 Found 2246 hits with Last Name = 'fura' and Initial = 'a'
Found 2246 hits with Last Name = 'fura' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324510
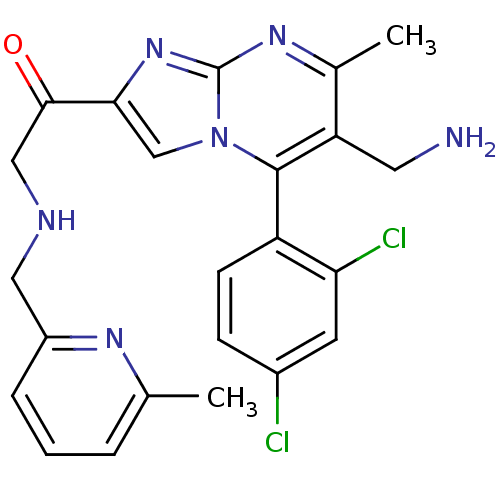
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324512
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
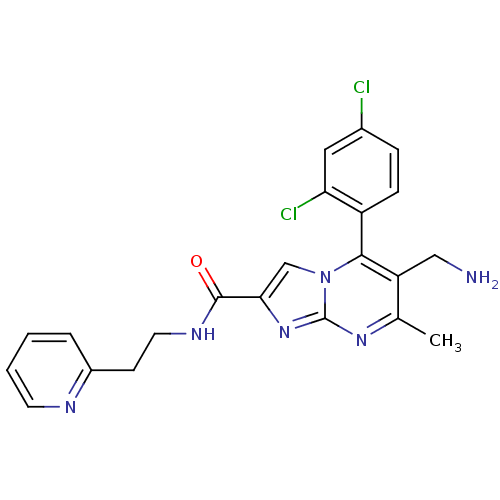
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324523
(CHEMBL1215018 | N-((3S)-1-(6-(aminomethyl)-5-(2,4-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CC[C@@H](C1)NS(C)(=O)=O |r,wD:25.30,(7.23,-5.85,;5.92,-6.66,;4.56,-5.95,;3.26,-6.75,;1.79,-6.32,;.92,-7.59,;1.87,-8.81,;3.31,-8.29,;4.66,-9.02,;5.97,-8.21,;7.33,-8.93,;8.64,-8.12,;4.63,-10.55,;3.28,-11.29,;3.25,-12.83,;4.57,-13.63,;4.54,-15.17,;5.92,-12.87,;5.95,-11.34,;7.29,-10.6,;-.62,-7.63,;-1.43,-6.33,;-1.34,-8.99,;-.88,-10.46,;-2.12,-11.36,;-3.37,-10.46,;-2.89,-8.99,;-4.91,-10.48,;-5.99,-9.37,;-7.4,-10.02,;-6.68,-7.99,;-4.84,-8.35,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-11-15(8-23)18(14-4-3-12(21)7-16(14)22)28-10-17(25-20(28)24-11)19(29)27-6-5-13(9-27)26-32(2,30)31/h3-4,7,10,13,26H,5-6,8-9,23H2,1-2H3/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
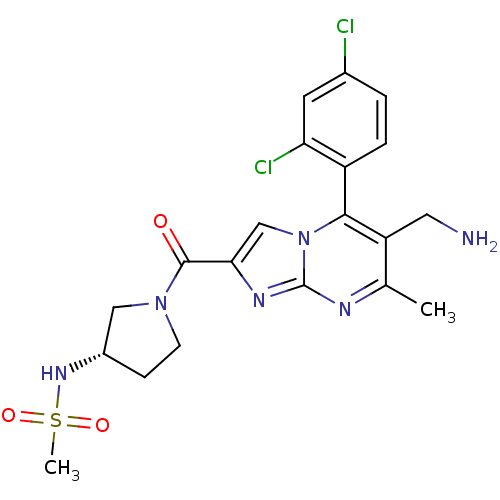
Androgen receptor
(Homo sapiens (Human)) | BDBM29321
(oxazolidin-2-imine, 6d)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | -54.8 | n/a | n/a | 19 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356582
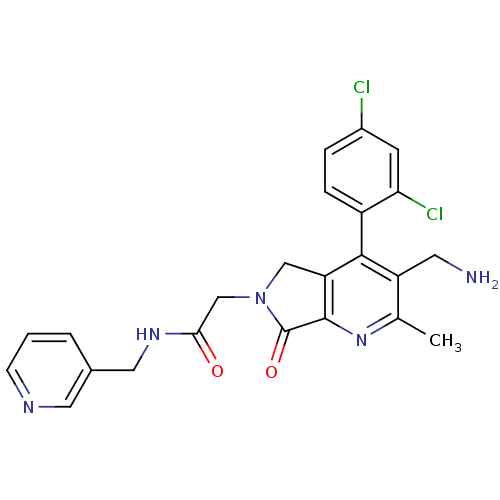
(CHEMBL1910126)Show SMILES Cc1nc2C(=O)N(CC(=O)NCc3cccnc3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(14.01,-46.7,;12.68,-47.48,;11.34,-46.71,;10.02,-47.49,;8.55,-47.01,;8.08,-45.55,;7.65,-48.25,;6.11,-48.26,;5.34,-49.59,;6.12,-50.92,;3.8,-49.59,;3.04,-50.93,;1.5,-50.93,;.74,-52.26,;-.8,-52.27,;-1.58,-50.93,;-.81,-49.6,;.73,-49.59,;8.55,-49.5,;10.01,-49.03,;11.35,-49.8,;12.68,-49.03,;14.02,-49.79,;15.35,-49.02,;11.35,-51.33,;10.01,-52.1,;10.01,-53.64,;11.35,-54.41,;11.35,-55.95,;12.69,-53.64,;12.68,-52.1,;14.01,-51.33,)| Show InChI InChI=1S/C23H21Cl2N5O2/c1-13-17(8-26)21(16-5-4-15(24)7-19(16)25)18-11-30(23(32)22(18)29-13)12-20(31)28-10-14-3-2-6-27-9-14/h2-7,9H,8,10-12,26H2,1H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324512

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccccn1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.93,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-6-5-14(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-7-15-4-2-3-8-26-15/h2-6,8,10,12H,7,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356591

(CHEMBL1910117)Show SMILES CN(C)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(23.14,-19.79,;23.91,-21.12,;23.14,-22.45,;25.45,-21.12,;26.22,-22.45,;26.22,-19.78,;27.75,-19.78,;28.65,-21.03,;30.12,-20.55,;30.12,-19.01,;31.45,-18.24,;32.78,-19,;34.12,-18.23,;32.79,-20.55,;34.13,-21.32,;35.46,-20.55,;31.45,-21.32,;31.46,-22.86,;30.12,-23.63,;30.12,-25.17,;31.45,-25.94,;31.45,-27.48,;32.79,-25.16,;32.79,-23.62,;34.12,-22.85,;28.66,-18.54,;28.18,-17.07,)| Show InChI InChI=1S/C19H20Cl2N4O2/c1-10-13(7-22)17(12-5-4-11(20)6-15(12)21)14-8-25(9-16(26)24(2)3)19(27)18(14)23-10/h4-6H,7-9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324510

