

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50562795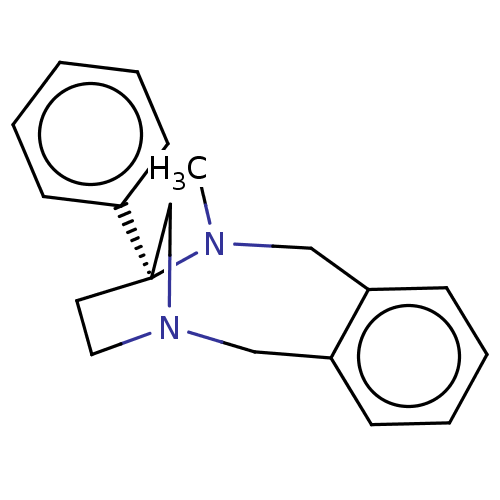 (CHEMBL4751700) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to mu opioid receptor (unknown origin) | Citation and Details Article DOI: 10.1016/j.bmcl.2021.127790 BindingDB Entry DOI: 10.7270/Q21R6V78 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50304619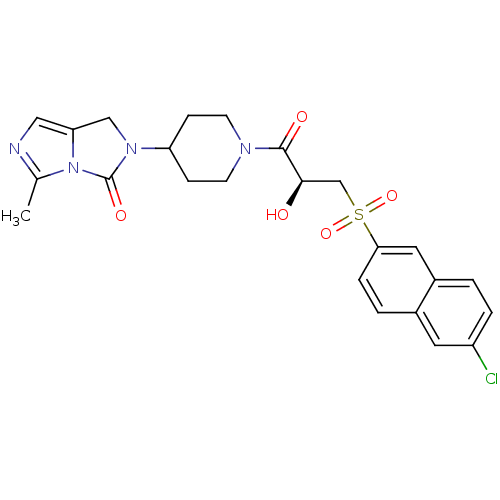 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human thrombin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50304620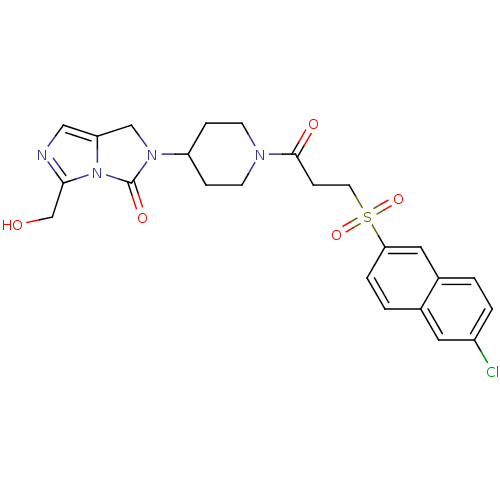 (2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human thrombin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50304620 (2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50304619 ((S)-2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)-2-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human plasmin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50304620 (2-(1-(3-(6-chloronaphthalen-2-ylsulfonyl)propanoyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company, Ltd