 Found 122 hits with Last Name = 'fujino' and Initial = 'm'
Found 122 hits with Last Name = 'fujino' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melanin-concentrating hormone receptor 1
(RAT) | BDBM50150715
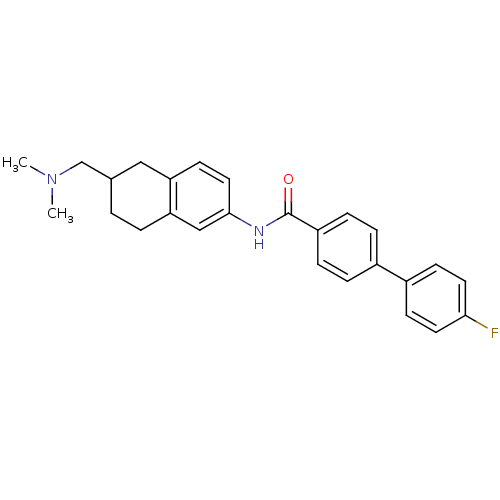
(4''-Fluoro-biphenyl-4-carboxylic acid (6-dimethyla...)Show SMILES CN(C)CC1CCc2cc(NC(=O)c3ccc(cc3)-c3ccc(F)cc3)ccc2C1 Show InChI InChI=1S/C26H27FN2O/c1-29(2)17-18-3-4-23-16-25(14-11-22(23)15-18)28-26(30)21-7-5-19(6-8-21)20-9-12-24(27)13-10-20/h5-14,16,18H,3-4,15,17H2,1-2H3,(H,28,30) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by PDSP Ki Database
| |
Eur J Pharmacol 438: 129-35 (2002)
Article DOI: 10.1016/s0014-2999(02)01314-6
BindingDB Entry DOI: 10.7270/Q2GX495R |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122654

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C36H31F2N5O4S/c1-41(20-23-10-5-3-6-11-23)21-28-31-33(44)43(26-12-7-4-8-13-26)36(46)42(22-27-29(37)14-9-15-30(27)38)34(31)48-32(28)24-16-18-25(19-17-24)39-35(45)40-47-2/h3-19H,20-22H2,1-2H3,(H2,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for inhibition of arachidonic acid(AA) release from CHO cells in Human |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122652
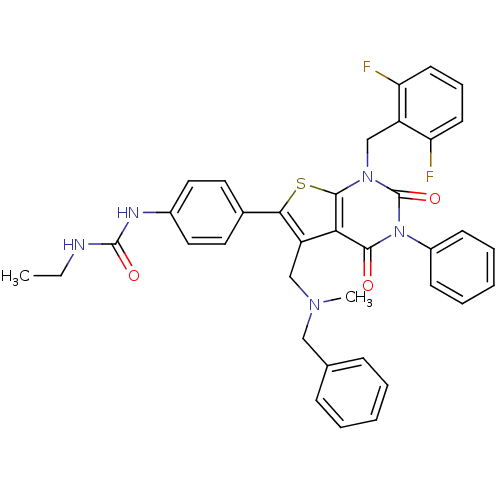
(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H33F2N5O3S/c1-3-40-36(46)41-26-19-17-25(18-20-26)33-29(22-42(2)21-24-11-6-4-7-12-24)32-34(45)44(27-13-8-5-9-14-27)37(47)43(35(32)48-33)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H2,40,41,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for inhibition of arachidonic acid(AA) release from CHO cells in Human |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50043326
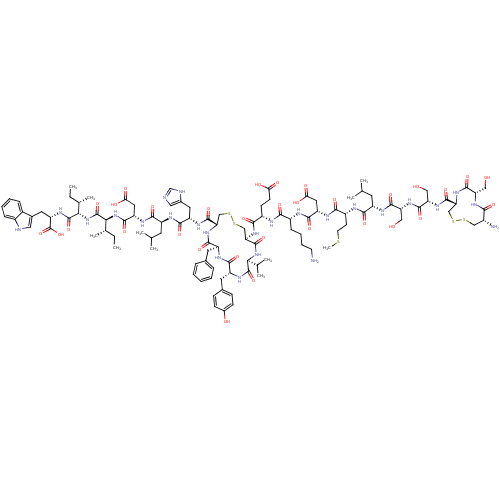
(CHEMBL438733 | c(Cys-Ser-Cys)-Ser-Ser-Leu-Met-Asp-...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC(=O)[C@H]2CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(160)81-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66+,67-,68+,69-,70-,71-,72+,73-,74-,75-,76-,77-,78+,79-,80-,81+,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122652

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H33F2N5O3S/c1-3-40-36(46)41-26-19-17-25(18-20-26)33-29(22-42(2)21-24-11-6-4-7-12-24)32-34(45)44(27-13-8-5-9-14-27)37(47)43(35(32)48-33)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H2,40,41,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122654