(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES Cc1cccc(CNCC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n1 |(-4.59,-8.35,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.31,;-2.42,-4.28,;-1.62,-5.59,;-.08,-5.54,;.65,-4.18,;2.19,-4.14,;2.92,-2.78,;2.11,-1.48,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.17,-3.27,;9.45,-1.81,;10.76,-1,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.48,-6.48,;10.83,-5.74,;-2.34,-6.93,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-13-4-3-5-16(28-13)10-27-11-21(32)20-12-31-22(17-7-6-15(24)8-19(17)25)18(9-26)14(2)29-23(31)30-20/h3-8,12,27H,9-11,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356589
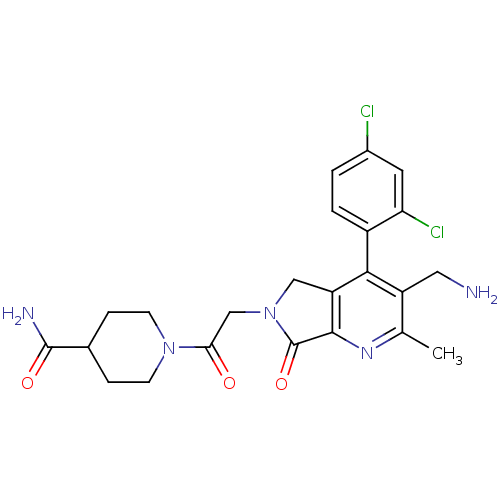
(CHEMBL1910119)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCC(CC3)C(N)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(6.41,-30.16,;5.08,-30.93,;3.75,-30.17,;2.42,-30.95,;.95,-30.47,;.48,-29,;.05,-31.71,;-1.49,-31.71,;-2.25,-33.05,;-1.48,-34.38,;-3.79,-33.05,;-4.56,-31.72,;-6.09,-31.72,;-6.87,-33.05,;-6.1,-34.38,;-4.56,-34.39,;-8.41,-33.04,;-9.18,-31.71,;-9.18,-34.38,;.95,-32.96,;2.41,-32.48,;3.75,-33.26,;5.09,-32.48,;6.42,-33.25,;7.75,-32.48,;3.75,-34.79,;2.41,-35.56,;2.41,-37.1,;3.75,-37.87,;3.75,-39.41,;5.09,-37.09,;5.08,-35.56,;6.41,-34.78,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-12-16(9-26)20(15-3-2-14(24)8-18(15)25)17-10-30(23(33)21(17)28-12)11-19(31)29-6-4-13(5-7-29)22(27)32/h2-3,8,13H,4-7,9-11,26H2,1H3,(H2,27,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356585

(CHEMBL1910123)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(22.17,-39.13,;20.84,-39.9,;19.5,-39.14,;18.17,-39.91,;16.71,-39.44,;16.24,-37.97,;15.8,-40.68,;14.27,-40.68,;13.5,-42.02,;14.28,-43.35,;11.96,-42.02,;11.05,-40.78,;9.59,-41.26,;9.59,-42.8,;11.06,-43.27,;16.71,-41.93,;18.17,-41.45,;19.51,-42.22,;20.84,-41.45,;22.18,-42.22,;23.51,-41.45,;19.51,-43.76,;18.17,-44.53,;18.17,-46.07,;19.51,-46.84,;19.51,-48.38,;20.84,-46.06,;20.84,-44.52,;22.17,-43.75,)| Show InChI InChI=1S/C21H22Cl2N4O2/c1-12-15(9-24)19(14-5-4-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-6-2-3-7-26/h4-5,8H,2-3,6-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
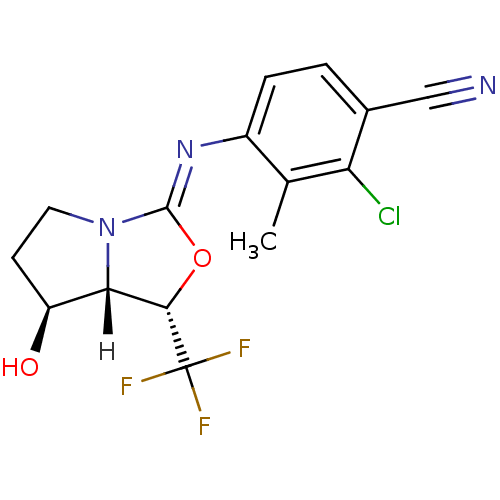
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442192

(CHEMBL2441845)Show SMILES Cc1[nH]c2cn(CCC(O)=O)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(11.05,-4.3,;9.72,-5.08,;8.38,-4.32,;7.05,-5.08,;5.58,-4.61,;4.67,-5.87,;3.13,-5.88,;2.36,-4.55,;3.12,-3.21,;2.34,-1.88,;4.66,-3.2,;5.59,-7.12,;5.12,-8.58,;7.06,-6.63,;8.39,-7.4,;9.72,-6.63,;11.06,-7.4,;12.39,-6.62,;8.38,-8.94,;7.05,-9.7,;7.04,-11.24,;8.37,-12.01,;8.37,-13.55,;9.71,-11.24,;9.71,-9.71,;11.05,-8.93,)| Show InChI InChI=1S/C18H17Cl2N3O3/c1-9-12(7-21)16(11-3-2-10(19)6-13(11)20)17-14(22-9)8-23(18(17)26)5-4-15(24)25/h2-3,6,8,22H,4-5,7,21H2,1H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29323
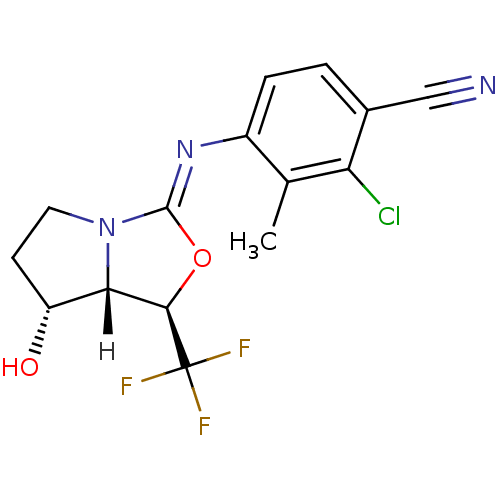
(oxazolidin-2-imine, 6f)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12+,13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 1.40 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29320

(BMS-665139 | oxazolidin-2-imine, 6c)Show SMILES [H][C@@]12[C@@H](O)CCN1\C(O[C@H]2C(F)(F)F)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)21-14-22-5-4-10(23)12(22)13(24-14)15(17,18)19/h2-3,10,12-13,23H,4-5H2,1H3/b21-14-/t10-,12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 0.200 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM29319
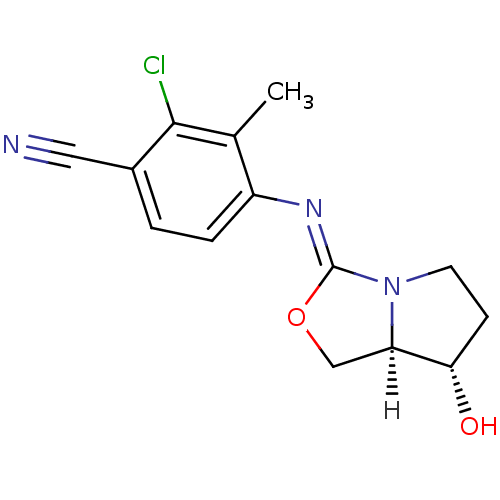
(oxazolidin-2-imine, 6b)Show SMILES [H][C@]12CO\C(=N/c3ccc(C#N)c(Cl)c3C)N1CC[C@@H]2O |r| Show InChI InChI=1S/C14H14ClN3O2/c1-8-10(3-2-9(6-16)13(8)15)17-14-18-5-4-12(19)11(18)7-20-14/h2-3,11-12,19H,4-5,7H2,1H3/b17-14-/t11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 14 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18161

