Entry DOI: 10.7270/Q2PN986S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543405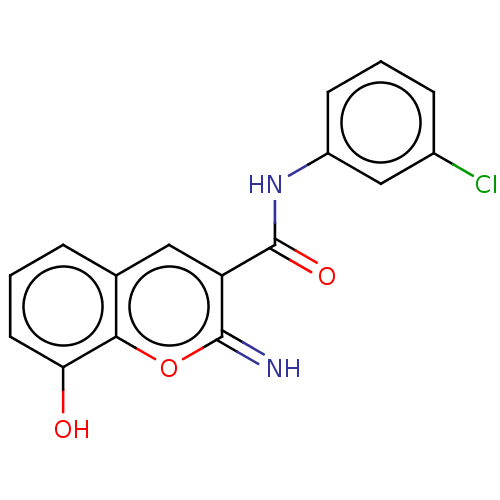 (CHEMBL4639417) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50203179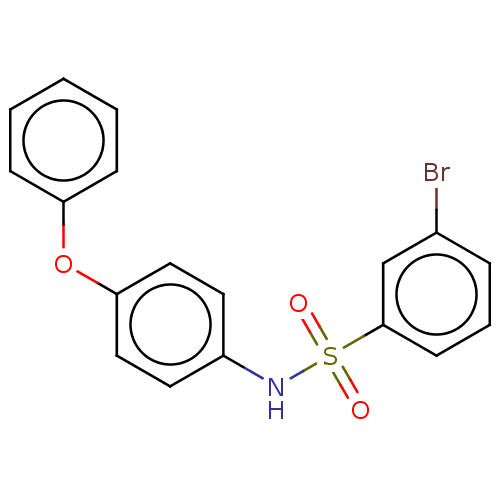 (CHEMBL3917144) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Displacement of [1,2,6,7-3H]-PG from recombinant human GST-tagged PR-LBD (657 to 933 residues) expressed in baculovirus infected insect cells measure... | ACS Med Chem Lett 7: 1028-1033 (2016) Article DOI: 10.1021/acsmedchemlett.6b00184 BindingDB Entry DOI: 10.7270/Q22Z17HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543406 (CHEMBL4637597) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543411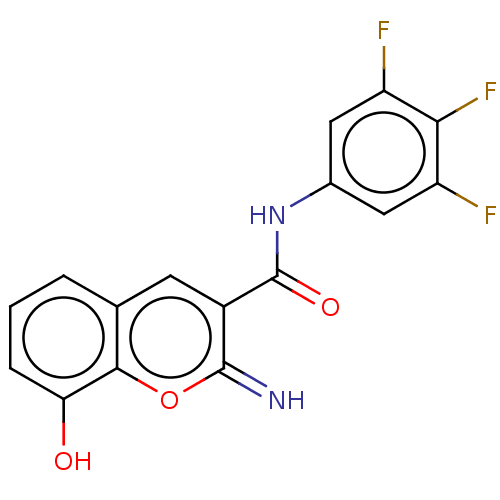 (CHEMBL4634140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| STE20/SPS1-related proline-alanine-rich protein kinase (Homo sapiens (Human)) | BDBM50560445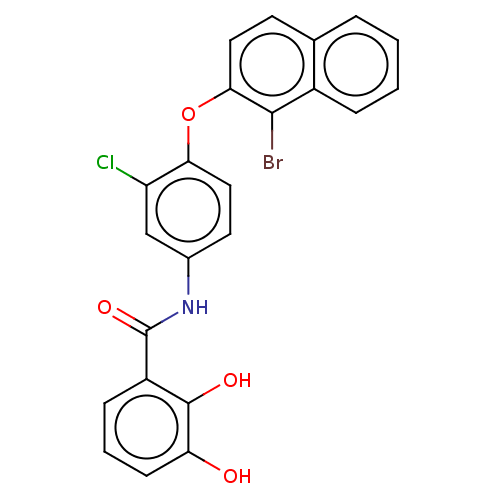 (CHEMBL4792789) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of SPAK (unknown origin) by ELISA | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127408 BindingDB Entry DOI: 10.7270/Q25D8WJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543410 (CHEMBL4633866) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543415 (CHEMBL4641799) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543414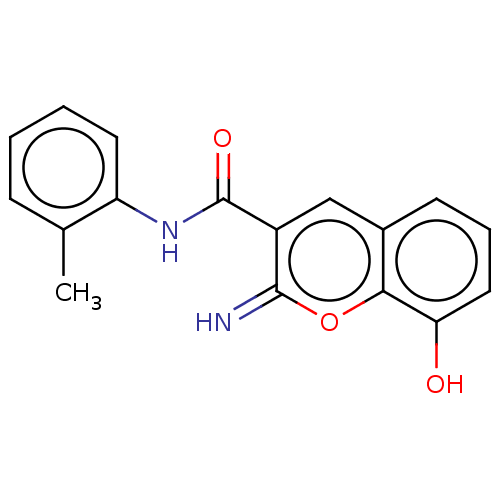 (CHEMBL4636345) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062741 (CHEMBL3397781) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Displacement of [1,2,6,7-3H]progesterone from human recombinant PR LBD after 24 hrs by liquid scintillation counting analysis | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062745 (CHEMBL3397778) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-inducted alkaline phosphatase expression after 24 hrs by plate r... | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543413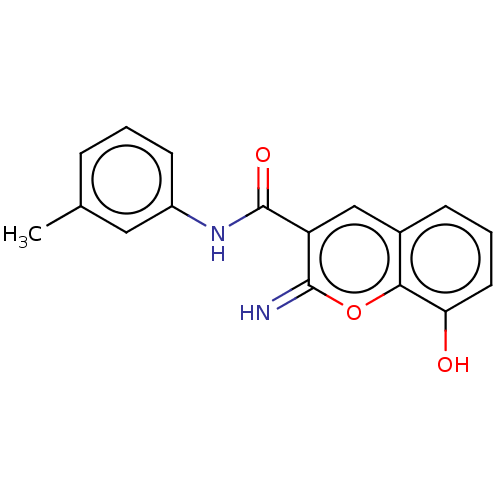 (CHEMBL4649502) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50198662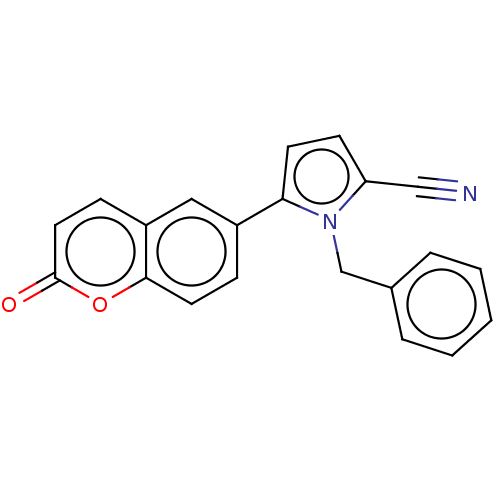 (CHEMBL3966957) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Ochanomizu University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase expression after 24 hrs by alkaline... | Bioorg Med Chem 24: 5602-5610 (2016) Article DOI: 10.1016/j.bmc.2016.09.020 BindingDB Entry DOI: 10.7270/Q2R49SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543400 (CHEMBL4640248) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543408 (CHEMBL4640782) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50062746 (CHEMBL3397780) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Antagonist activity at PR in human T47D cells assessed as inhibition of progesterone-inducted alkaline phosphatase expression after 24 hrs by plate r... | Bioorg Med Chem 23: 803-9 (2015) Article DOI: 10.1016/j.bmc.2014.12.047 BindingDB Entry DOI: 10.7270/Q2XG9SS2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50213370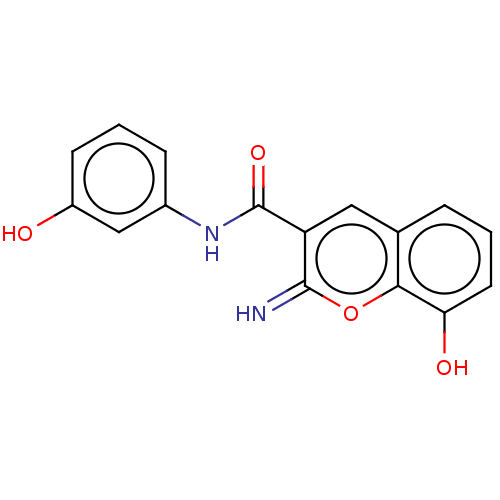 (CHEMBL82085) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50203158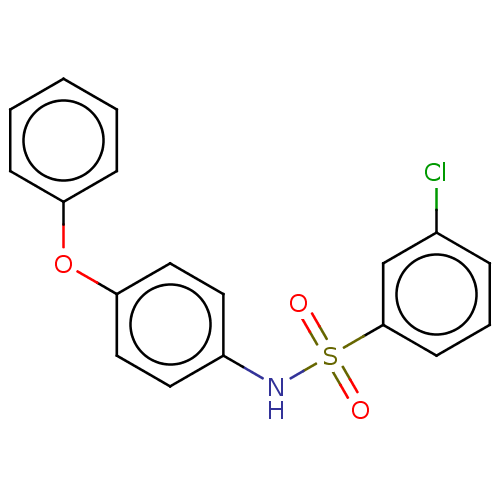 (CHEMBL3912293) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University Curated by ChEMBL | Assay Description Displacement of [1,2,6,7-3H]-PG from recombinant human GST-tagged PR-LBD (657 to 933 residues) expressed in baculovirus infected insect cells measure... | ACS Med Chem Lett 7: 1028-1033 (2016) Article DOI: 10.1021/acsmedchemlett.6b00184 BindingDB Entry DOI: 10.7270/Q22Z17HW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldo-keto reductase family 1 member C3 (Homo sapiens (Human)) | BDBM50543403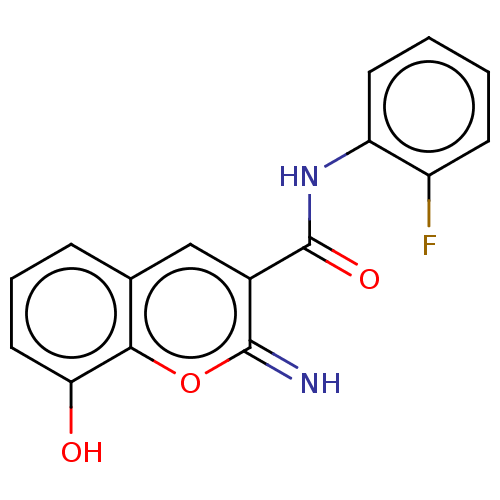 (CHEMBL4642678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of recombinant AKR1C3 (unknown origin) assessed as reduction in S-tetralol-induced dehydrogenase activity by measuring NADPH level | J Med Chem 63: 10396-10411 (2020) Article DOI: 10.1021/acs.jmedchem.0c00939 BindingDB Entry DOI: 10.7270/Q2V40ZSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 665 total ) | Next | Last >> |