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C36H31F2N5O4S/c1-41(20-23-10-5-3-6-11-23)21-28-31-33(44)43(26-12-7-4-8-13-26)36(46)42(22-27-29(37)14-9-15-30(27)38)34(31)48-32(28)24-16-18-25(19-17-24)39-35(45)40-47-2/h3-19H,20-22H2,1-2H3,(H2,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50409694
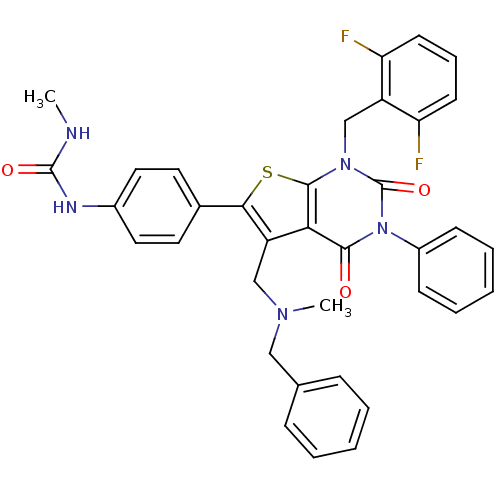
(CHEMBL2092994)Show SMILES CNC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C36H31F2N5O3S/c1-39-35(45)40-25-18-16-24(17-19-25)32-28(21-41(2)20-23-10-5-3-6-11-23)31-33(44)43(26-12-7-4-8-13-26)36(46)42(34(31)47-32)22-27-29(37)14-9-15-30(27)38/h3-19H,20-22H2,1-2H3,(H2,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50043322
(CHEMBL427748 | c(Cys-Ser-c(Cys-Ser-Ser-Leu-Met-Asp...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-57(10)88(108(164)126-77(109(165)166)39-60-43-113-65-23-17-16-22-63(60)65)134-100(156)71(36-55(6)7)119-99(155)76(42-86(143)144)124-94(150)70(35-54(4)5)118-97(153)74(40-61-44-112-52-114-61)122-104(160)81-49-169-168-48-64(111)89(145)127-78(45-135)103(159)132-82-50-170-171-51-83(106(162)133-87(56(8)9)107(163)125-73(38-59-25-27-62(138)28-26-59)95(151)121-72(96(152)131-81)37-58-20-14-13-15-21-58)130-91(147)67(29-30-84(139)140)116-90(146)66(24-18-19-32-110)115-98(154)75(41-85(141)142)123-92(148)68(31-33-167-11)117-93(149)69(34-53(2)3)120-101(157)79(46-136)128-102(158)80(47-137)129-105(82)161/h13-17,20-23,25-28,43-44,52-57,64,66-83,87-88,113,135-138H,12,18-19,24,29-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,155)(H,120,157)(H,121,151)(H,122,160)(H,123,148)(H,124,150)(H,125,163)(H,126,164)(H,127,145)(H,128,158)(H,129,161)(H,130,147)(H,131,152)(H,132,159)(H,133,162)(H,134,156)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t57-,64+,66+,67-,68+,69-,70-,71-,72-,73+,74-,75-,76-,77-,78-,79+,80-,81-,82+,83+,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50368776
(CHEMBL1790962)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)[C@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H161N25O31S5/c1-13-57(10)87(107(162)125-77(109(164)165)40-61-43-113-66-24-18-17-23-64(61)66)133-98(153)72(37-55(6)7)123-108(163)88(58(11)138)134-99(154)71(36-54(4)5)118-96(151)75(41-62-44-112-52-114-62)121-103(158)81-49-168-167-48-65(111)89(144)126-78(45-135)102(157)131-82-50-169-170-51-83(105(160)132-86(56(8)9)106(161)124-74(39-60-26-28-63(139)29-27-60)94(149)120-73(95(150)130-81)38-59-21-15-14-16-22-59)129-91(146)68(30-31-84(140)141)116-90(145)67(25-19-20-33-110)115-97(152)76(42-85(142)143)122-92(147)69(32-34-166-12)117-93(148)70(35-53(2)3)119-100(155)79(46-136)127-101(156)80(47-137)128-104(82)159/h14-18,21-24,26-29,43-44,52-58,65,67-83,86-88,113,135-139H,13,19-20,25,30-42,45-51,110-111H2,1-12H3,(H,112,114)(H,115,152)(H,116,145)(H,117,148)(H,118,151)(H,119,155)(H,120,149)(H,121,158)(H,122,147)(H,123,163)(H,124,161)(H,125,162)(H,126,144)(H,127,156)(H,128,159)(H,129,146)(H,130,150)(H,131,157)(H,132,160)(H,133,153)(H,134,154)(H,140,141)(H,142,143)(H,164,165)/t57-,58-,65+,67+,68-,69+,70?,71-,72-,73-,74+,75-,76-,77-,78-,79+,80-,81-,82+,83+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50368773
(CHEMBL1790959)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)[C@@H](C)O)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C108H159N25O31S5/c1-13-56(10)86(106(161)123-75(108(163)164)39-60-42-112-65-24-18-17-23-63(60)65)132-105(160)85(55(8)9)131-107(162)87(57(11)137)133-97(152)70(36-53(4)5)117-95(150)73(40-61-43-111-51-113-61)120-101(156)79-48-167-166-47-64(110)88(143)124-76(44-134)100(155)129-80-49-168-169-50-81(103(158)130-84(54(6)7)104(159)122-72(38-59-26-28-62(138)29-27-59)93(148)119-71(94(149)128-79)37-58-21-15-14-16-22-58)127-90(145)67(30-31-82(139)140)115-89(144)66(25-19-20-33-109)114-96(151)74(41-83(141)142)121-91(146)68(32-34-165-12)116-92(147)69(35-52(2)3)118-98(153)77(45-135)125-99(154)78(46-136)126-102(80)157/h14-18,21-24,26-29,42-43,51-57,64,66-81,84-87,112,134-138H,13,19-20,25,30-41,44-50,109-110H2,1-12H3,(H,111,113)(H,114,151)(H,115,144)(H,116,147)(H,117,150)(H,118,153)(H,119,148)(H,120,156)(H,121,146)(H,122,159)(H,123,161)(H,124,143)(H,125,154)(H,126,157)(H,127,145)(H,128,149)(H,129,155)(H,130,158)(H,131,162)(H,132,160)(H,133,152)(H,139,140)(H,141,142)(H,163,164)/t56-,57+,64+,66+,67-,68+,69-,70-,71-,72+,73-,74-,75-,76-,77+,78-,79-,80+,81+,84-,85-,86-,87-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50368774

(CHEMBL1790963)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H161N25O31S5/c1-13-56(9)86(106(161)124-76(109(164)165)40-61-43-113-66-25-19-18-24-64(61)66)132-107(162)87(57(10)14-2)133-108(163)88(58(11)138)134-98(153)71(37-54(5)6)118-96(151)74(41-62-44-112-52-114-62)121-102(157)80-49-168-167-48-65(111)89(144)125-77(45-135)101(156)130-81-50-169-170-51-82(104(159)131-85(55(7)8)105(160)123-73(39-60-27-29-63(139)30-28-60)94(149)120-72(95(150)129-80)38-59-22-16-15-17-23-59)128-91(146)68(31-32-83(140)141)116-90(145)67(26-20-21-34-110)115-97(152)75(42-84(142)143)122-92(147)69(33-35-166-12)117-93(148)70(36-53(3)4)119-99(154)78(46-136)126-100(155)79(47-137)127-103(81)158/h15-19,22-25,27-30,43-44,52-58,65,67-82,85-88,113,135-139H,13-14,20-21,26,31-42,45-51,110-111H2,1-12H3,(H,112,114)(H,115,152)(H,116,145)(H,117,148)(H,118,151)(H,119,154)(H,120,149)(H,121,157)(H,122,147)(H,123,160)(H,124,161)(H,125,144)(H,126,155)(H,127,158)(H,128,146)(H,129,150)(H,130,156)(H,131,159)(H,132,162)(H,133,163)(H,134,153)(H,140,141)(H,142,143)(H,164,165)/t56-,57-,58+,65+,67+,68-,69+,70-,71-,72-,73+,74-,75-,76-,77-,78+,79-,80-,81+,82+,85-,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50067485
(3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...)Show SMILES CC(C)OC(=O)c1cn(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1 Show InChI InChI=1S/C37H37F2N3O4S/c1-22(2)35(44)40-26-16-14-25(15-17-26)34-28(19-41(5)18-24-10-7-6-8-11-24)32-33(43)29(37(45)46-23(3)4)21-42(36(32)47-34)20-27-30(38)12-9-13-31(27)39/h6-17,21-23H,18-20H2,1-5H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50067485