((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...)Show SMILES [H][C@@]12CC[C@H](O)[C@@]1(C)CC[C@@]1([H])[C@@]2([H])CC[C@@]2([H])CC(=O)CC[C@]12C |r| Show InChI InChI=1S/C19H30O2/c1-18-9-7-13(20)11-12(18)3-4-14-15-5-6-17(21)19(15,2)10-8-16(14)18/h12,14-17,21H,3-11H2,1-2H3/t12-,14-,15-,16-,17-,18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.300 | -53.8 | n/a | n/a | 2.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 49: 7596-9 (2006)
Article DOI: 10.1021/jm061101w
BindingDB Entry DOI: 10.7270/Q2862DQ9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324511
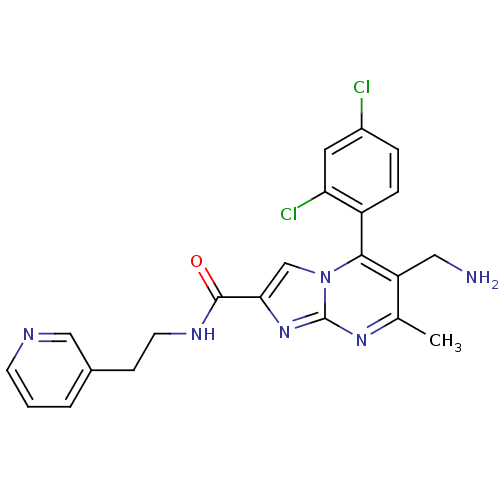
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1cccnc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.94,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.32,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-5-4-15(23)9-18(16)24)30-12-19(29-22(30)28-13)21(31)27-8-6-14-3-2-7-26-11-14/h2-5,7,9,11-12H,6,8,10,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324525
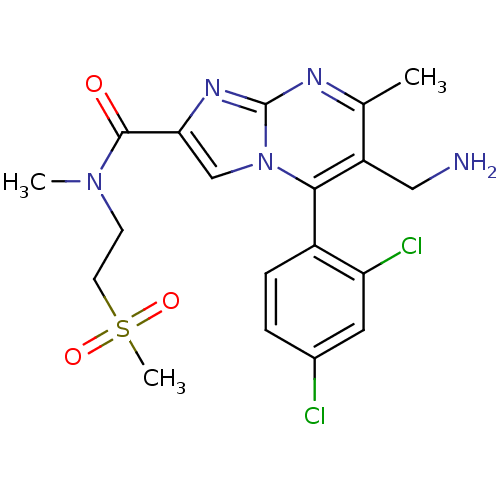
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCS(C)(=O)=O)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(2.96,-5.01,;2.15,-3.7,;.61,-3.75,;-.19,-2.43,;-1.73,-2.48,;-2.54,-1.17,;-3.23,-2.88,;-1.74,-4.02,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.91,;10.83,-3.64,;12.13,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.25,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,)| Show InChI InChI=1S/C19H21Cl2N5O3S/c1-11-14(9-22)17(13-5-4-12(20)8-15(13)21)26-10-16(24-19(26)23-11)18(27)25(2)6-7-30(3,28)29/h4-5,8,10H,6-7,9,22H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356584
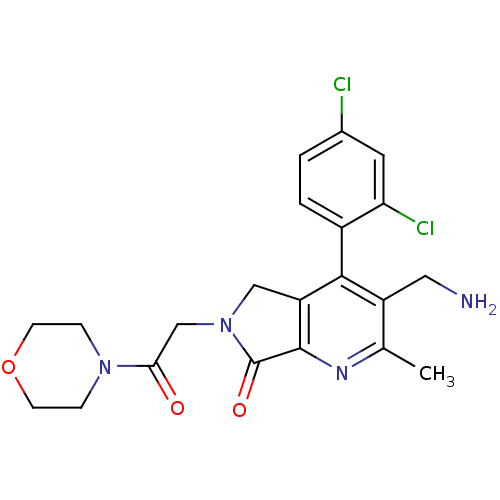
(CHEMBL1910124)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCOCC3)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(23.9,-37.72,;22.57,-38.49,;21.24,-37.73,;19.91,-38.51,;18.45,-38.03,;17.97,-36.56,;17.54,-39.27,;16.01,-39.27,;15.24,-40.61,;16.01,-41.94,;13.7,-40.61,;12.93,-39.28,;11.4,-39.28,;10.62,-40.61,;11.39,-41.94,;12.94,-41.95,;18.44,-40.52,;19.91,-40.04,;21.24,-40.82,;22.58,-40.04,;23.91,-40.81,;25.25,-40.04,;21.24,-42.35,;19.91,-43.12,;19.91,-44.66,;21.24,-45.43,;21.24,-46.97,;22.58,-44.65,;22.57,-43.12,;23.9,-42.34,)| Show InChI InChI=1S/C21H22Cl2N4O3/c1-12-15(9-24)19(14-3-2-13(22)8-17(14)23)16-10-27(21(29)20(16)25-12)11-18(28)26-4-6-30-7-5-26/h2-3,8H,4-7,9-11,24H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356592

(CHEMBL1910116)Show SMILES CNC(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(7.18,-19.79,;7.95,-21.12,;9.49,-21.12,;10.26,-22.45,;10.26,-19.78,;11.79,-19.78,;12.69,-21.03,;14.16,-20.55,;14.16,-19.01,;15.49,-18.24,;16.82,-19,;18.16,-18.23,;16.83,-20.55,;18.17,-21.32,;19.5,-20.55,;15.49,-21.32,;15.5,-22.86,;14.16,-23.63,;14.16,-25.17,;15.49,-25.94,;15.49,-27.48,;16.83,-25.16,;16.83,-23.62,;18.16,-22.85,;12.7,-18.54,;12.22,-17.07,)| Show InChI InChI=1S/C18H18Cl2N4O2/c1-9-12(6-21)16(11-4-3-10(19)5-14(11)20)13-7-24(8-15(25)22-2)18(26)17(13)23-9/h3-5H,6-8,21H2,1-2H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356583

(CHEMBL1910125)Show SMILES Cc1nc2C(=O)N(CC(=O)Nc3ccnn3C)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(39.71,-37.55,;38.38,-38.32,;37.04,-37.56,;35.71,-38.33,;34.25,-37.86,;33.78,-36.39,;33.34,-39.1,;31.81,-39.1,;31.04,-40.44,;31.81,-41.77,;29.5,-40.44,;28.73,-41.77,;27.21,-41.94,;26.89,-43.44,;28.22,-44.21,;29.37,-43.18,;30.87,-43.5,;34.25,-40.35,;35.71,-39.87,;37.05,-40.64,;38.38,-39.87,;39.72,-40.64,;41.05,-39.87,;37.05,-42.18,;35.71,-42.95,;35.71,-44.49,;37.04,-45.26,;37.05,-46.8,;38.38,-44.48,;38.38,-42.94,;39.71,-42.17,)| Show InChI InChI=1S/C21H20Cl2N6O2/c1-11-14(8-24)19(13-4-3-12(22)7-16(13)23)15-9-29(21(31)20(15)26-11)10-18(30)27-17-5-6-25-28(17)2/h3-7H,8-10,24H2,1-2H3,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324500
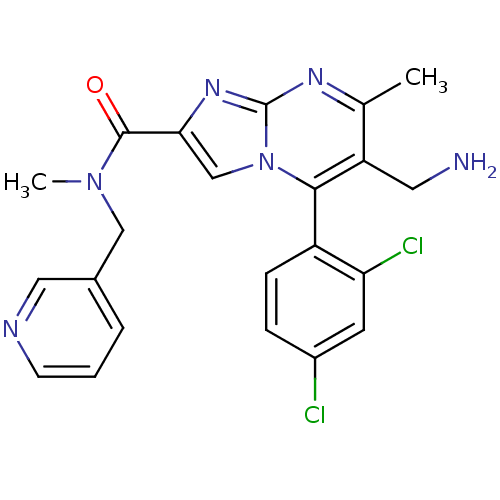
(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES CN(Cc1cccnc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.9,-13.73,;4.13,-12.4,;2.59,-12.4,;1.82,-13.73,;.28,-13.72,;-.49,-15.05,;.28,-16.39,;1.83,-16.4,;2.59,-15.06,;4.91,-11.07,;4.14,-9.74,;6.44,-11.06,;7.34,-12.29,;8.8,-11.82,;10.13,-12.59,;11.47,-11.82,;12.8,-12.6,;14.14,-11.85,;11.46,-10.28,;12.8,-9.51,;10.13,-9.51,;8.8,-10.28,;7.34,-9.8,;10.13,-14.13,;8.79,-14.9,;8.8,-16.44,;10.13,-17.21,;10.14,-18.75,;11.45,-16.44,;11.45,-14.9,;12.8,-14.11,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(9-25)20(16-6-5-15(23)8-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-4-3-7-26-10-14/h3-8,10,12H,9,11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324524
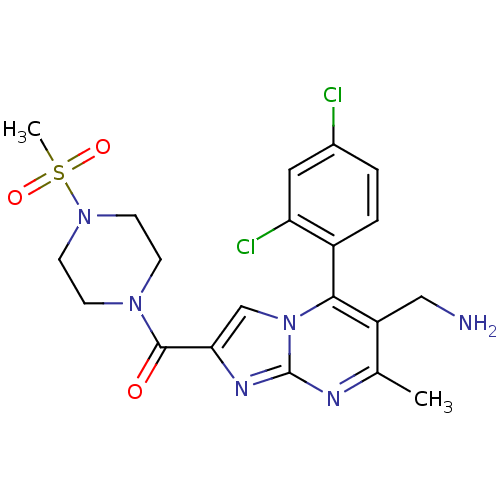
((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356590

