 Found 257 hits with Last Name = 'furch' and Initial = 'ja'
Found 257 hits with Last Name = 'furch' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Androgen receptor
(Homo sapiens (Human)) | BDBM50372090
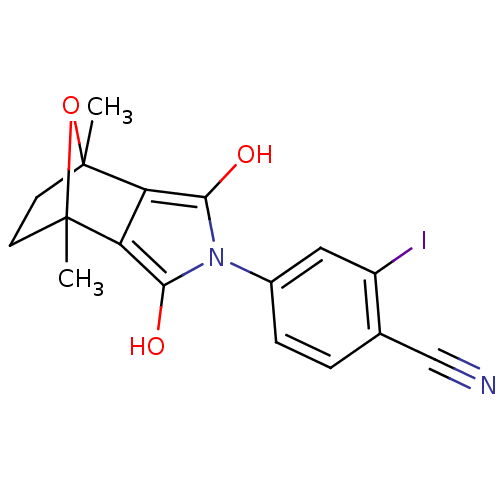
(CHEMBL403668)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H15IN2O3/c1-16-5-6-17(2,23-16)13-12(16)14(21)20(15(13)22)10-4-3-9(8-19)11(18)7-10/h3-4,7,21-22H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372078
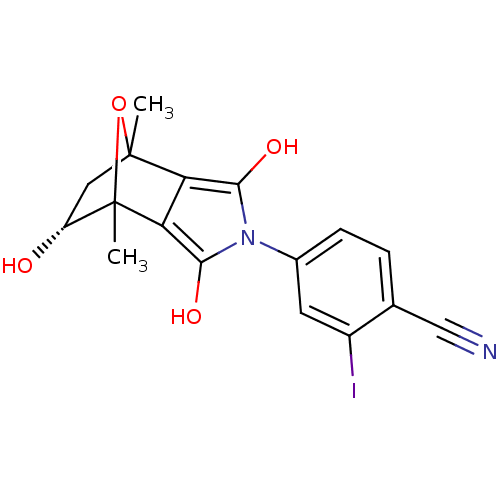
(CHEMBL272574)Show SMILES CC12C[C@@H](O)C(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |TLB:4:3:7:8.14,THB:12:14:7:2.3,9:8:7:2.3| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11,21-23H,6H2,1-2H3/t11-,16?,17?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372088
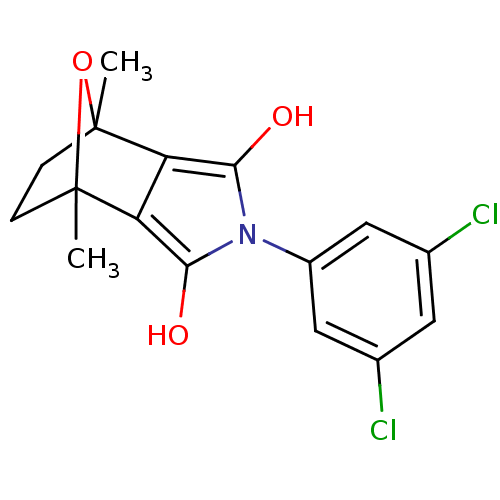
(CHEMBL257668)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1cc(Cl)cc(Cl)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C16H15Cl2NO3/c1-15-3-4-16(2,22-15)12-11(15)13(20)19(14(12)21)10-6-8(17)5-9(18)7-10/h5-7,20-21H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372089

(CHEMBL272509)Show SMILES CSc1cc(ccc1C#N)-n1c(O)c2c(c1O)C1(C)CCC2(C)O1 |THB:11:13:23:20.19,15:14:23:20.19| Show InChI InChI=1S/C18H18N2O3S/c1-17-6-7-18(2,23-17)14-13(17)15(21)20(16(14)22)11-5-4-10(9-19)12(8-11)24-3/h4-5,8,21-22H,6-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372082
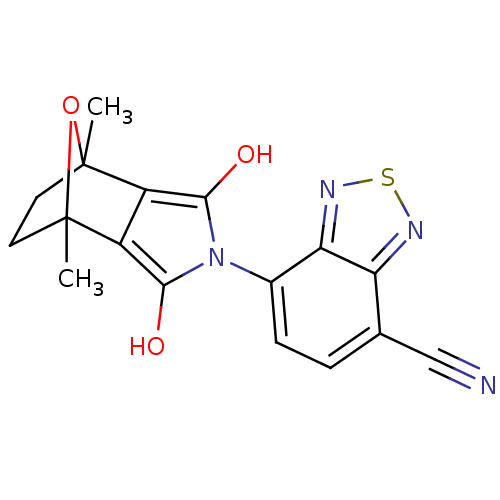
(CHEMBL446626)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c2nsnc12 |THB:11:13:6:2.3,8:7:6:2.3,(28.74,-48.8,;27.41,-49.58,;28.3,-50.89,;27.39,-51.64,;26.52,-50.54,;25.81,-51.91,;27.75,-48.2,;25.03,-50.57,;23.98,-49.8,;22.75,-50.2,;24.39,-48.57,;25.69,-48.58,;26.33,-47.45,;26.08,-49.81,;23.05,-47.8,;21.7,-48.58,;20.36,-47.8,;20.37,-46.25,;19.03,-45.49,;17.68,-44.73,;21.69,-45.47,;22.02,-43.95,;23.56,-43.79,;24.19,-45.21,;23.04,-46.25,)| Show