(3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...)Show SMILES CC(C)OC(=O)c1cn(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1 Show InChI InChI=1S/C37H37F2N3O4S/c1-22(2)35(44)40-26-16-14-25(15-17-26)34-28(19-41(5)18-24-10-7-6-8-11-24)32-33(43)29(37(45)46-23(3)4)21-42(36(32)47-34)20-27-30(38)12-9-13-31(27)39/h6-17,21-23H,18-20H2,1-5H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
The Compound was tested for the concentration to inhibit 50% of [125 I ]leuprorelin binding to the cloned human Leutinizing releasing hormone recepto... |
J Med Chem 41: 4190-5 (1998)
Article DOI: 10.1021/jm9803673
BindingDB Entry DOI: 10.7270/Q2CR5V19 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122660
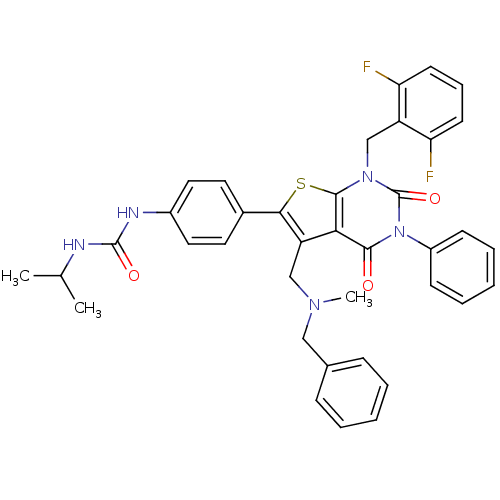
(1-{4-[5-[(Benzyl-methyl-amino)-methyl]-1-(2,6-difl...)Show SMILES CC(C)NC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C38H35F2N5O3S/c1-24(2)41-37(47)42-27-19-17-26(18-20-27)34-30(22-43(3)21-25-11-6-4-7-12-25)33-35(46)45(28-13-8-5-9-14-28)38(48)44(36(33)49-34)23-29-31(39)15-10-16-32(29)40/h4-20,24H,21-23H2,1-3H3,(H2,41,42,47) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122659
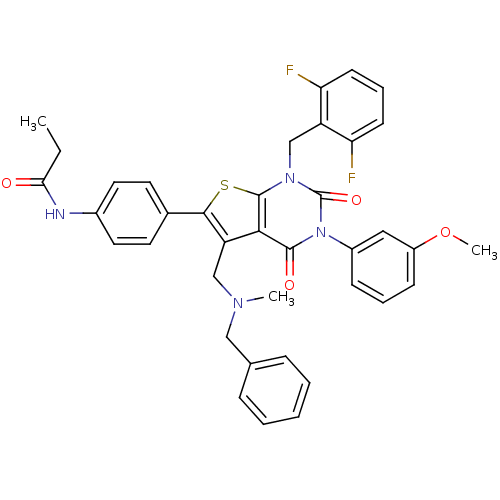
(CHEMBL20511 | N-{4-[5-[(Benzyl-methyl-amino)-methy...)Show SMILES CCC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3cccc(OC)c3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C38H34F2N4O4S/c1-4-33(45)41-26-18-16-25(17-19-26)35-30(22-42(2)21-24-10-6-5-7-11-24)34-36(46)44(27-12-8-13-28(20-27)48-3)38(47)43(37(34)49-35)23-29-31(39)14-9-15-32(29)40/h5-20H,4,21-23H2,1-3H3,(H,41,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50368775
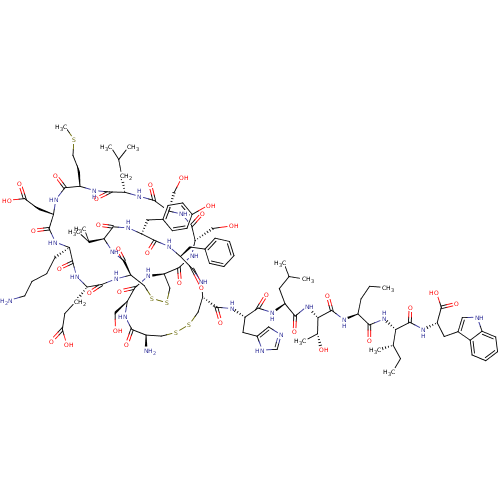
(CHEMBL1790960)Show SMILES CCC[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)[C@@H](C)O)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C108H159N25O31S5/c1-12-21-66(92(147)132-86(56(9)13-2)106(161)124-76(108(163)164)40-60-43-112-65-25-18-17-24-63(60)65)117-107(162)87(57(10)137)133-98(153)71(37-54(5)6)118-96(151)74(41-61-44-111-52-113-61)121-102(157)80-49-167-166-48-64(110)88(143)125-77(45-134)101(156)130-81-50-168-169-51-82(104(159)131-85(55(7)8)105(160)123-73(39-59-27-29-62(138)30-28-59)94(149)120-72(95(150)129-80)38-58-22-15-14-16-23-58)128-90(145)68(31-32-83(139)140)115-89(144)67(26-19-20-34-109)114-97(152)75(42-84(141)142)122-91(146)69(33-35-165-11)116-93(148)70(36-53(3)4)119-99(154)78(46-135)126-100(155)79(47-136)127-103(81)158/h14-18,22-25,27-30,43-44,52-57,64,66-82,85-87,112,134-138H,12-13,19-21,26,31-42,45-51,109-110H2,1-11H3,(H,111,113)(H,114,152)(H,115,144)(H,116,148)(H,117,162)(H,118,151)(H,119,154)(H,120,149)(H,121,157)(H,122,146)(H,123,160)(H,124,161)(H,125,143)(H,126,155)(H,127,158)(H,128,145)(H,129,150)(H,130,156)(H,131,159)(H,132,147)(H,133,153)(H,139,140)(H,141,142)(H,163,164)/t56-,57+,64+,66-,67+,68-,69+,70-,71-,72-,73+,74-,75-,76-,77-,78+,79-,80-,81+,82+,85-,86-,87-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122667
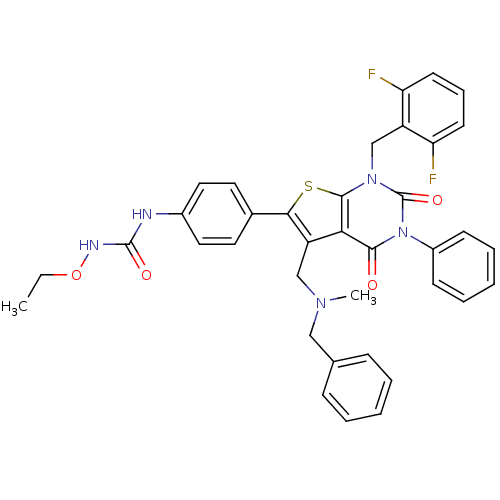
(1-{4-[5-[(Benzyl-methyl-amino)-methyl]-1-(2,6-difl...)Show SMILES CCONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H33F2N5O4S/c1-3-48-41-36(46)40-26-19-17-25(18-20-26)33-29(22-42(2)21-24-11-6-4-7-12-24)32-34(45)44(27-13-8-5-9-14-27)37(47)43(35(32)49-33)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H2,40,41,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50368772
(CHEMBL2373291)Show SMILES [H][C@@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC1=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C112H165N25O31S5/c1-11-59(8)90(110(165)128-80(112(167)168)43-64-46-116-69-27-19-18-26-67(64)69)136-102(157)77(41-62-24-16-13-17-25-62)127-111(166)91(60(9)141)137-101(156)74(39-57(4)5)121-99(154)78(44-65-47-115-55-117-65)124-106(161)84-52-171-170-51-68(114)92(147)129-81(48-138)105(160)134-85-53-172-173-54-86(108(163)135-89(58(6)7)109(164)126-76(42-63-29-31-66(142)32-30-63)97(152)123-75(98(153)133-84)40-61-22-14-12-15-23-61)132-94(149)71(33-34-87(143)144)119-93(148)70(28-20-21-36-113)118-100(155)79(45-88(145)146)125-95(150)72(35-37-169-10)120-96(151)73(38-56(2)3)122-103(158)82(49-139)130-104(159)83(50-140)131-107(85)162/h12,14-15,18-19,22-23,26-27,29-32,46-47,55-60,62,68,70-86,89-91,116,138-142H,11,13,16-17,20-21,24-25,28,33-45,48-54,113-114H2,1-10H3,(H,115,117)(H,118,155)(H,119,148)(H,120,151)(H,121,154)(H,122,158)(H,123,152)(H,124,161)(H,125,150)(H,126,164)(H,127,166)(H,128,165)(H,129,147)(H,130,159)(H,131,162)(H,132,149)(H,133,153)(H,134,160)(H,135,163)(H,136,157)(H,137,156)(H,143,144)(H,145,146)(H,167,168)/t59-,60+,68+,70+,71-,72+,73-,74-,75-,76+,77-,78-,79?,80-,81-,82+,83-,84-,85+,86-,89-,90-,91-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122656
(CHEMBL280365 | N-{4-[5-[(Benzyl-methyl-amino)-meth...)Show SMILES COc1cccc(c1)-n1c(=O)n(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1 Show InChI InChI=1S/C39H36F2N4O4S/c1-24(2)36(46)42-27-18-16-26(17-19-27)35-31(22-43(3)21-25-10-6-5-7-11-25)34-37(47)45(28-12-8-13-29(20-28)49-4)39(48)44(38(34)50-35)23-30-32(40)14-9-15-33(30)41/h5-20,24H,21-23H2,1-4H3,(H,42,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50115988
(3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OC(C)C)c(=O)n2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C35H36F2N6O4/c1-5-38-34(46)39-25-16-14-24(15-17-25)31-30(21-41(4)18-23-10-7-6-8-11-23)43-32(44)27(33(45)47-22(2)3)20-42(35(43)40-31)19-26-28(36)12-9-13-29(26)37/h6-17,20,22H,5,18-19,21H2,1-4H3,(H2,38,39,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells |
Bioorg Med Chem Lett 12: 2073-7 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G32 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50369395
(ELIGARD | LEUPROLIDE)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
The Compound was tested for the concentration to inhibit 50% of [125 I ]leuprorelin binding to the cloned human Leutinizing releasing hormone recepto... |
J Med Chem 41: 4190-5 (1998)
Article DOI: 10.1021/jm9803673
BindingDB Entry DOI: 10.7270/Q2CR5V19 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122657
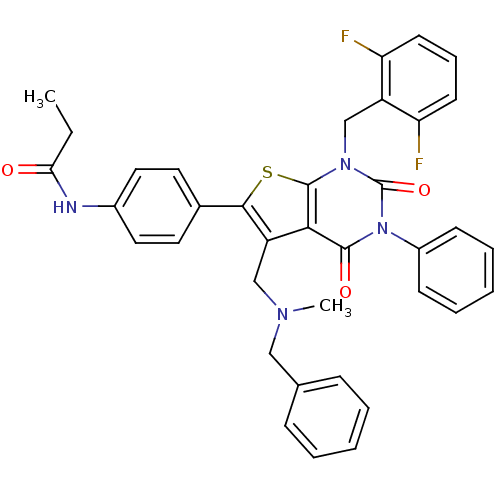
(CHEMBL21126 | N-{4-[5-[(Benzyl-methyl-amino)-methy...)Show SMILES CCC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H32F2N4O3S/c1-3-32(44)40-26-19-17-25(18-20-26)34-29(22-41(2)21-24-11-6-4-7-12-24)33-35(45)43(27-13-8-5-9-14-27)37(46)42(36(33)47-34)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122652