(CHEMBL1910118)Show SMILES CCN(CC)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(39.88,-17.76,;39.11,-19.1,;39.89,-20.43,;39.12,-21.76,;39.89,-23.09,;41.43,-20.43,;42.2,-21.76,;42.19,-19.09,;43.73,-19.09,;44.63,-20.33,;46.09,-19.86,;46.1,-18.32,;47.43,-17.55,;48.76,-18.31,;50.09,-17.53,;48.77,-19.86,;50.1,-20.63,;51.43,-19.86,;47.43,-20.63,;47.43,-22.17,;46.09,-22.94,;46.09,-24.47,;47.43,-25.25,;47.43,-26.79,;48.77,-24.47,;48.76,-22.93,;50.09,-22.16,;44.63,-17.84,;44.16,-16.38,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-16-19(14-7-6-13(22)8-17(14)23)15(9-24)12(3)25-20(16)21(27)29/h6-8H,4-5,9-11,24H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356587
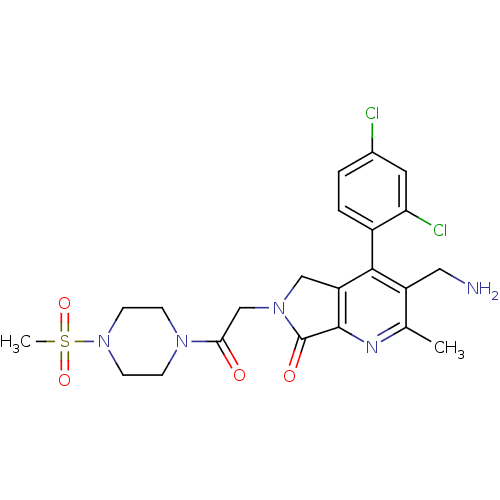
(CHEMBL1910121)Show SMILES Cc1nc2C(=O)N(CC(=O)N3CCN(CC3)S(C)(=O)=O)Cc2c(c1CN)-c1ccc(Cl)cc1Cl |(48.61,-29.07,;47.28,-29.84,;45.95,-29.08,;44.62,-29.86,;43.16,-29.38,;42.68,-27.91,;42.25,-30.62,;40.72,-30.62,;39.95,-31.96,;40.72,-33.29,;38.41,-31.96,;37.64,-30.63,;36.11,-30.63,;35.33,-31.96,;36.1,-33.29,;37.65,-33.3,;33.79,-31.95,;33.03,-30.62,;32.45,-32.72,;33.78,-33.49,;43.15,-31.87,;44.62,-31.4,;45.95,-32.17,;47.29,-31.39,;48.62,-32.16,;49.96,-31.39,;45.95,-33.7,;44.62,-34.47,;44.62,-36.01,;45.95,-36.78,;45.95,-38.32,;47.29,-36,;47.28,-34.47,;48.61,-33.69,)| Show InChI InChI=1S/C22H25Cl2N5O4S/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-28(22(31)21(17)26-13)12-19(30)27-5-7-29(8-6-27)34(2,32)33/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324524

((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442198
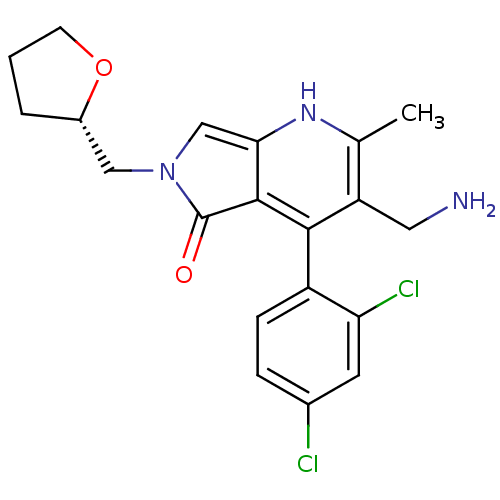
(CHEMBL2441839)Show SMILES Cc1[nH]c2cn(C[C@@H]3CCCO3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |r,wD:7.6,(24.71,-38.82,;23.38,-39.6,;22.05,-38.83,;20.72,-39.6,;19.24,-39.13,;18.34,-40.39,;16.8,-40.39,;16.02,-39.06,;14.47,-39,;14.06,-37.52,;15.35,-36.67,;16.55,-37.63,;19.25,-41.63,;18.79,-43.1,;20.72,-41.15,;22.05,-41.92,;23.39,-41.15,;24.72,-41.91,;26.05,-41.14,;22.05,-43.46,;20.71,-44.22,;20.71,-45.76,;22.04,-46.53,;22.04,-48.07,;23.38,-45.76,;23.38,-44.22,;24.71,-43.45,)| Show InChI InChI=1S/C20H21Cl2N3O2/c1-11-15(8-23)18(14-5-4-12(21)7-16(14)22)19-17(24-11)10-25(20(19)26)9-13-3-2-6-27-13/h4-5,7,10,13,24H,2-3,6,8-9,23H2,1H3/t13-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18188
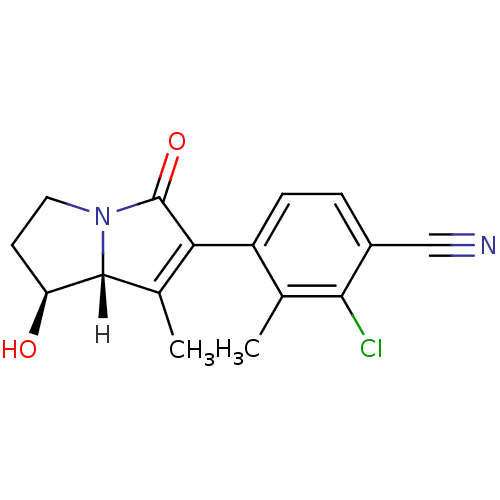
(4-[(1S,7aR)-1-hydroxy-7-methyl-5-oxo-2,3,5,7a-tetr...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)C(=C2C)c1ccc(C#N)c(Cl)c1C |r,c:10| Show InChI InChI=1S/C16H15ClN2O2/c1-8-11(4-3-10(7-18)14(8)17)13-9(2)15-12(20)5-6-19(15)16(13)21/h3-4,12,15,20H,5-6H2,1-2H3/t12-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.5 | -52.6 | n/a | n/a | 2 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324504
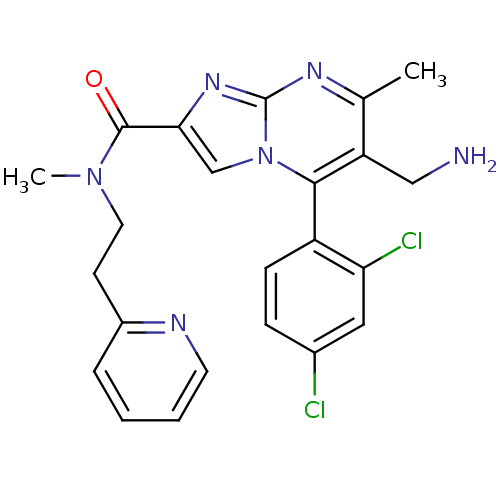
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCc1ccccn1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(7.39,-9.52,;6.58,-8.21,;5.04,-8.26,;4.23,-6.94,;2.69,-6.99,;1.89,-5.67,;.35,-5.72,;-.38,-7.08,;.44,-8.4,;1.97,-8.34,;7.3,-6.85,;6.49,-5.54,;8.85,-6.8,;9.79,-8.02,;11.23,-7.5,;12.59,-8.23,;13.89,-7.42,;15.25,-8.15,;16.56,-7.34,;13.85,-5.88,;15.15,-5.06,;12.48,-5.16,;11.19,-5.97,;9.71,-5.54,;12.55,-9.76,;11.2,-10.5,;11.17,-12.04,;12.49,-12.84,;12.58,-14.44,;13.85,-12.09,;13.87,-10.56,;15.21,-9.81,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-14-18(12-26)21(17-7-6-15(24)11-19(17)25)31-13-20(29-23(31)28-14)22(32)30(2)10-8-16-5-3-4-9-27-16/h3-7,9,11,13H,8,10,12,26H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356588