InChI InChI=1S/C17H14N4O3S/c1-16-5-6-17(2,24-16)11-10(16)14(22)21(15(11)23)9-4-3-8(7-18)12-13(9)20-25-19-12/h3-4,22-23H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372103

(CHEMBL402807)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc([N+]([O-])=O)c2ccccc12 |THB:9:8:7:5.4,1:2:7:5.4,(7.88,-13.23,;8.78,-12.29,;10.06,-12.46,;11.36,-11.72,;12.65,-12.28,;13.1,-11.18,;11.68,-10.45,;11.33,-9.07,;10.63,-11.29,;9.69,-10.39,;9.71,-9.09,;8.54,-11.01,;6.99,-10.97,;6.18,-12.3,;4.63,-12.26,;3.89,-10.89,;2.34,-10.85,;1.6,-9.49,;1.53,-12.17,;4.7,-9.57,;3.96,-8.22,;4.76,-6.91,;6.31,-6.94,;7.04,-8.3,;6.24,-9.61,)| Show InChI InChI=1S/C18H14N2O5/c21-17-15-13-7-8-14(25-13)16(15)18(22)19(17)11-5-6-12(20(23)24)10-4-2-1-3-9(10)11/h1-6,13-14,21-22H,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372086
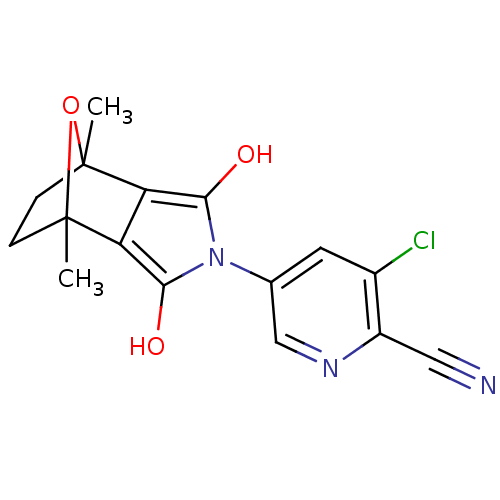
(CHEMBL403213)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1cnc(C#N)c(Cl)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C16H14ClN3O3/c1-15-3-4-16(2,23-15)12-11(15)13(21)20(14(12)22)8-5-9(17)10(6-18)19-7-8/h5,7,21-22H,3-4H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372079

(CHEMBL255331)Show SMILES CC12C[C@H](O)C(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |TLB:4:3:7:14.8,THB:9:8:7:3.2,12:14:7:3.2| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11,21-23H,6H2,1-2H3/t11-,16?,17?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372085
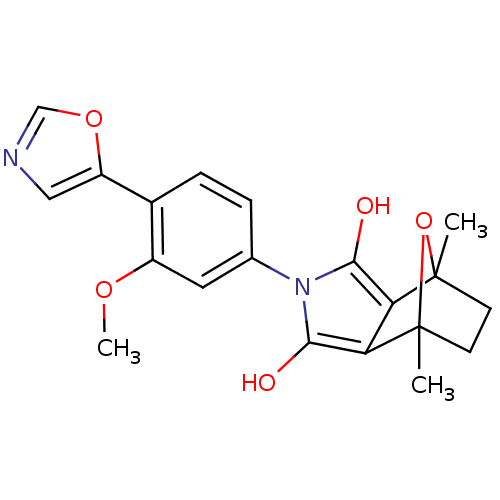
(CHEMBL403061)Show SMILES COc1cc(ccc1-c1cnco1)-n1c(O)c2c(c1O)C1(C)CCC2(C)O1 |THB:14:16:26:23.22,18:17:26:23.22| Show InChI InChI=1S/C20H20N2O5/c1-19-6-7-20(2,27-19)16-15(19)17(23)22(18(16)24)11-4-5-12(13(8-11)25-3)14-9-21-10-26-14/h4-5,8-10,23-24H,6-7H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372087