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C37H33F2N5O3S/c1-3-40-36(46)41-26-19-17-25(18-20-26)33-29(22-42(2)21-24-11-6-4-7-12-24)32-34(45)44(27-13-8-5-9-14-27)37(47)43(35(32)48-33)23-28-30(38)15-10-16-31(28)39/h4-20H,3,21-23H2,1-2H3,(H2,40,41,46) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for antagonist concentration required to inhibit specific binding of [125I]leuprorelin to Leutinizing releasing hormone receptor in monkey (ch... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50368774

(CHEMBL1790963)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H161N25O31S5/c1-13-56(9)86(106(161)124-76(109(164)165)40-61-43-113-66-25-19-18-24-64(61)66)132-107(162)87(57(10)14-2)133-108(163)88(58(11)138)134-98(153)71(37-54(5)6)118-96(151)74(41-62-44-112-52-114-62)121-102(157)80-49-168-167-48-65(111)89(144)125-77(45-135)101(156)130-81-50-169-170-51-82(104(159)131-85(55(7)8)105(160)123-73(39-60-27-29-63(139)30-28-60)94(149)120-72(95(150)129-80)38-59-22-16-15-17-23-59)128-91(146)68(31-32-83(140)141)116-90(145)67(26-20-21-34-110)115-97(152)75(42-84(142)143)122-92(147)69(33-35-166-12)117-93(148)70(36-53(3)4)119-99(154)78(46-136)126-100(155)79(47-137)127-103(81)158/h15-19,22-25,27-30,43-44,52-58,65,67-82,85-88,113,135-139H,13-14,20-21,26,31-42,45-51,110-111H2,1-12H3,(H,112,114)(H,115,152)(H,116,145)(H,117,148)(H,118,151)(H,119,154)(H,120,149)(H,121,157)(H,122,147)(H,123,160)(H,124,161)(H,125,144)(H,126,155)(H,127,158)(H,128,146)(H,129,150)(H,130,156)(H,131,159)(H,132,162)(H,133,163)(H,134,153)(H,140,141)(H,142,143)(H,164,165)/t56-,57-,58+,65+,67+,68-,69+,70-,71-,72-,73+,74-,75-,76-,77-,78+,79-,80-,81+,82+,85-,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122671
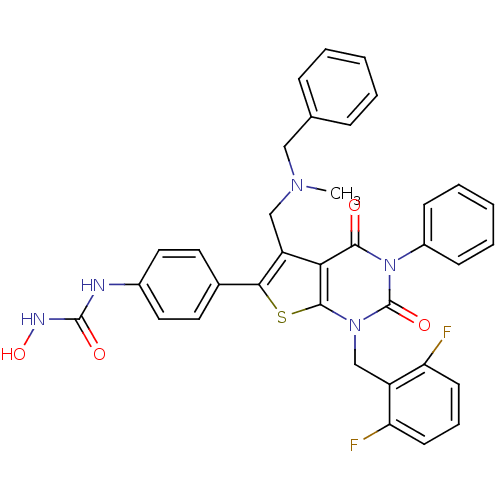
(1-{4-[5-[(Benzyl-methyl-amino)-methyl]-1-(2,6-difl...)Show SMILES CN(Cc1c(sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c12)-c1ccc(NC(=O)NO)cc1)Cc1ccccc1 Show InChI InChI=1S/C35H29F2N5O4S/c1-40(19-22-9-4-2-5-10-22)20-27-30-32(43)42(25-11-6-3-7-12-25)35(45)41(21-26-28(36)13-8-14-29(26)37)33(30)47-31(27)23-15-17-24(18-16-23)38-34(44)39-46/h2-18,46H,19-21H2,1H3,(H2,38,39,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50115989
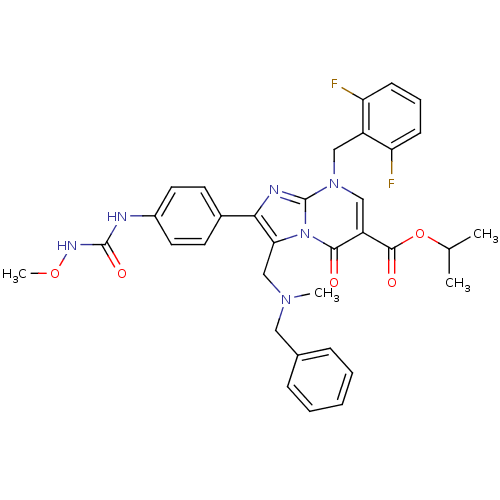
(3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...)Show SMILES CONC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OC(C)C)c(=O)n2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C34H34F2N6O5/c1-21(2)47-32(44)26-19-41(18-25-27(35)11-8-12-28(25)36)34-38-30(23-13-15-24(16-14-23)37-33(45)39-46-4)29(42(34)31(26)43)20-40(3)17-22-9-6-5-7-10-22/h5-16,19,21H,17-18,20H2,1-4H3,(H2,37,39,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells |
Bioorg Med Chem Lett 12: 2073-7 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G32 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122674
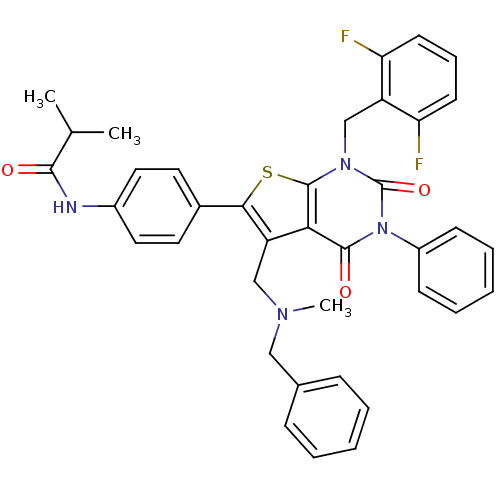
(CHEMBL280213 | N-{4-[5-[(Benzyl-methyl-amino)-meth...)Show SMILES CC(C)C(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C38H34F2N4O3S/c1-24(2)35(45)41-27-19-17-26(18-20-27)34-30(22-42(3)21-25-11-6-4-7-12-25)33-36(46)44(28-13-8-5-9-14-28)38(47)43(37(33)48-34)23-29-31(39)15-10-16-32(29)40/h4-20,24H,21-23H2,1-3H3,(H,41,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50368777
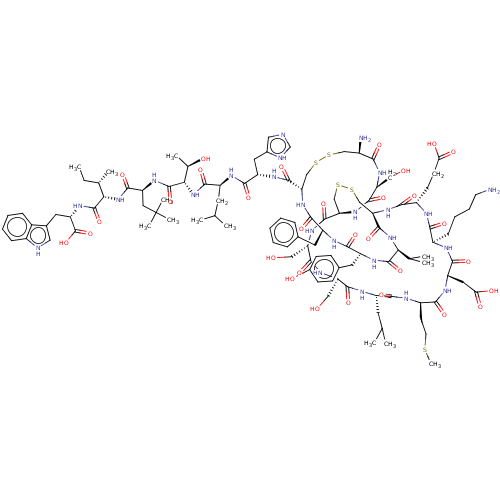