(CHEMBL1910120)Show SMILES CC(=O)N1CCN(CC1)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(11.15,-31.36,;11.92,-32.69,;11.15,-34.03,;13.46,-32.7,;14.24,-31.37,;15.77,-31.37,;16.54,-32.7,;15.78,-34.04,;14.23,-34.03,;18.08,-32.7,;18.85,-34.03,;18.84,-31.36,;20.38,-31.36,;21.28,-32.61,;22.75,-32.13,;22.75,-30.6,;24.08,-29.82,;25.41,-30.58,;26.74,-29.81,;25.42,-32.13,;26.75,-32.9,;28.08,-32.13,;24.08,-32.91,;24.08,-34.44,;22.75,-35.21,;22.74,-36.75,;24.08,-37.52,;24.08,-39.06,;25.42,-36.74,;25.41,-35.21,;26.74,-34.43,;21.28,-30.12,;20.81,-28.65,)| Show InChI InChI=1S/C23H25Cl2N5O3/c1-13-17(10-26)21(16-4-3-15(24)9-19(16)25)18-11-30(23(33)22(18)27-13)12-20(32)29-7-5-28(6-8-29)14(2)31/h3-4,9H,5-8,10-12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324513
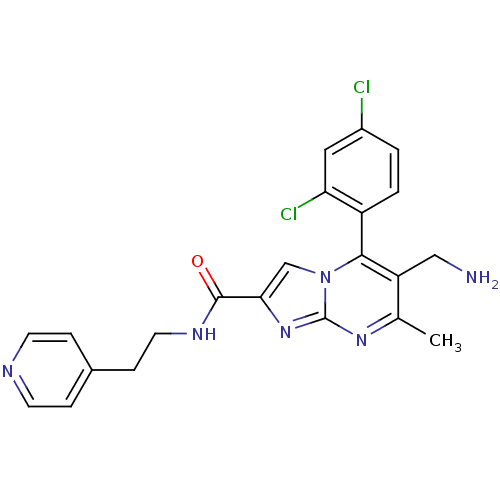
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1ccncc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.42,-4.28,;-3.96,-4.32,;-4.69,-5.68,;-3.87,-6.99,;-2.34,-6.94,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(11-25)20(16-3-2-15(23)10-18(16)24)30-12-19(29-22(30)28-13)21(31)27-9-6-14-4-7-26-8-5-14/h2-5,7-8,10,12H,6,9,11,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324511

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)NCCc1cccnc1 |(10.76,-1,;9.45,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.48,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.08,-5.54,;-1.62,-5.59,;-2.34,-6.94,;-3.87,-6.99,;-4.69,-5.68,;-3.96,-4.32,;-2.42,-4.28,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-5-4-15(23)9-18(16)24)30-12-19(29-22(30)28-13)21(31)27-8-6-14-3-2-7-26-11-14/h2-5,7,9,11-12H,6,8,10,25H2,1H3,(H,27,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324500

(1-(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl...)Show SMILES CN(Cc1cccnc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.9,-13.73,;4.13,-12.4,;2.59,-12.4,;1.82,-13.73,;.28,-13.72,;-.49,-15.05,;.28,-16.39,;1.83,-16.4,;2.59,-15.06,;4.91,-11.07,;4.14,-9.74,;6.44,-11.06,;7.34,-12.29,;8.8,-11.82,;10.13,-12.59,;11.47,-11.82,;12.8,-12.6,;14.14,-11.85,;11.46,-10.28,;12.8,-9.51,;10.13,-9.51,;8.8,-10.28,;7.34,-9.8,;10.13,-14.13,;8.79,-14.9,;8.8,-16.44,;10.13,-17.21,;10.14,-18.75,;11.45,-16.44,;11.45,-14.9,;12.8,-14.11,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(9-25)20(16-6-5-15(23)8-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-4-3-7-26-10-14/h3-8,10,12H,9,11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324497
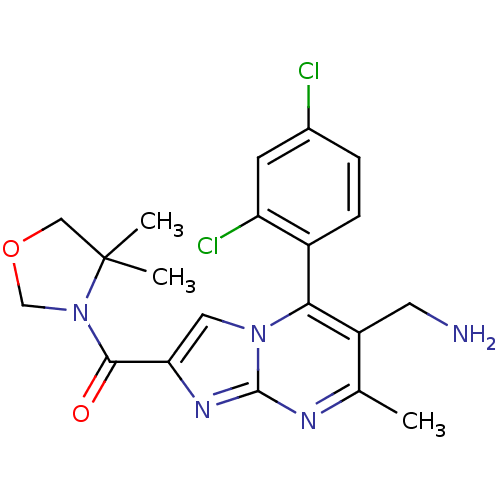
((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1COCC1(C)C |(10.72,-.55,;9.42,-1.37,;8.06,-.65,;6.76,-1.46,;5.28,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.46,-2.91,;10.82,-3.64,;12.13,-2.83,;8.13,-5.25,;6.78,-5.99,;6.74,-7.53,;8.07,-8.33,;8.03,-9.87,;9.42,-7.58,;9.44,-6.05,;10.79,-5.3,;2.88,-2.34,;2.07,-1.03,;2.15,-3.7,;2.66,-5.14,;1.44,-6.08,;.17,-5.2,;.61,-3.72,;-.99,-3.83,;-.16,-2.39,)| Show InChI InChI=1S/C20H21Cl2N5O2/c1-11-14(7-23)17(13-5-4-12(21)6-15(13)22)26-8-16(25-19(26)24-11)18(28)27-10-29-9-20(27,2)3/h4-6,8H,7,9-10,23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324508
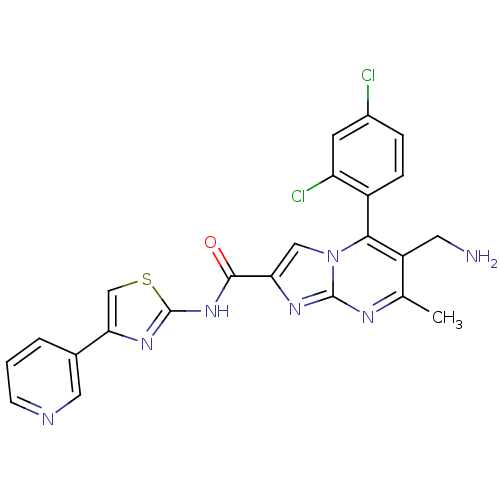
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methyl-N-...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)Nc1nc(cs1)-c1cccnc1 |(10.76,-1,;9.46,-1.81,;8.1,-1.09,;6.8,-1.9,;5.33,-1.46,;4.46,-2.74,;5.4,-3.96,;6.85,-3.44,;8.2,-4.17,;9.51,-3.36,;10.87,-4.08,;12.18,-3.27,;8.17,-5.7,;6.82,-6.44,;6.79,-7.98,;8.11,-8.78,;8.08,-10.32,;9.46,-8.02,;9.49,-6.49,;10.83,-5.74,;2.92,-2.78,;2.11,-1.48,;2.19,-4.14,;.65,-4.18,;-.23,-5.46,;-1.7,-5.02,;-1.75,-3.48,;-.3,-2.97,;-2.84,-6.06,;-2.83,-7.6,;-4.15,-8.37,;-5.5,-7.61,;-5.51,-6.06,;-4.17,-5.29,)| Show InChI InChI=1S/C23H17Cl2N7OS/c1-12-16(8-26)20(15-5-4-14(24)7-17(15)25)32-10-18(29-22(32)28-12)21(33)31-23-30-19(11-34-23)13-3-2-6-27-9-13/h2-7,9-11H,8,26H2,1H3,(H,30,31,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50356586