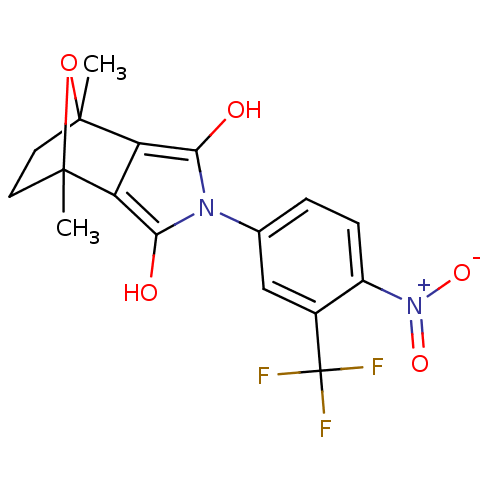
(CHEMBL403214)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H15F3N2O5/c1-15-5-6-16(2,27-15)12-11(15)13(23)21(14(12)24)8-3-4-10(22(25)26)9(7-8)17(18,19)20/h3-4,7,23-24H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372091
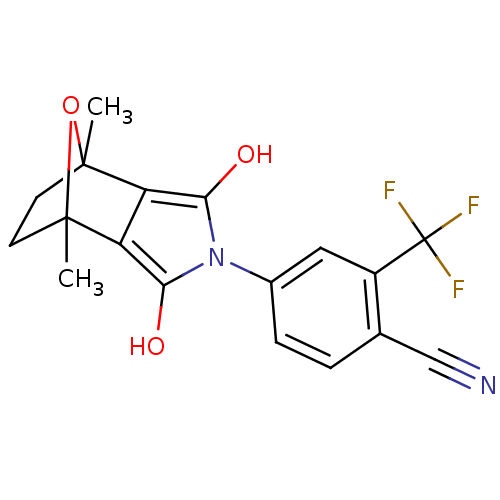
(CHEMBL403669)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C18H15F3N2O3/c1-16-5-6-17(2,26-16)13-12(16)14(24)23(15(13)25)10-4-3-9(8-22)11(7-10)18(19,20)21/h3-4,7,24-25H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372080

(CHEMBL403039)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc([N+]([O-])=O)c2c(Br)cccc12 |THB:11:13:6:2.3,8:7:6:2.3,(31.1,-48.31,;29.77,-49.09,;30.66,-50.4,;29.75,-51.15,;28.88,-50.05,;28.17,-51.42,;30.11,-47.72,;27.39,-50.08,;26.35,-49.32,;25.12,-49.71,;26.75,-48.09,;28.05,-48.09,;28.69,-46.97,;28.44,-49.33,;25.41,-47.32,;25.4,-45.77,;24.06,-44.99,;22.74,-45.77,;21.39,-45.01,;21.39,-43.46,;20.06,-45.78,;22.74,-47.32,;21.41,-48.09,;20.07,-47.32,;21.41,-49.64,;22.75,-50.4,;24.08,-49.63,;24.07,-48.09,)| Show InChI InChI=1S/C20H17BrN2O5/c1-19-8-9-20(2,28-19)16-15(19)17(24)22(18(16)25)12-6-7-13(23(26)27)14-10(12)4-3-5-11(14)21/h3-7,24-25H,8-9H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372084

(CHEMBL271262)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1c(N)ccc2ncsc12 |THB:11:13:6:2.3,8:7:6:2.3,(43.68,-48.16,;42.36,-48.94,;43.25,-50.24,;42.34,-50.99,;41.47,-49.89,;40.77,-51.26,;42.7,-47.56,;39.99,-49.92,;38.94,-49.16,;38.06,-49.55,;39.35,-47.93,;40.64,-47.94,;41.28,-46.81,;41.04,-49.17,;38.01,-47.17,;36.67,-47.94,;36.67,-49.48,;35.34,-47.17,;35.34,-45.62,;36.66,-44.84,;36.98,-43.33,;38.52,-43.17,;39.15,-44.59,;38,-45.62,)| Show InChI InChI=1S/C17H17N3O3S/c1-16-5-6-17(2,23-16)11-10(16)14(21)20(15(11)22)12-8(18)3-4-9-13(12)24-7-19-9/h3-4,7,21-22H,5-6,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372081