(CHEMBL2373292)Show SMILES [H][C@@]12CSSC[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC1=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C110H163N25O31S5/c1-14-57(8)87(107(163)125-76(109(165)166)40-61-44-114-66-25-19-18-24-64(61)66)134-99(155)77(43-110(10,11)12)126-108(164)88(58(9)139)135-98(154)71(37-55(4)5)119-96(152)74(41-62-45-113-53-115-62)122-103(159)81-50-169-168-49-65(112)89(145)127-78(46-136)102(158)132-82-51-170-171-52-83(105(161)133-86(56(6)7)106(162)124-73(39-60-27-29-63(140)30-28-60)94(150)121-72(95(151)131-81)38-59-22-16-15-17-23-59)130-91(147)68(31-32-84(141)142)117-90(146)67(26-20-21-34-111)116-97(153)75(42-85(143)144)123-92(148)69(33-35-167-13)118-93(149)70(36-54(2)3)120-100(156)79(47-137)128-101(157)80(48-138)129-104(82)160/h15-19,22-25,27-30,44-45,53-58,65,67-83,86-88,114,136-140H,14,20-21,26,31-43,46-52,111-112H2,1-13H3,(H,113,115)(H,116,153)(H,117,146)(H,118,149)(H,119,152)(H,120,156)(H,121,150)(H,122,159)(H,123,148)(H,124,162)(H,125,163)(H,126,164)(H,127,145)(H,128,157)(H,129,160)(H,130,147)(H,131,151)(H,132,158)(H,133,161)(H,134,155)(H,135,154)(H,141,142)(H,143,144)(H,165,166)/t57-,58+,65+,67+,68-,69+,70?,71-,72-,73+,74-,75-,76-,77-,78-,79+,80-,81-,82+,83+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50368776

(CHEMBL1790962)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)[C@H](C)O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H161N25O31S5/c1-13-57(10)87(107(162)125-77(109(164)165)40-61-43-113-66-24-18-17-23-64(61)66)133-98(153)72(37-55(6)7)123-108(163)88(58(11)138)134-99(154)71(36-54(4)5)118-96(151)75(41-62-44-112-52-114-62)121-103(158)81-49-168-167-48-65(111)89(144)126-78(45-135)102(157)131-82-50-169-170-51-83(105(160)132-86(56(8)9)106(161)124-74(39-60-26-28-63(139)29-27-60)94(149)120-73(95(150)130-81)38-59-21-15-14-16-22-59)129-91(146)68(30-31-84(140)141)116-90(145)67(25-19-20-33-110)115-97(152)76(42-85(142)143)122-92(147)69(32-34-166-12)117-93(148)70(35-53(2)3)119-100(155)79(46-136)127-101(156)80(47-137)128-104(82)159/h14-18,21-24,26-29,43-44,52-58,65,67-83,86-88,113,135-139H,13,19-20,25,30-42,45-51,110-111H2,1-12H3,(H,112,114)(H,115,152)(H,116,145)(H,117,148)(H,118,151)(H,119,155)(H,120,149)(H,121,158)(H,122,147)(H,123,163)(H,124,161)(H,125,162)(H,126,144)(H,127,156)(H,128,159)(H,129,146)(H,130,150)(H,131,157)(H,132,160)(H,133,153)(H,134,154)(H,140,141)(H,142,143)(H,164,165)/t57-,58-,65+,67+,68-,69+,70?,71-,72-,73-,74+,75-,76-,77-,78-,79+,80-,81-,82+,83+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(Bos taurus) | BDBM50043324
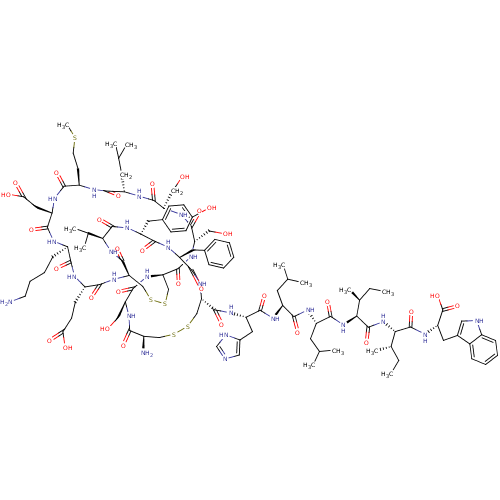
(CHEMBL411171 | c(Cys-Ser-c(Cys-Ser-Ser-Leu-Met-Asp...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C111H165N25O30S5/c1-14-59(11)89(109(163)127-79(111(165)166)42-63-45-115-68-26-20-19-25-66(63)68)136-110(164)90(60(12)15-2)135-101(155)74(39-57(7)8)121-96(150)73(38-56(5)6)120-99(153)77(43-64-46-114-54-116-64)124-105(159)83-51-169-168-50-67(113)91(145)128-80(47-137)104(158)133-84-52-170-171-53-85(107(161)134-88(58(9)10)108(162)126-76(41-62-28-30-65(140)31-29-62)97(151)123-75(98(152)132-83)40-61-23-17-16-18-24-61)131-93(147)70(32-33-86(141)142)118-92(146)69(27-21-22-35-112)117-100(154)78(44-87(143)144)125-94(148)71(34-36-167-13)119-95(149)72(37-55(3)4)122-102(156)81(48-138)129-103(157)82(49-139)130-106(84)160/h16-20,23-26,28-31,45-46,54-60,67,69-85,88-90,115,137-140H,14-15,21-22,27,32-44,47-53,112-113H2,1-13H3,(H,114,116)(H,117,154)(H,118,146)(H,119,149)(H,120,153)(H,121,150)(H,122,156)(H,123,151)(H,124,159)(H,125,148)(H,126,162)(H,127,163)(H,128,145)(H,129,157)(H,130,160)(H,131,147)(H,132,152)(H,133,158)(H,134,161)(H,135,155)(H,136,164)(H,141,142)(H,143,144)(H,165,166)/t59-,60-,67+,69+,70-,71+,72-,73-,74-,75-,76+,77-,78-,79-,80-,81+,82-,83-,84+,85+,88-,89-,90-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in bovine cerebrum membrane for Endothelin B receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50369395