(CHEMBL1910122)Show SMILES CN1CCN(CC1)C(=O)CN1Cc2c(nc(C)c(CN)c2-c2ccc(Cl)cc2Cl)C1=O |(-10,-42.49,;-8.46,-42.49,;-7.69,-41.16,;-6.16,-41.16,;-5.39,-42.49,;-6.15,-43.83,;-7.69,-43.83,;-3.85,-42.49,;-3.08,-43.82,;-3.08,-41.16,;-1.55,-41.15,;-.64,-42.4,;.82,-41.93,;.82,-40.39,;2.15,-39.61,;3.49,-40.38,;4.82,-39.6,;3.49,-41.93,;4.83,-42.69,;6.16,-41.92,;2.15,-42.7,;2.16,-44.23,;.82,-45,;.82,-46.54,;2.15,-47.31,;2.16,-48.85,;3.49,-46.53,;3.49,-45,;4.82,-44.22,;-.64,-39.91,;-1.11,-38.44,)| Show InChI InChI=1S/C22H25Cl2N5O2/c1-13-16(10-25)20(15-4-3-14(23)9-18(15)24)17-11-29(22(31)21(17)26-13)12-19(30)28-7-5-27(2)6-8-28/h3-4,9H,5-8,10-12,25H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of DPP4 |
Bioorg Med Chem Lett 21: 6646-51 (2011)
Article DOI: 10.1016/j.bmcl.2011.09.074
BindingDB Entry DOI: 10.7270/Q28W3DQK |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514
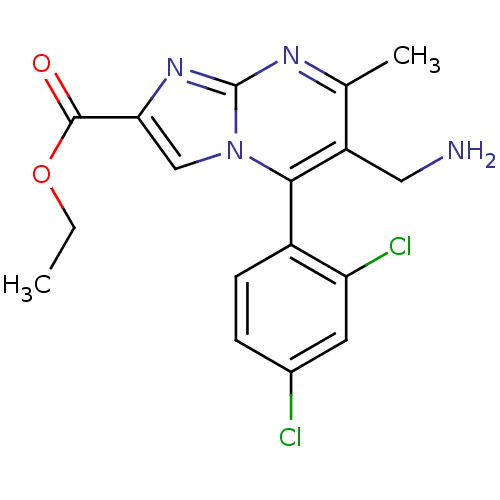
((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324514

((+)-Ethyl 6-(aminomethyl)-5-(2,4-dichlorophenyl)-7...)Show SMILES CCOC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-9.34,-.68,;-8.61,.68,;-7.07,.72,;-6.34,2.08,;-7.15,3.39,;-4.8,2.13,;-3.86,.91,;-2.41,1.43,;-1.06,.7,;.25,1.51,;1.61,.79,;2.92,1.59,;.2,3.06,;1.51,3.87,;-1.16,3.77,;-2.46,2.96,;-3.93,3.4,;-1.01,-.83,;-2.32,-1.64,;-2.28,-3.18,;-.92,-3.91,;-.87,-5.44,;.4,-3.09,;.34,-1.55,;1.65,-.74,)| Show InChI InChI=1S/C17H16Cl2N4O2/c1-3-25-16(24)14-8-23-15(11-5-4-10(18)6-13(11)19)12(7-20)9(2)21-17(23)22-14/h4-6,8H,3,7,20H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50324494
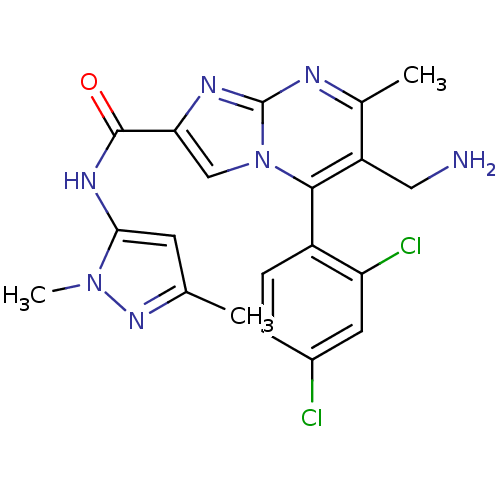
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(1,3-dime...)Show SMILES Cc1cc(NC(=O)c2cn3c(c(CN)c(C)nc3n2)-c2ccc(Cl)cc2Cl)n(C)n1 |(-2.95,-5.54,;-1.73,-4.59,;-.25,-5.02,;.61,-3.75,;2.15,-3.7,;2.88,-2.34,;2.07,-1.03,;4.42,-2.29,;5.36,-3.51,;6.81,-2.99,;8.16,-3.72,;9.47,-2.92,;10.83,-3.64,;12.14,-2.83,;9.42,-1.37,;10.73,-.55,;8.06,-.65,;6.76,-1.46,;5.29,-1.03,;8.13,-5.26,;6.78,-5.99,;6.75,-7.53,;8.07,-8.33,;8.04,-9.87,;9.42,-7.58,;9.45,-6.05,;10.79,-5.3,;-.33,-2.53,;.1,-1.06,;-1.78,-3.05,)| Show InChI InChI=1S/C20H19Cl2N7O/c1-10-6-17(28(3)27-10)26-19(30)16-9-29-18(13-5-4-12(21)7-15(13)22)14(8-23)11(2)24-20(29)25-16/h4-7,9H,8,23H2,1-3H3,(H,26,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP8 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18183
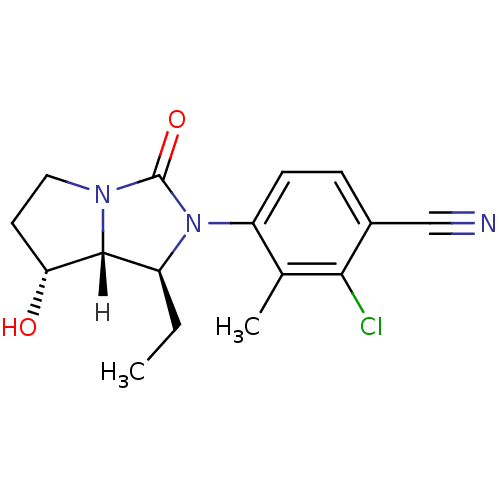
(4-[(1S,7R,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...)Show SMILES [H][C@@]12[C@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H18ClN3O2/c1-3-11-15-13(21)6-7-19(15)16(22)20(11)12-5-4-10(8-18)14(17)9(12)2/h4-5,11,13,15,21H,3,6-7H2,1-2H3/t11-,13+,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | 2.60 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29324
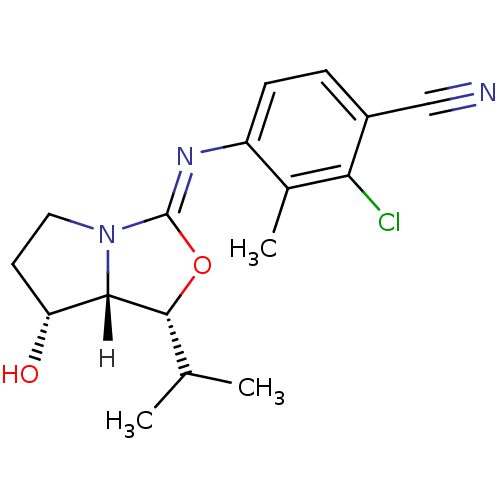
(oxazolidin-2-imine, 6g)Show SMILES [H][C@@]12[C@H](O)CCN1\C(O[C@@H]2C(C)C)=N\c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C17H20ClN3O2/c1-9(2)16-15-13(22)6-7-21(15)17(23-16)20-12-5-4-11(8-19)14(18)10(12)3/h4-5,9,13,15-16,22H,6-7H2,1-3H3/b20-17-/t13-,15+,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | -51.7 | n/a | n/a | 3.70 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50205111
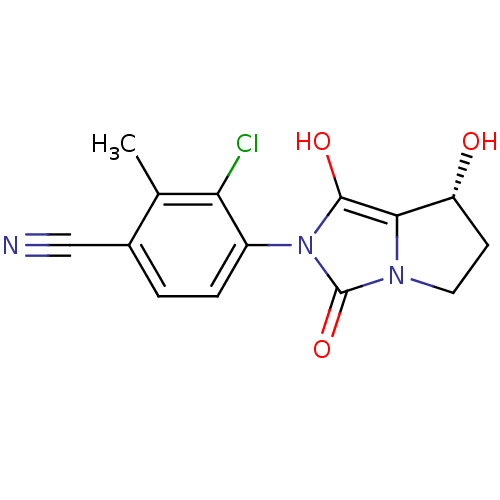
(3-chloro-4-((7R,7aS)-7-hydroxy-1,3-dioxo-tetrahydr...)Show SMILES Cc1c(Cl)c(ccc1C#N)-n1c(O)c2[C@H](O)CCn2c1=O |wU:14.15,(22.97,-37.01,;22.21,-35.67,;20.67,-35.65,;19.89,-36.98,;19.91,-34.31,;20.69,-32.99,;22.22,-32.99,;22.99,-34.34,;24.53,-34.34,;26.08,-34.34,;18.37,-34.31,;17.48,-33.06,;17.96,-31.6,;16.01,-33.53,;14.55,-33.04,;14.08,-31.57,;13.63,-34.28,;14.54,-35.53,;16,-35.06,;17.46,-35.55,;17.93,-37.02,)| Show InChI InChI=1S/C14H12ClN3O3/c1-7-8(6-16)2-3-9(11(7)15)18-13(20)12-10(19)4-5-17(12)14(18)21/h2-3,10,19-20H,4-5H2,1H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human AR |
Bioorg Med Chem Lett 17: 1860-4 (2007)
Article DOI: 10.1016/j.bmcl.2007.01.076
BindingDB Entry DOI: 10.7270/Q2S46RN1 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 4
(Homo sapiens (Human)) | BDBM50324504