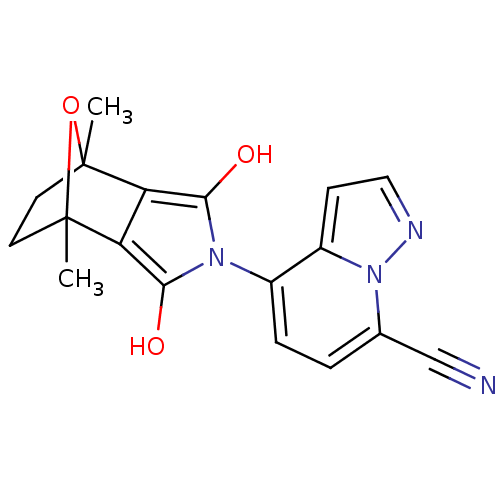
(CHEMBL255216)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)n2nccc12 |THB:11:13:6:2.3,8:7:6:2.3,(29.8,-48.84,;28.47,-49.62,;29.36,-50.93,;28.45,-51.68,;27.58,-50.57,;26.88,-51.94,;28.81,-48.25,;26.1,-50.61,;25.06,-49.84,;23.83,-50.24,;25.46,-48.62,;26.76,-48.62,;27.39,-47.5,;27.15,-49.85,;24.13,-47.85,;22.79,-48.62,;21.45,-47.85,;21.45,-46.31,;20.12,-45.55,;18.78,-44.79,;22.77,-45.53,;23.1,-44.01,;24.64,-43.86,;25.27,-45.27,;24.11,-46.3,)| Show InChI InChI=1S/C18H16N4O3/c1-17-6-7-18(2,25-17)14-13(17)15(23)21(16(14)24)11-4-3-10(9-19)22-12(11)5-8-20-22/h3-5,8,23-24H,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372104
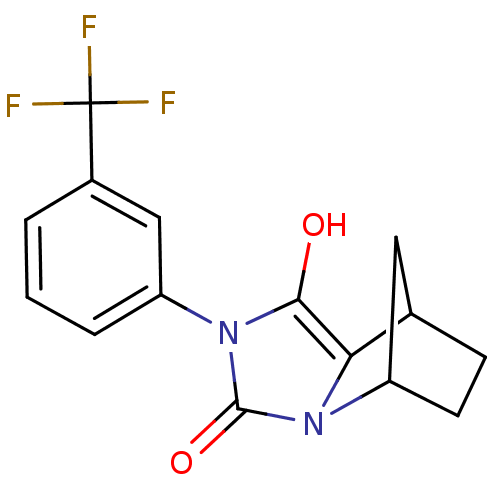
(CHEMBL255776)Show SMILES Oc1c2C3CCC(C3)n2c(=O)n1-c1cccc(c1)C(F)(F)F |TLB:9:8:5.4:7,THB:1:2:5.4:7| Show InChI InChI=1S/C15H13F3N2O2/c16-15(17,18)9-2-1-3-10(7-9)20-13(21)12-8-4-5-11(6-8)19(12)14(20)22/h1-3,7-8,11,21H,4-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372083

(CHEMBL271261)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1c(N)ccc2nccn12 |THB:11:13:6:2.3,8:7:6:2.3,(28.01,-48.56,;26.67,-49.34,;27.57,-50.65,;26.65,-51.4,;25.78,-50.29,;25.08,-51.67,;27.02,-47.96,;24.29,-50.33,;23.24,-49.56,;22.24,-49.9,;23.65,-48.33,;24.95,-48.33,;25.59,-47.2,;25.35,-49.57,;22.31,-47.56,;20.96,-48.33,;20.96,-49.88,;19.62,-47.56,;19.62,-46.01,;20.95,-45.22,;21.27,-43.7,;22.82,-43.54,;23.45,-44.96,;22.29,-46,)| Show InChI InChI=1S/C17H18N4O3/c1-16-5-6-17(2,24-16)12-11(16)14(22)21(15(12)23)13-9(18)3-4-10-19-7-8-20(10)13/h3-4,7-8,22-23H,5-6,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50156506