Curated by ChEMBL | Assay Description Inhibition of human plasmin by para-nitroanilide release assay | Bioorg Med Chem 17: 7993-8002 (2009) Article DOI: 10.1016/j.bmc.2009.10.009 BindingDB Entry DOI: 10.7270/Q2HX1DM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434413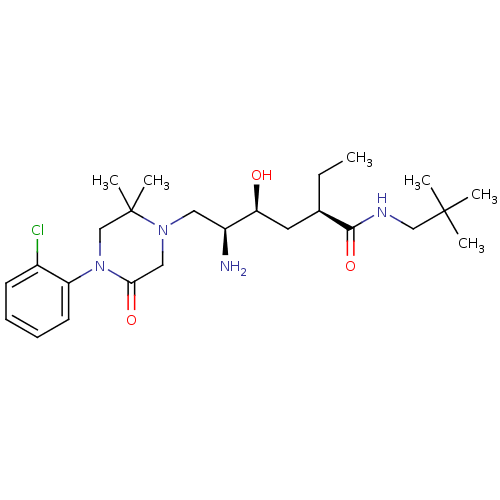 (CHEMBL2387447) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256877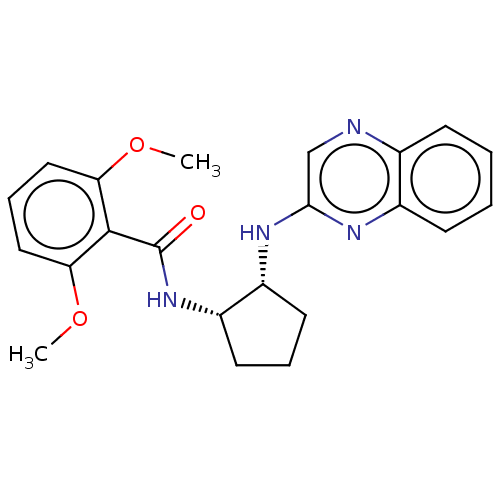 (US9493432, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50392953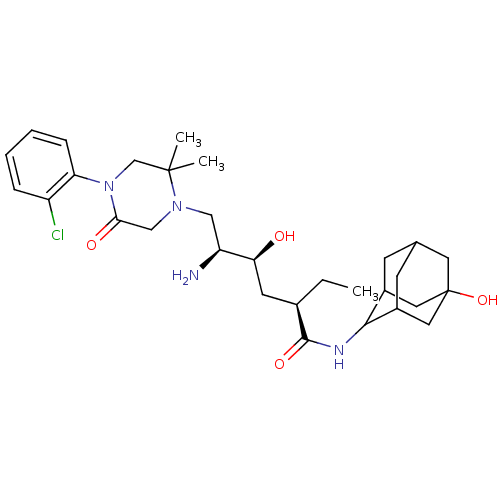 (CHEMBL2152353) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin compound treated for 10 mins before substrate addition measured after 90 mins by fluorescence method | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434425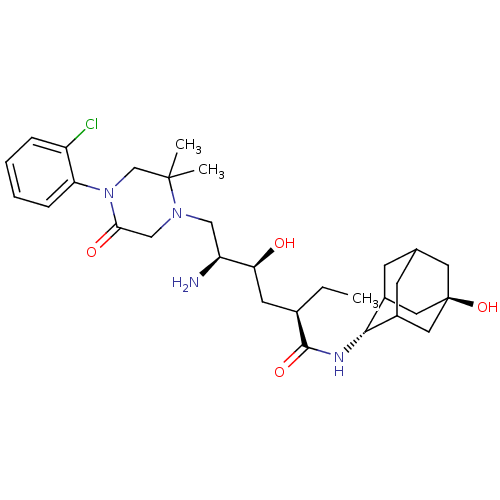 (CHEMBL2387567) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434435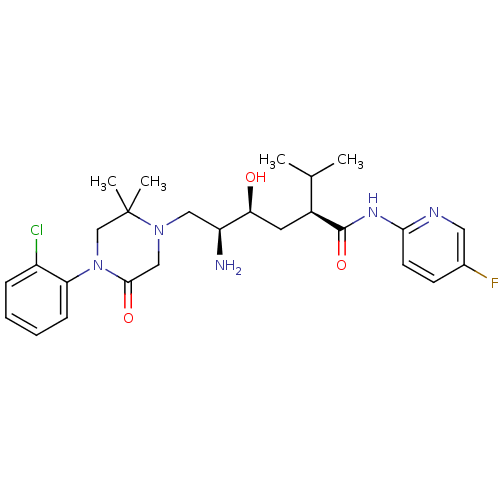 (CHEMBL2387557) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50387262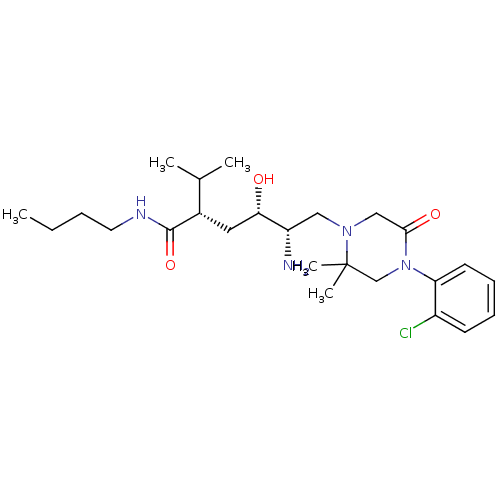 (CHEMBL2048702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434414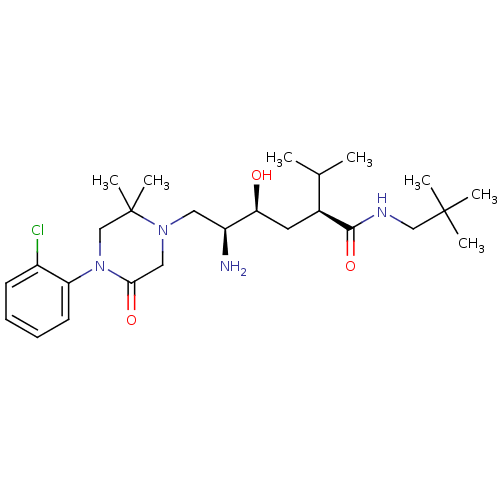 (CHEMBL2387446) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256955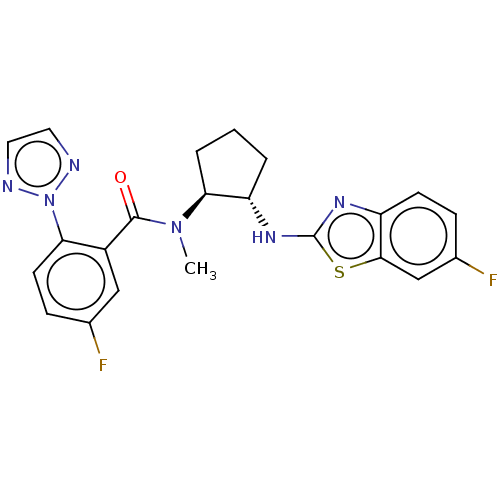 (US9493432, 81) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256957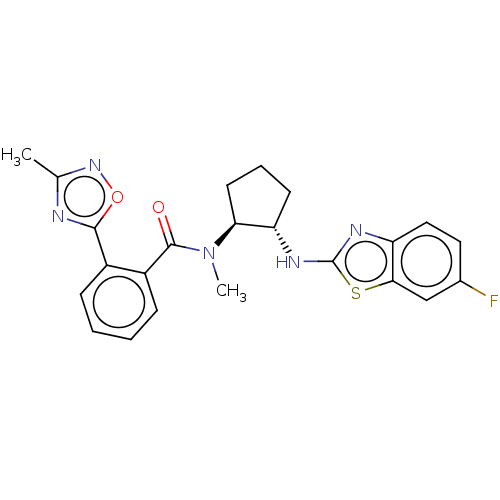 (US9493432, 83) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434412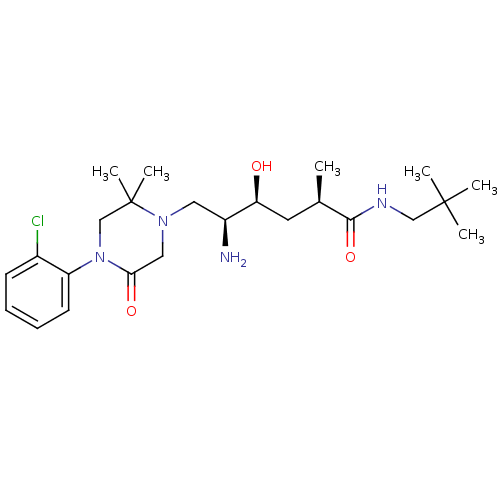 (CHEMBL2387448) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434429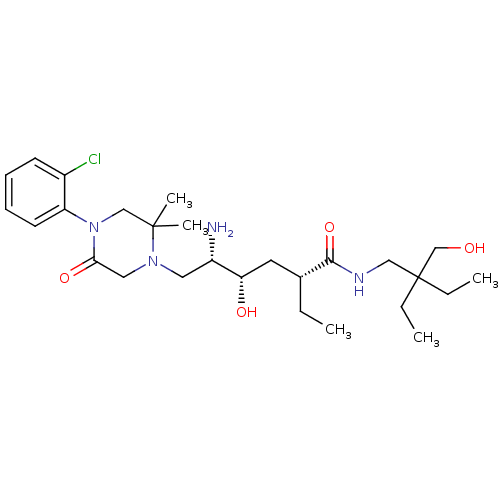 (CHEMBL2387563) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256954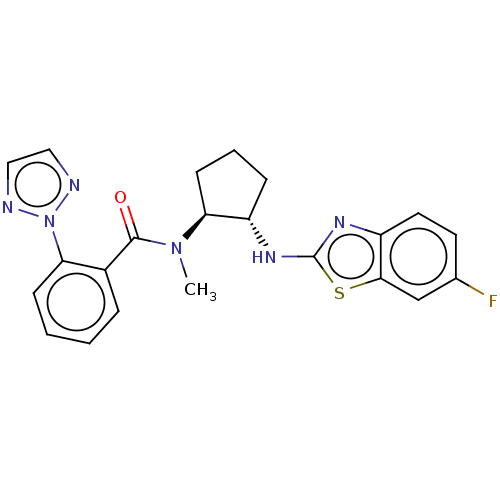 (US9493432, 80) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50439255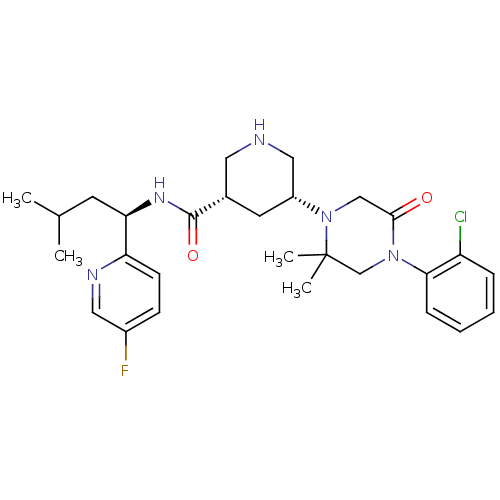 (CHEMBL2419040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM256953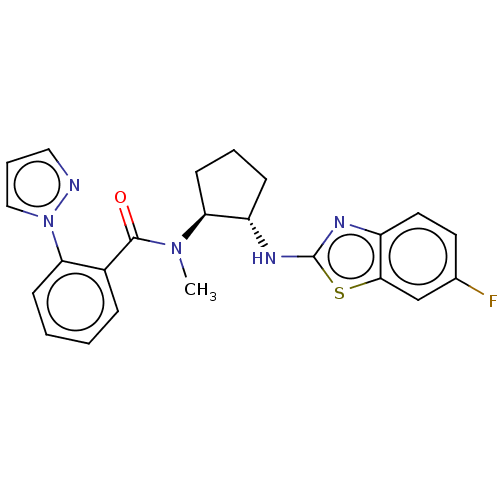 (US9493432, 79) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | 37 |