(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(CCc1ccccn1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(7.39,-9.52,;6.58,-8.21,;5.04,-8.26,;4.23,-6.94,;2.69,-6.99,;1.89,-5.67,;.35,-5.72,;-.38,-7.08,;.44,-8.4,;1.97,-8.34,;7.3,-6.85,;6.49,-5.54,;8.85,-6.8,;9.79,-8.02,;11.23,-7.5,;12.59,-8.23,;13.89,-7.42,;15.25,-8.15,;16.56,-7.34,;13.85,-5.88,;15.15,-5.06,;12.48,-5.16,;11.19,-5.97,;9.71,-5.54,;12.55,-9.76,;11.2,-10.5,;11.17,-12.04,;12.49,-12.84,;12.58,-14.44,;13.85,-12.09,;13.87,-10.56,;15.21,-9.81,)| Show InChI InChI=1S/C23H22Cl2N6O/c1-14-18(12-26)21(17-7-6-15(24)11-19(17)25)31-13-20(29-23(31)28-14)22(32)30(2)10-8-16-5-3-4-9-27-16/h3-7,9,11,13H,8,10,12,26H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant DPP4 assessed as Gly-Pro-pNA cleavage |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 8
(Homo sapiens (Human)) | BDBM50442191

(CHEMBL2441846)Show SMILES CCN(CC)C(=O)Cn1cc2[nH]c(C)c(CN)c(-c3ccc(Cl)cc3Cl)c2c1=O |(18.85,-1.37,;18.08,-2.7,;16.54,-2.71,;15.77,-1.38,;14.23,-1.39,;15.78,-4.05,;14.24,-4.06,;16.56,-5.38,;18.1,-5.37,;19,-4.11,;20.48,-4.59,;21.8,-3.82,;23.14,-4.58,;24.47,-3.81,;23.14,-6.13,;24.48,-6.9,;25.81,-6.13,;21.81,-6.9,;21.81,-8.44,;20.47,-9.2,;20.47,-10.74,;21.8,-11.52,;21.8,-13.06,;23.14,-10.74,;23.14,-9.21,;24.47,-8.44,;20.48,-6.13,;19.01,-6.62,;18.54,-8.09,)| Show InChI InChI=1S/C21H24Cl2N4O2/c1-4-26(5-2)18(28)11-27-10-17-20(21(27)29)19(15(9-24)12(3)25-17)14-7-6-13(22)8-16(14)23/h6-8,10,25H,4-5,9,11,24H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP8 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50442183
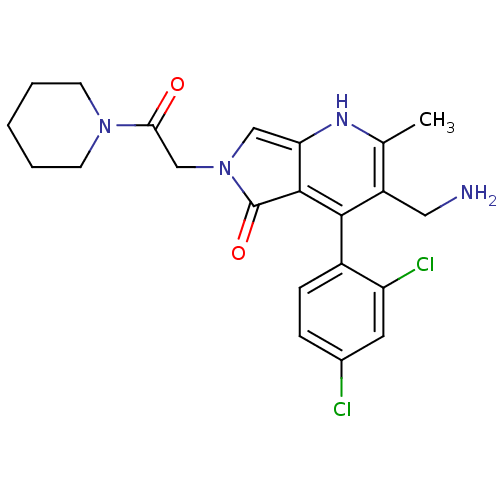
(CHEMBL2441955)Show SMILES Cc1[nH]c2cn(CC(=O)N3CCCCC3)c(=O)c2c(c1CN)-c1ccc(Cl)cc1Cl |(49.34,-35.7,;48.01,-36.47,;46.68,-35.71,;45.35,-36.47,;43.87,-36,;42.97,-37.26,;41.43,-37.27,;40.65,-35.94,;39.11,-35.95,;41.42,-34.6,;42.95,-34.6,;43.71,-33.27,;42.94,-31.94,;41.4,-31.95,;40.63,-33.28,;43.89,-38.51,;43.42,-39.98,;45.36,-38.02,;46.69,-38.79,;48.02,-38.02,;49.35,-38.79,;50.69,-38.01,;46.68,-40.33,;45.35,-41.09,;45.34,-42.63,;46.67,-43.41,;46.67,-44.95,;48.01,-42.63,;48.01,-41.1,;49.34,-40.32,)| Show InChI InChI=1S/C22H24Cl2N4O2/c1-13-16(10-25)20(15-6-5-14(23)9-17(15)24)21-18(26-13)11-28(22(21)30)12-19(29)27-7-3-2-4-8-27/h5-6,9,11,26H,2-4,7-8,10,12,25H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of DPP9 (unknown origin) |
J Med Chem 56: 7343-57 (2013)
Article DOI: 10.1021/jm4008906
BindingDB Entry DOI: 10.7270/Q2N01806 |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM29318

(oxazolidin-2-imine, 6a)Show SMILES [H][C@]12CO\C(=N/c3ccc(C#N)c(Cl)c3C)N1CC[C@H]2O |r| Show InChI InChI=1S/C14H14ClN3O2/c1-8-10(3-2-9(6-16)13(8)15)17-14-18-5-4-12(19)11(18)7-20-14/h2-3,11-12,19H,4-5,7H2,1H3/b17-14-/t11-,12-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.800 | -51.4 | n/a | n/a | 4.80 | n/a | n/a | 7.6 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Binding assays were conducted by incubating test compound at various concentrations with [3H]DHT with human cancer epithelial breast cell lines MDAMB... |
J Med Chem 52: 2794-8 (2009)
Article DOI: 10.1021/jm801583j
BindingDB Entry DOI: 10.7270/Q2W66J30 |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324524