(ELIGARD | LEUPROLIDE)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested for the concentration to inhibit 50% of [125 I]leuprorelin binding to Leutinizing releasing hormone receptor in the membrane ... |
J Med Chem 41: 4190-5 (1998)
Article DOI: 10.1021/jm9803673
BindingDB Entry DOI: 10.7270/Q2CR5V19 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50115998

(3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...)Show SMILES CCNC(=O)Nc1ccc(cc1)-c1nc2n(Cc3c(F)cccc3F)cc(C(=O)OCC)c(=O)n2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C34H34F2N6O4/c1-4-37-33(45)38-24-16-14-23(15-17-24)30-29(21-40(3)18-22-10-7-6-8-11-22)42-31(43)26(32(44)46-5-2)20-41(34(42)39-30)19-25-27(35)12-9-13-28(25)36/h6-17,20H,4-5,18-19,21H2,1-3H3,(H2,37,38,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells |
Bioorg Med Chem Lett 12: 2073-7 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G32 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50067485

(3-[(Benzyl-methyl-amino)-methyl]-7-(2,6-difluoro-b...)Show SMILES CC(C)OC(=O)c1cn(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(NC(=O)C(C)C)cc1 Show InChI InChI=1S/C37H37F2N3O4S/c1-22(2)35(44)40-26-16-14-25(15-17-26)34-28(19-41(5)18-24-10-7-6-8-11-24)32-33(43)29(37(45)46-23(3)4)21-42(36(32)47-34)20-27-30(38)12-9-13-31(27)39/h6-17,21-23H,18-20H2,1-5H3,(H,40,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of LHRH-stimulated arachidonic acid (AA) release from CHO cells expressing human Leutinizing releasing hormone receptor |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122661

(1-{4-[5-[(Benzyl-methyl-amino)-methyl]-1-(2,6-difl...)Show SMILES CC(C)ONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C38H35F2N5O4S/c1-24(2)49-42-37(47)41-27-19-17-26(18-20-27)34-30(22-43(3)21-25-11-6-4-7-12-25)33-35(46)45(28-13-8-5-9-14-28)38(48)44(36(33)50-34)23-29-31(39)15-10-16-32(29)40/h4-20,24H,21-23H2,1-3H3,(H2,41,42,47) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122654

(1-(4-(1-(2,6-difluorobenzyl)-5-((benzyl(methyl)ami...)Show SMILES CONC(=O)Nc1ccc(cc1)-c1sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c2c1CN(C)Cc1ccccc1 Show InChI InChI=1S/C36H31F2N5O4S/c1-41(20-23-10-5-3-6-11-23)21-28-31-33(44)43(26-12-7-4-8-13-26)36(46)42(22-27-29(37)14-9-15-30(27)38)34(31)48-32(28)24-16-18-25(19-17-24)39-35(45)40-47-2/h3-19H,20-22H2,1-2H3,(H2,39,40,45) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for antagonist concentration required to inhibit specific binding of [125I]leuprorelin to LHRH receptor in monkey (chinese hamster ovary (CHO)... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50368777

(CHEMBL2373292)Show SMILES [H][C@@]12CSSC[C@@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC1=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC(C)(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C110H163N25O31S5/c1-14-57(8)87(107(163)125-76(109(165)166)40-61-44-114-66-25-19-18-24-64(61)66)134-99(155)77(43-110(10,11)12)126-108(164)88(58(9)139)135-98(154)71(37-55(4)5)119-96(152)74(41-62-45-113-53-115-62)122-103(159)81-50-169-168-49-65(112)89(145)127-78(46-136)102(158)132-82-51-170-171-52-83(105(161)133-86(56(6)7)106(162)124-73(39-60-27-29-63(140)30-28-60)94(150)121-72(95(151)131-81)38-59-22-16-15-17-23-59)130-91(147)68(31-32-84(141)142)117-90(146)67(26-20-21-34-111)116-97(153)75(42-85(143)144)123-92(148)69(33-35-167-13)118-93(149)70(36-54(2)3)120-100(156)79(47-137)128-101(157)80(48-138)129-104(82)160/h15-19,22-25,27-30,44-45,53-58,65,67-83,86-88,114,136-140H,14,20-21,26,31-43,46-52,111-112H2,1-13H3,(H,113,115)(H,116,153)(H,117,146)(H,118,149)(H,119,152)(H,120,156)(H,121,150)(H,122,159)(H,123,148)(H,124,162)(H,125,163)(H,126,164)(H,127,145)(H,128,157)(H,129,160)(H,130,147)(H,131,151)(H,132,158)(H,133,161)(H,134,155)(H,135,154)(H,141,142)(H,143,144)(H,165,166)/t57-,58+,65+,67+,68-,69+,70?,71-,72-,73+,74-,75-,76-,77-,78-,79+,80-,81-,82+,83+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50368772