Takeda Pharmaceuticals Company Limited US Patent | Assay Description Orexin antagonist activity was determined by measuring changes in intracellular calcium levels using a Ca2+ sensitive fluorescent dye. The changes in... | US Patent US9493432 (2016) BindingDB Entry DOI: 10.7270/Q2FQ9VJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434434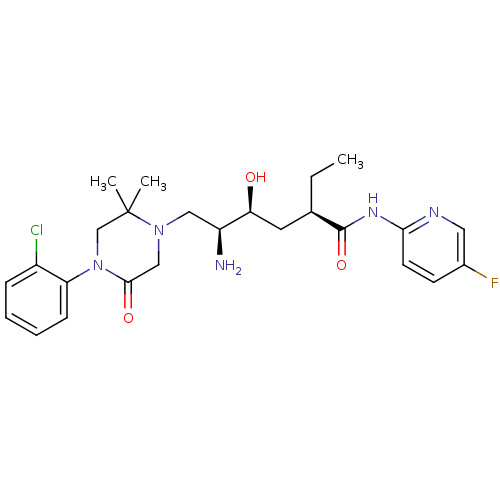 (CHEMBL2387558) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50336806 (1-(3-(aminomethyl)-2-isobutyl-4-p-tolylquinolin-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DPP4 in human Caco2 cells after 60 mins | J Med Chem 54: 831-50 (2012) Article DOI: 10.1021/jm101236h BindingDB Entry DOI: 10.7270/Q2QR4Z4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50333177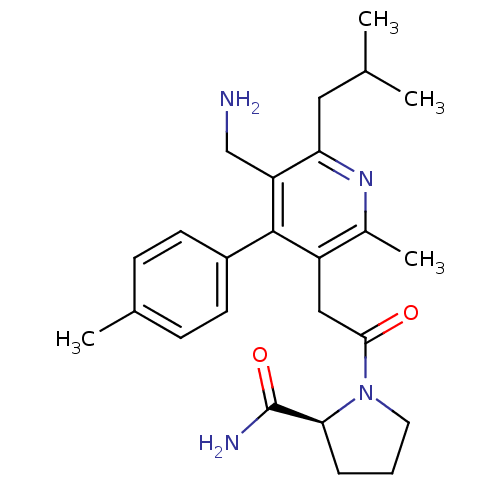 (1-{[5-(Aminomethyl)-2-methyl-4-(4-methylphenyl)-6-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry | Bioorg Med Chem 19: 172-85 (2011) Article DOI: 10.1016/j.bmc.2010.11.038 BindingDB Entry DOI: 10.7270/Q2TB174K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50333175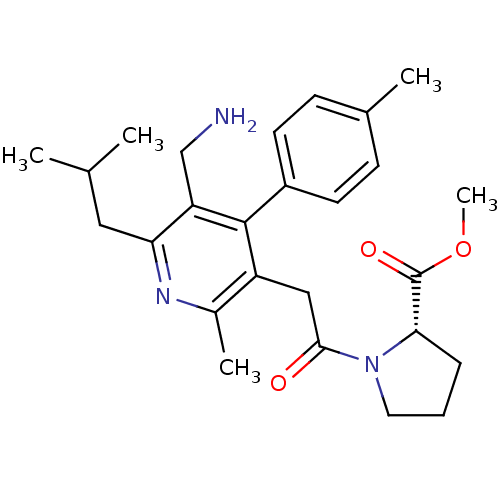 (CHEMBL1644848 | Methyl 1-{[5-(aminomethyl)-2-methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceutical Company Limited Curated by ChEMBL | Assay Description Inhibition of DDP4 in human Caco2 cells after 60 mins by spectrophotometry | Bioorg Med Chem 19: 172-85 (2011) Article DOI: 10.1016/j.bmc.2010.11.038 BindingDB Entry DOI: 10.7270/Q2TB174K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434411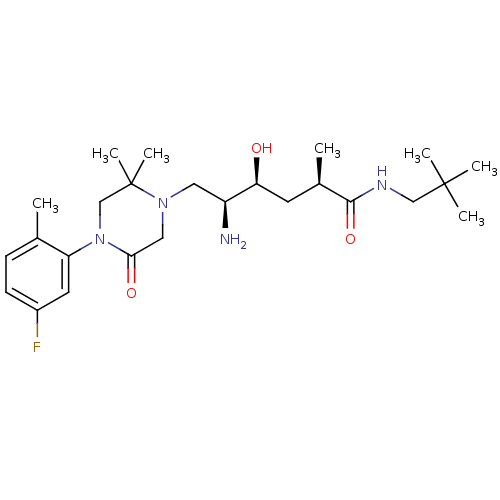 (CHEMBL2387449) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50439253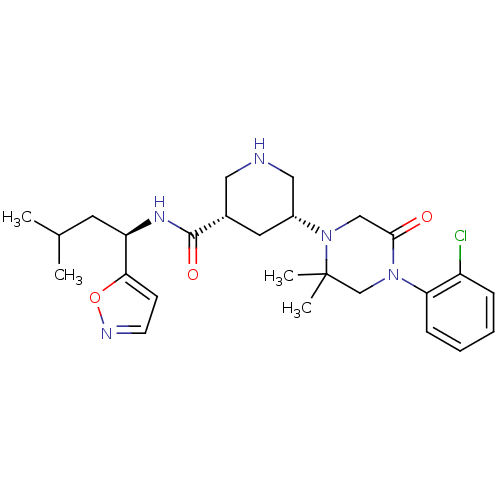 (CHEMBL2419042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434428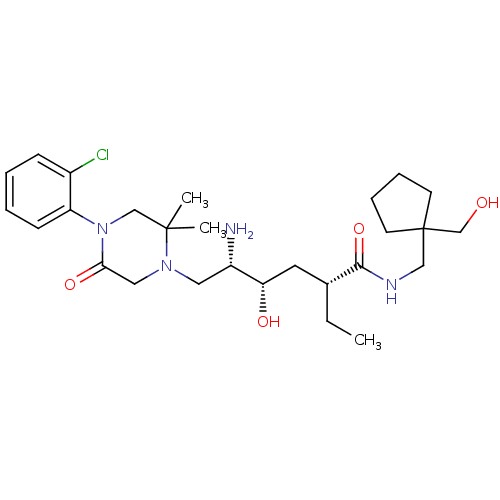 (CHEMBL2387564) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434427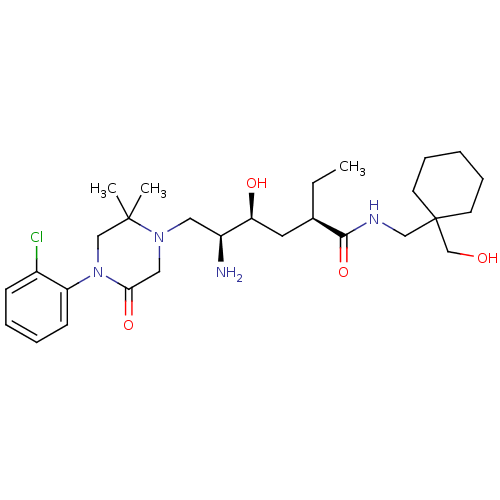 (CHEMBL2387565) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434424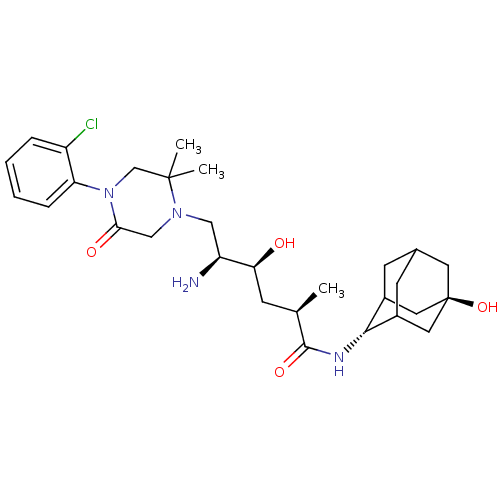 (CHEMBL2387568) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434423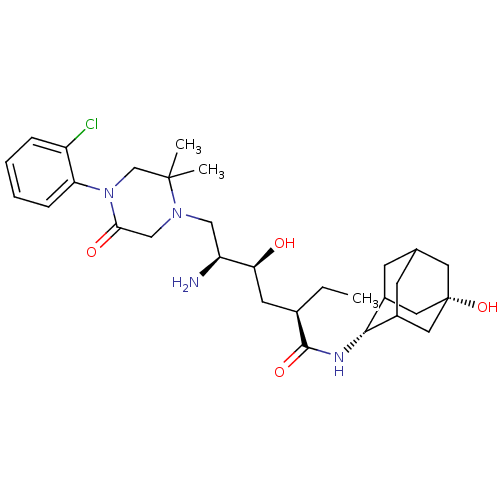 (CHEMBL2387569) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50434421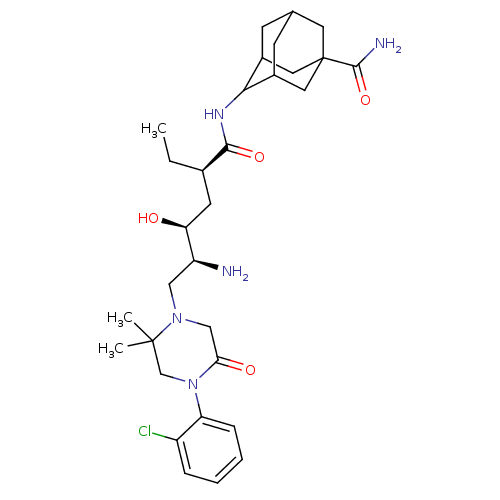 (CHEMBL2387571) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of renin in cynomolgus monkey plasma after 1 hr by RIA | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50439251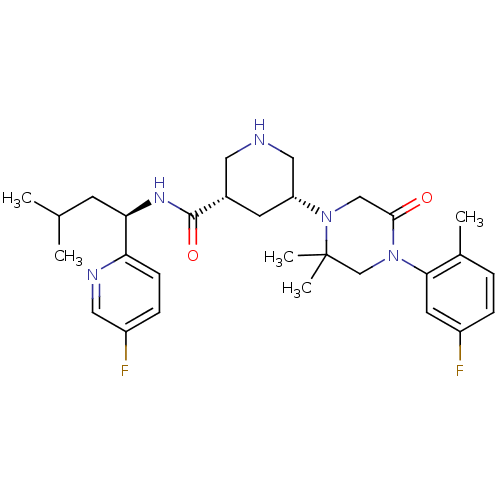 (CHEMBL2419045) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434421 (CHEMBL2387571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402219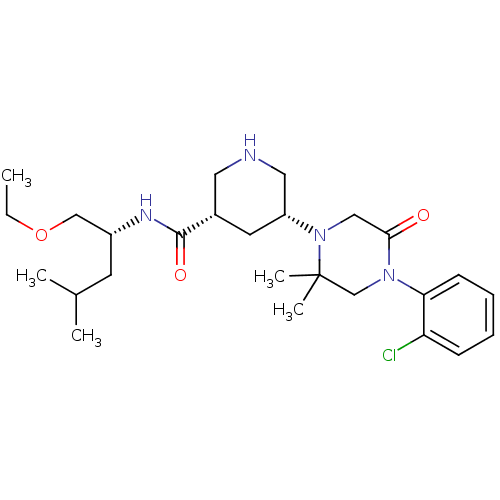 (CHEMBL2204732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434426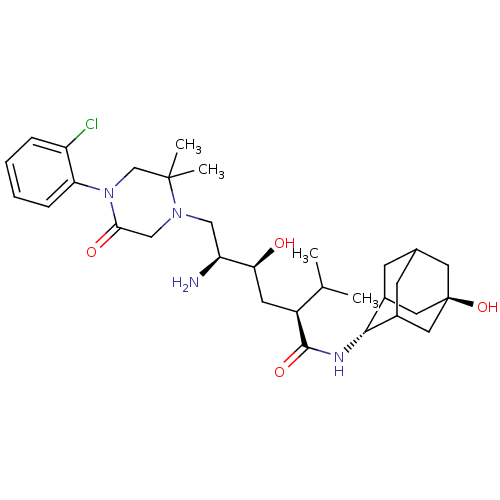 (CHEMBL2387566) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434419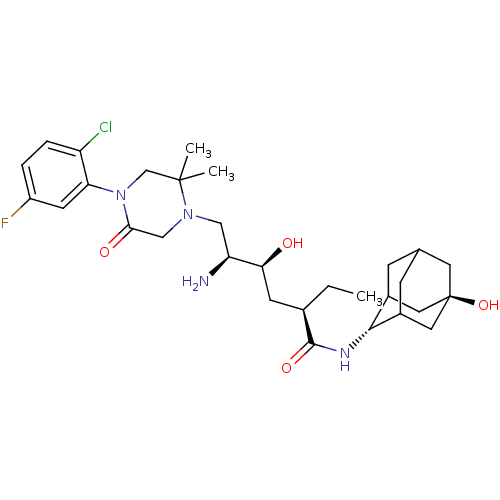 (CHEMBL2387573) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434441 (CHEMBL2387551) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50387268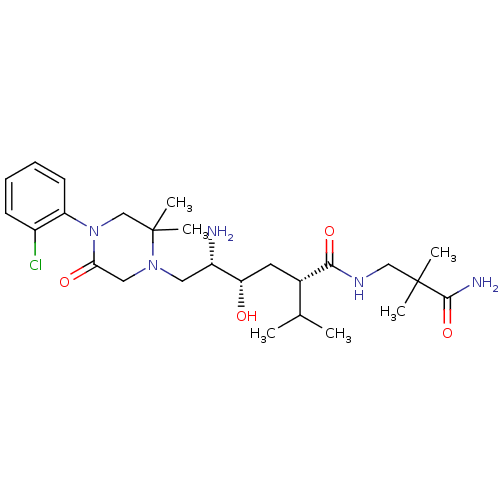 (CHEMBL2048571) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434448 (CHEMBL2387544) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434446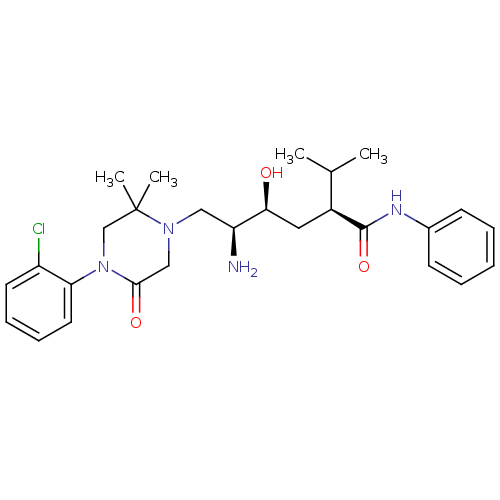 (CHEMBL2387546) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434438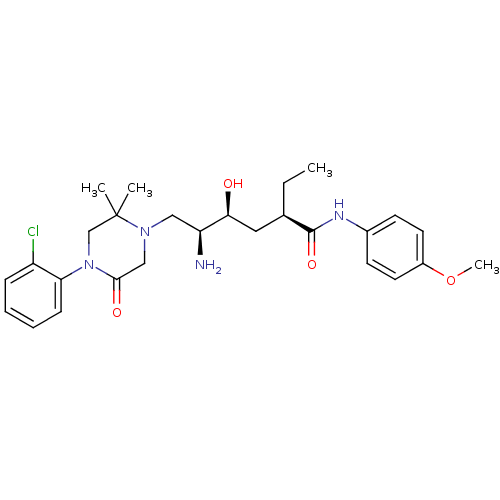 (CHEMBL2387554) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434416 (CHEMBL2387444) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Macaca fascicularis) | BDBM50402219 (CHEMBL2204732) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of cynomolgus monkey plasma renin assessed as angiotensin 1 level after 60 mins by competitive radioimmunoassay | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50439249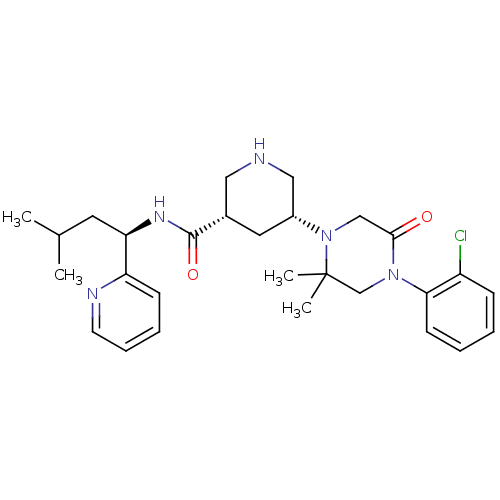 (CHEMBL2419046) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of human recombinant renin compound treated for 10 mins before substrate addition measured after 90 mins by fluorescence method | ACS Med Chem Lett 3: 754-758 (2012) Article DOI: 10.1021/ml300168e BindingDB Entry DOI: 10.7270/Q29S1S41 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50439250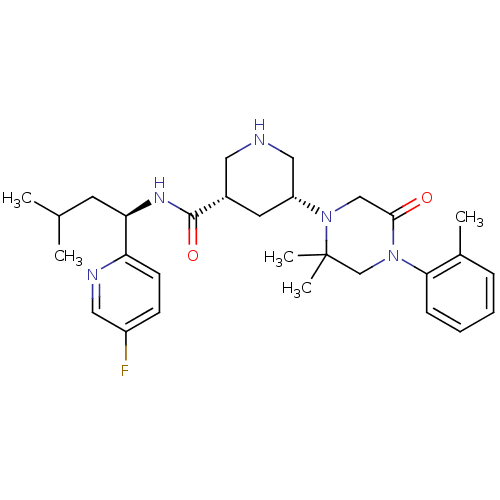 (CHEMBL2419044) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434417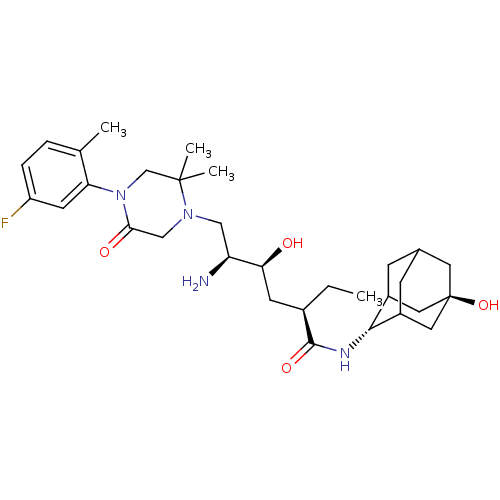 (CHEMBL2387575) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50402224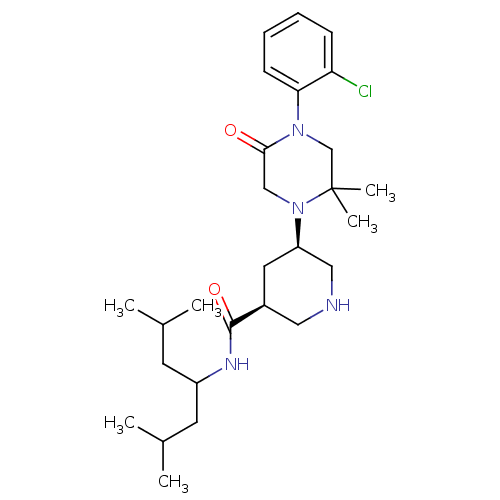 (CHEMBL2204739) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of trypsin-activated human recombinant renin using (Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys(DABCYL)-Arg) as substrate i... | Bioorg Med Chem 21: 5907-22 (2013) Article DOI: 10.1016/j.bmc.2013.06.057 BindingDB Entry DOI: 10.7270/Q2TH8P4B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50434407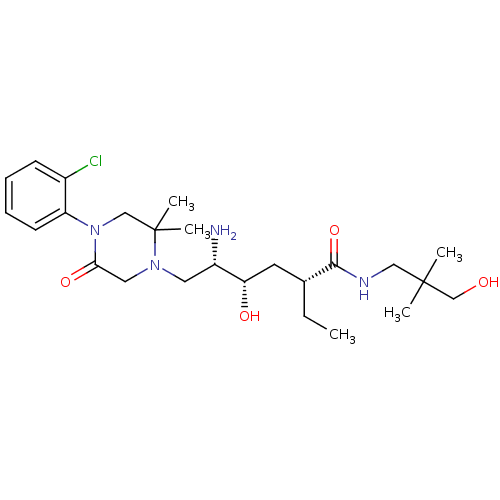 (CHEMBL2387453) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Daiichi Sankyo Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant renin using Arg-Glu(EDANS)-Ile-His-Pro-Phe-His-Leu-Val-Ile-His-Thr-Lys (DABCYL)-Arg as substrate incubated for 10 min... | Bioorg Med Chem 21: 3175-96 (2013) Article DOI: 10.1016/j.bmc.2013.03.022 BindingDB Entry DOI: 10.7270/Q2GT5PJK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2925 total ) | Next | Last >> |