((6-(aminomethyl)-5-(2,4-dichlorophenyl)-7-methylim...)Show SMILES Cc1nc2nc(cn2c(c1CN)-c1ccc(Cl)cc1Cl)C(=O)N1CCN(CC1)S(C)(=O)=O |(11.6,.54,;10.29,-.27,;8.93,.44,;7.63,-.37,;6.16,.07,;5.29,-1.2,;6.24,-2.42,;7.68,-1.9,;9.03,-2.63,;10.34,-1.82,;11.7,-2.54,;13.01,-1.73,;9,-4.16,;7.65,-4.9,;7.62,-6.44,;8.94,-7.24,;8.91,-8.78,;10.29,-6.48,;10.32,-4.95,;11.66,-4.21,;3.75,-1.24,;2.94,.06,;3.02,-2.6,;3.82,-3.91,;3.09,-5.26,;1.55,-5.3,;.75,-3.99,;1.48,-2.64,;.82,-6.65,;-.72,-6.7,;.4,-8.14,;2.15,-7.42,)| Show InChI InChI=1S/C20H22Cl2N6O3S/c1-12-15(10-23)18(14-4-3-13(21)9-16(14)22)28-11-17(25-20(28)24-12)19(29)26-5-7-27(8-6-26)32(2,30)31/h3-4,9,11H,5-8,10,23H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324505
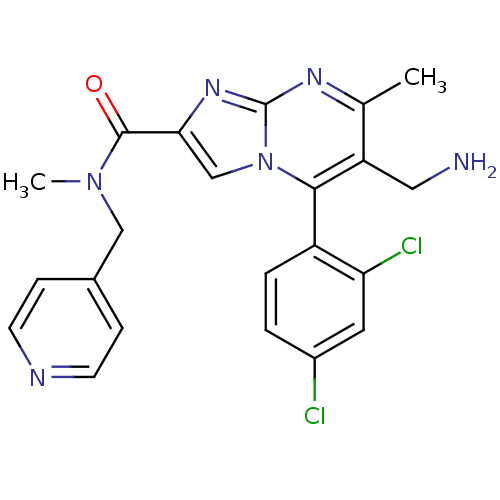
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N,7-dimethy...)Show SMILES CN(Cc1ccncc1)C(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(4.39,-7.36,;3.62,-6.02,;2.08,-6.02,;1.31,-7.35,;2.08,-8.69,;1.31,-10.03,;-.23,-10.03,;-1,-8.68,;-.23,-7.35,;4.4,-4.69,;3.63,-3.36,;5.94,-4.68,;6.84,-5.92,;8.3,-5.44,;9.63,-6.21,;10.97,-5.44,;12.31,-6.23,;12.3,-7.77,;10.97,-3.9,;12.31,-3.13,;9.64,-3.13,;8.31,-3.9,;6.84,-3.42,;9.63,-7.75,;8.3,-8.53,;8.3,-10.07,;9.63,-10.84,;9.65,-12.38,;10.96,-10.07,;10.96,-8.52,;12.3,-7.74,)| Show InChI InChI=1S/C22H20Cl2N6O/c1-13-17(10-25)20(16-4-3-15(23)9-18(16)24)30-12-19(28-22(30)27-13)21(31)29(2)11-14-5-7-26-8-6-14/h3-9,12H,10-11,25H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.860 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18177
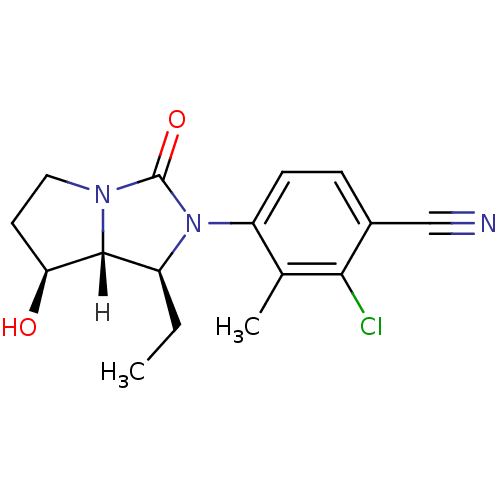
(4-[(1S,7S,7aR)-1-ethyl-7-hydroxy-3-oxo-hexahydro-1...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)N([C@H]2CC)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C16H18ClN3O2/c1-3-11-15-13(21)6-7-19(15)16(22)20(11)12-5-4-10(8-18)14(17)9(12)2/h4-5,11,13,15,21H,3,6-7H2,1-2H3/t11-,13-,15+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | -51.1 | n/a | n/a | 1.80 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18178
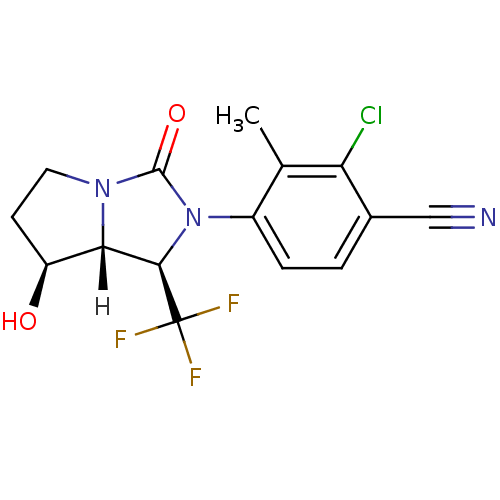
(4-[(1R,7S,7aR)-7-hydroxy-3-oxo-1-(trifluoromethyl)...)Show SMILES [H][C@@]12[C@@H](O)CCN1C(=O)N([C@H]2C(F)(F)F)c1ccc(C#N)c(Cl)c1C |r| Show InChI InChI=1S/C15H13ClF3N3O2/c1-7-9(3-2-8(6-20)11(7)16)22-13(15(17,18)19)12-10(23)4-5-21(12)14(22)24/h2-3,10,12-13,23H,4-5H2,1H3/t10-,12-,13+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | -51.1 | n/a | n/a | 2.5 | n/a | n/a | 7.4 | 22 |
Bristol-Myers Squibb Pharmaceutical Research Institute
| Assay Description
Receptor Binding Assay (Ki)-Binding determined through direct displacement of ligand with [3H]-DHT in the MDA-453 cell line. Transactivation Assay (E... |
J Med Chem 50: 3015-3025 (2007)
Article DOI: 10.1021/jm070312d
BindingDB Entry DOI: 10.7270/Q24F1P1F |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 9
(Homo sapiens (Human)) | BDBM50324501
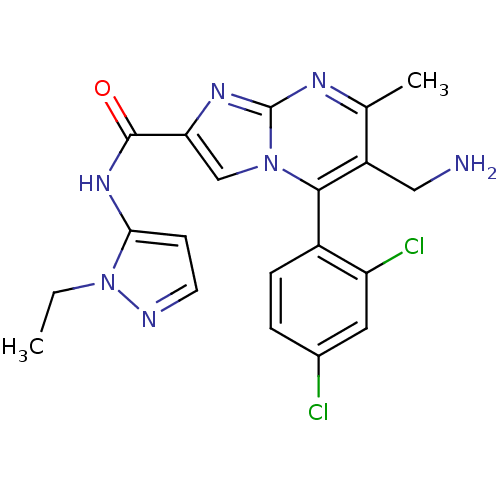
(6-(aminomethyl)-5-(2,4-dichlorophenyl)-N-(1-ethyl-...)Show SMILES CCn1nccc1NC(=O)c1cn2c(c(CN)c(C)nc2n1)-c1ccc(Cl)cc1Cl |(-3.58,-1.22,;-2.53,-2.33,;-2.95,-3.81,;-4.4,-4.33,;-4.36,-5.87,;-2.88,-6.31,;-2.01,-5.04,;-.47,-4.99,;.26,-3.63,;-.55,-2.32,;1.8,-3.58,;2.74,-4.81,;4.19,-4.28,;5.54,-5.01,;6.85,-4.21,;8.21,-4.93,;9.52,-4.12,;6.8,-2.66,;8.11,-1.84,;5.44,-1.94,;4.14,-2.75,;2.67,-2.32,;5.51,-6.55,;4.16,-7.29,;4.13,-8.83,;5.45,-9.63,;5.42,-11.17,;6.8,-8.87,;6.83,-7.34,;8.17,-6.59,)| Show InChI InChI=1S/C20H19Cl2N7O/c1-3-29-17(6-7-24-29)27-19(30)16-10-28-18(13-5-4-12(21)8-15(13)22)14(9-23)11(2)25-20(28)26-16/h4-8,10H,3,9,23H2,1-2H3,(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human DPP9 |
J Med Chem 53: 5620-8 (2010)
Article DOI: 10.1021/jm100634a
BindingDB Entry DOI: 10.7270/Q2S182PX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data