(CHEMBL2373291)Show SMILES [H][C@@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC1=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1c[nH]cn1)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C112H165N25O31S5/c1-11-59(8)90(110(165)128-80(112(167)168)43-64-46-116-69-27-19-18-26-67(64)69)136-102(157)77(41-62-24-16-13-17-25-62)127-111(166)91(60(9)141)137-101(156)74(39-57(4)5)121-99(154)78(44-65-47-115-55-117-65)124-106(161)84-52-171-170-51-68(114)92(147)129-81(48-138)105(160)134-85-53-172-173-54-86(108(163)135-89(58(6)7)109(164)126-76(42-63-29-31-66(142)32-30-63)97(152)123-75(98(153)133-84)40-61-22-14-12-15-23-61)132-94(149)71(33-34-87(143)144)119-93(148)70(28-20-21-36-113)118-100(155)79(45-88(145)146)125-95(150)72(35-37-169-10)120-96(151)73(38-56(2)3)122-103(158)82(49-139)130-104(159)83(50-140)131-107(85)162/h12,14-15,18-19,22-23,26-27,29-32,46-47,55-60,62,68,70-86,89-91,116,138-142H,11,13,16-17,20-21,24-25,28,33-45,48-54,113-114H2,1-10H3,(H,115,117)(H,118,155)(H,119,148)(H,120,151)(H,121,154)(H,122,158)(H,123,152)(H,124,161)(H,125,150)(H,126,164)(H,127,166)(H,128,165)(H,129,147)(H,130,159)(H,131,162)(H,132,149)(H,133,153)(H,134,160)(H,135,163)(H,136,157)(H,137,156)(H,143,144)(H,145,146)(H,167,168)/t59-,60+,68+,70+,71-,72+,73-,74-,75-,76+,77-,78-,79?,80-,81-,82+,83-,84-,85+,86-,89-,90-,91-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50115995
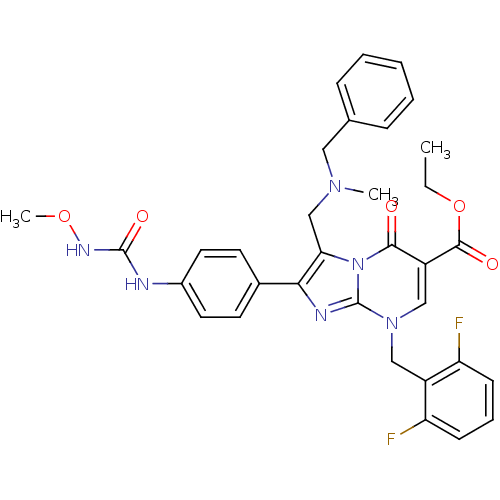
(3-[(Benzyl-methyl-amino)-methyl]-8-(2,6-difluoro-b...)Show SMILES CCOC(=O)c1cn(Cc2c(F)cccc2F)c2nc(c(CN(C)Cc3ccccc3)n2c1=O)-c1ccc(NC(=O)NOC)cc1 Show InChI InChI=1S/C33H32F2N6O5/c1-4-46-31(43)25-19-40(18-24-26(34)11-8-12-27(24)35)33-37-29(22-13-15-23(16-14-22)36-32(44)38-45-3)28(41(33)30(25)42)20-39(2)17-21-9-6-5-7-10-21/h5-16,19H,4,17-18,20H2,1-3H3,(H2,36,38,44) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of specific [125I]-leuprorelin binding to the cloned human LHRH receptor expressed in chinese hamster ovary cells |
Bioorg Med Chem Lett 12: 2073-7 (2002)
BindingDB Entry DOI: 10.7270/Q2FJ2G32 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(Sus scrofa) | BDBM50043322

(CHEMBL427748 | c(Cys-Ser-c(Cys-Ser-Ser-Leu-Met-Asp...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N[C@@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CO)NC(=O)[C@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-57(10)88(108(164)126-77(109(165)166)39-60-43-113-65-23-17-16-22-63(60)65)134-100(156)71(36-55(6)7)119-99(155)76(42-86(143)144)124-94(150)70(35-54(4)5)118-97(153)74(40-61-44-112-52-114-61)122-104(160)81-49-169-168-48-64(111)89(145)127-78(45-135)103(159)132-82-50-170-171-51-83(106(162)133-87(56(8)9)107(163)125-73(38-59-25-27-62(138)28-26-59)95(151)121-72(96(152)131-81)37-58-20-14-13-15-21-58)130-91(147)67(29-30-84(139)140)116-90(146)66(24-18-19-32-110)115-98(154)75(41-85(141)142)123-92(148)68(31-33-167-11)117-93(149)69(34-53(2)3)120-101(157)79(46-136)128-102(158)80(47-137)129-105(82)161/h13-17,20-23,25-28,43-44,52-57,64,66-83,87-88,113,135-138H,12,18-19,24,29-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,155)(H,120,157)(H,121,151)(H,122,160)(H,123,148)(H,124,150)(H,125,163)(H,126,164)(H,127,145)(H,128,158)(H,129,161)(H,130,147)(H,131,152)(H,132,159)(H,133,162)(H,134,156)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t57-,64+,66+,67-,68+,69-,70-,71-,72-,73+,74-,75-,76-,77-,78-,79+,80-,81-,82+,83+,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Tested for the competitive binding versus [125I]- ET-1 determined in porcine cardiac ventricular membrane for Endothelin A receptor |
J Med Chem 36: 4087-93 (1994)
BindingDB Entry DOI: 10.7270/Q2FT8MPC |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Rattus norvegicus) | BDBM50369395

(ELIGARD | LEUPROLIDE)Show SMILES CCNC(=O)[C@@H]1CCCN1C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](CO)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CCC(=O)N1 |r| Show InChI InChI=1S/C59H84N16O12/c1-6-63-57(86)48-14-10-22-75(48)58(87)41(13-9-21-64-59(60)61)68-51(80)42(23-32(2)3)69-52(81)43(24-33(4)5)70-53(82)44(25-34-15-17-37(77)18-16-34)71-56(85)47(30-76)74-54(83)45(26-35-28-65-39-12-8-7-11-38(35)39)72-55(84)46(27-36-29-62-31-66-36)73-50(79)40-19-20-49(78)67-40/h7-8,11-12,15-18,28-29,31-33,40-48,65,76-77H,6,9-10,13-14,19-27,30H2,1-5H3,(H,62,66)(H,63,86)(H,67,78)(H,68,80)(H,69,81)(H,70,82)(H,71,85)(H,72,84)(H,73,79)(H,74,83)(H4,60,61,64)/t40-,41-,42-,43+,44-,45-,46-,47-,48-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
The compound was tested for the concentration to inhibit 50% of [125 I]leuprorelin binding to Leutinizing releasing hormone receptor the membrane fra... |
J Med Chem 41: 4190-5 (1998)
Article DOI: 10.1021/jm9803673
BindingDB Entry DOI: 10.7270/Q2CR5V19 |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122655
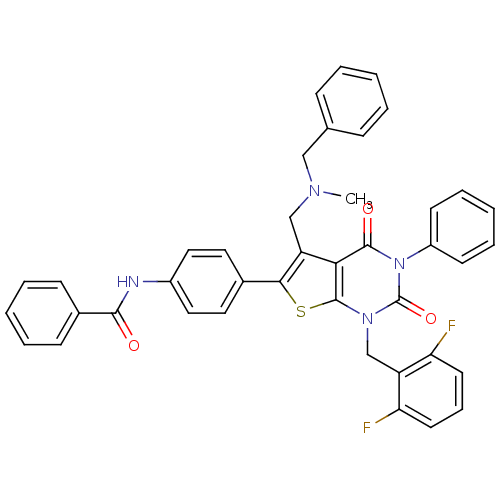
(CHEMBL280212 | N-(4-(1-(2,6-difluorobenzyl)-5-((be...)Show SMILES CN(Cc1c(sc2n(Cc3c(F)cccc3F)c(=O)n(-c3ccccc3)c(=O)c12)-c1ccc(NC(=O)c2ccccc2)cc1)Cc1ccccc1 Show InChI InChI=1S/C41H32F2N4O3S/c1-45(24-27-12-5-2-6-13-27)25-33-36-39(49)47(31-16-9-4-10-17-31)41(50)46(26-32-34(42)18-11-19-35(32)43)40(36)51-37(33)28-20-22-30(23-21-28)44-38(48)29-14-7-3-8-15-29/h2-23H,24-26H2,1H3,(H,44,48) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
Gonadotropin-releasing hormone receptor
(Homo sapiens (Human)) | BDBM50122653
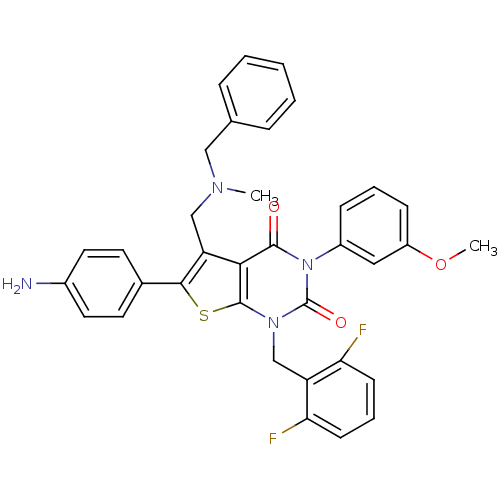
(6-(4-Amino-phenyl)-5-[(benzyl-methyl-amino)-methyl...)Show SMILES COc1cccc(c1)-n1c(=O)n(Cc2c(F)cccc2F)c2sc(c(CN(C)Cc3ccccc3)c2c1=O)-c1ccc(N)cc1 Show InChI InChI=1S/C35H30F2N4O3S/c1-39(19-22-8-4-3-5-9-22)20-28-31-33(42)41(25-10-6-11-26(18-25)44-2)35(43)40(21-27-29(36)12-7-13-30(27)37)34(31)45-32(28)23-14-16-24(38)17-15-23/h3-18H,19-21,38H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Antagonist concentration required to inhibit specific binding of [125I]leuprorelin to human luteinizing releasing hormone receptor in cloned chinese ... |
J Med Chem 46: 113-24 (2002)
Article DOI: 10.1021/jm020180i
BindingDB Entry DOI: 10.7270/Q26W9BTR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088321
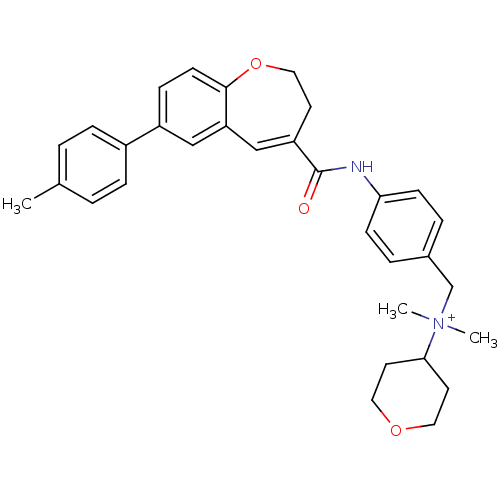
(CHEMBL292548 | Dimethyl-(tetrahydro-pyran-4-yl)-{4...)Show SMILES Cc1ccc(cc1)-c1ccc2OCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C32H36N2O3/c1-23-4-8-25(9-5-23)26-10-13-31-28(20-26)21-27(14-19-37-31)32(35)33-29-11-6-24(7-12-29)22-34(2,3)30-15-17-36-18-16-30/h4-13,20-21,30H,14-19,22H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES |
J Med Chem 43: 2049-63 (2000)
BindingDB Entry DOI: 10.7270/Q26D5S75 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES |
J Med Chem 43: 2049-63 (2000)
BindingDB Entry DOI: 10.7270/Q26D5S75 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088302
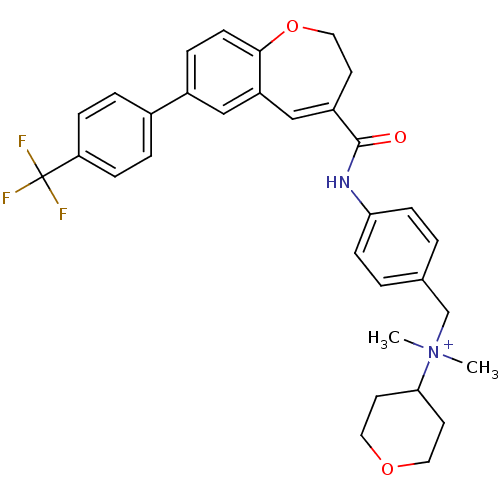
(CHEMBL56565 | Dimethyl-(tetrahydro-pyran-4-yl)-(4-...)Show SMILES C[N+](C)(Cc1ccc(NC(=O)C2=Cc3cc(ccc3OCC2)-c2ccc(cc2)C(F)(F)F)cc1)C1CCOCC1 |t:11| Show InChI InChI=1S/C32H33F3N2O3/c1-37(2,29-14-16-39-17-15-29)21-22-3-10-28(11-4-22)36-31(38)25-13-18-40-30-12-7-24(19-26(30)20-25)23-5-8-27(9-6-23)32(33,34)35/h3-12,19-20,29H,13-18,21H2,1-2H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES |
J Med Chem 43: 2049-63 (2000)
BindingDB Entry DOI: 10.7270/Q26D5S75 |
More data for this
Ligand-Target Pair | |
C-X-C chemokine receptor type 4
(Homo sapiens (Human)) | BDBM50517802

(CHEMBL4457992)Show SMILES C(N(Cc1ccccn1)Cc1ccccn1)c1ccc2c(CN3CCCNCCNCCCNCC3)cccc2c1 Show InChI InChI=1S/C34H45N7/c1-3-17-38-32(10-1)27-41(28-33-11-2-4-18-39-33)25-29-12-13-34-30(24-29)8-5-9-31(34)26-40-22-7-16-36-20-19-35-14-6-15-37-21-23-40/h1-5,8-13,17-18,24,35-37H,6-7,14-16,19-23,25-28H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Tokyo Medical and Dental University (TMDU)
Curated by ChEMBL
| Assay Description
Competitive inhibition of TAMRAAc-TZ14011 binding to CXCR4 (unknown origin) expressed in CHO cells in presence of ZnCl2 by NanoBRET assay relative to... |
Bioorg Med Chem 27: 1130-1138 (2019)
Article DOI: 10.1016/j.bmc.2019.02.013
BindingDB Entry DOI: 10.7270/Q2QR51HR |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088301

((E)-N,N-dimethyl-N-(4-(2-p-tolyl-6,7-dihydro-5H-be...)Show SMILES Cc1ccc(cc1)-c1ccc2CCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:15| Show InChI InChI=1S/C33H38N2O2/c1-24-7-11-27(12-8-24)28-14-13-26-5-4-6-29(22-30(26)21-28)33(36)34-31-15-9-25(10-16-31)23-35(2,3)32-17-19-37-20-18-32/h7-16,21-22,32H,4-6,17-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES |
J Med Chem 43: 2049-63 (2000)
BindingDB Entry DOI: 10.7270/Q26D5S75 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 5
(Homo sapiens (Human)) | BDBM50088322

((4-{[7-(4-Ethoxy-phenyl)-2,3-dihydro-benzo[b]oxepi...)Show SMILES CCOc1ccc(cc1)-c1ccc2OCCC(=Cc2c1)C(=O)Nc1ccc(C[N+](C)(C)C2CCOCC2)cc1 |c:17| Show InChI InChI=1S/C33H38N2O4/c1-4-38-31-12-7-25(8-13-31)26-9-14-32-28(21-26)22-27(15-20-39-32)33(36)34-29-10-5-24(6-11-29)23-35(2,3)30-16-18-37-19-17-30/h5-14,21-22,30H,4,15-20,23H2,1-3H3/p+1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Chemical Industries, Ltd.
Curated by ChEMBL
| Assay Description
Inhibitory effect on Chemokine binding to C-C chemokine receptor type 5 using [125I]-RANTES |
J Med Chem 43: 2049-63 (2000)
BindingDB Entry DOI: 10.7270/Q26D5S75 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

















