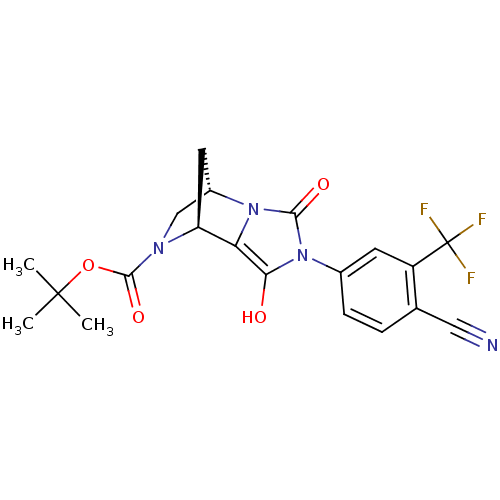
((1S,6S,7S)-4-(4-Cyano-3-trifluoromethyl-phenyl)-3,...)Show SMILES CC(C)(C)OC(=O)N1C[C@@H]2C[C@H]1c1c(O)n(-c3ccc(C#N)c(c3)C(F)(F)F)c(=O)n21 Show InChI InChI=1S/C20H19F3N4O4/c1-19(2,3)31-18(30)25-9-12-7-14(25)15-16(28)27(17(29)26(12)15)11-5-4-10(8-24)13(6-11)20(21,22)23/h4-6,12,14,28H,7,9H2,1-3H3/t12-,14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372092

(CHEMBL272510)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1cccc(c1)C(F)(F)F |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H16F3NO3/c1-15-6-7-16(2,24-15)12-11(15)13(22)21(14(12)23)10-5-3-4-9(8-10)17(18,19)20/h3-5,8,22-23H,6-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM35909
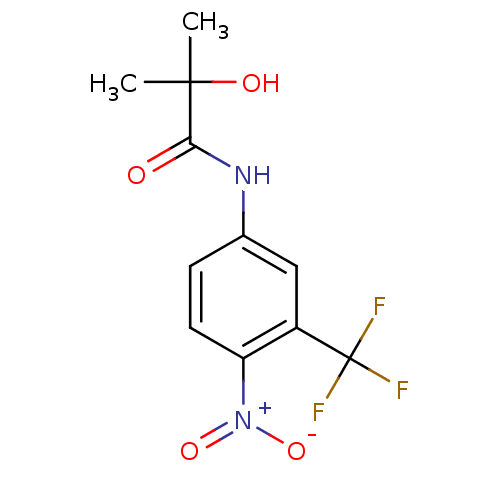
(2-Hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-ph...)Show SMILES CC(C)(O)C(=O)Nc1ccc(c(c1)C(F)(F)F)[N+]([O-])=O Show InChI InChI=1S/C11H11F3N2O4/c1-10(2,18)9(17)15-6-3-4-8(16(19)20)7(5-6)11(12,13)14/h3-5,18H,1-2H3,(H,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372102

(CHEMBL402930)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc(Br)c2ccccc12 |THB:9:8:7:5.4,1:2:7:5.4,(-5.34,-18.05,;-4.1,-17.65,;-3.05,-18.42,;-1.56,-18.38,;-.69,-19.49,;.23,-18.74,;-.67,-17.43,;-.32,-16.04,;-2,-17.66,;-2.4,-16.42,;-1.75,-15.29,;-3.7,-16.42,;-5.04,-15.65,;-6.39,-16.42,;-7.73,-15.65,;-7.73,-14.09,;-9.07,-13.32,;-6.39,-13.32,;-6.39,-11.78,;-5.07,-11.01,;-3.72,-11.78,;-3.72,-13.32,;-5.05,-14.09,)| Show InChI InChI=1S/C18H14BrNO3/c19-11-5-6-12(10-4-2-1-3-9(10)11)20-17(21)15-13-7-8-14(23-13)16(15)18(20)22/h1-6,13-14,21-22H,7-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM18525
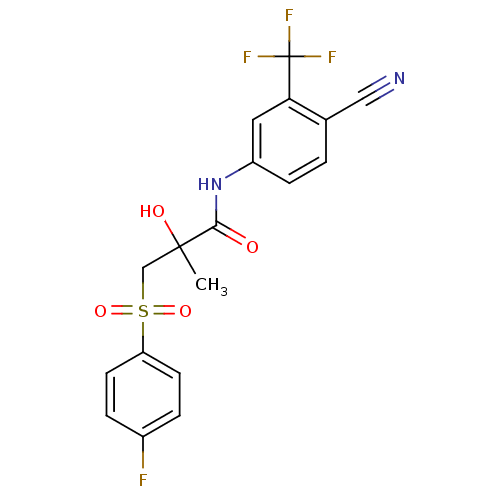
(Bicalutamide | CHEMBL409 | N-[4-cyano-3-(trifluoro...)Show SMILES CC(O)(CS(=O)(=O)c1ccc(F)cc1)C(=O)Nc1ccc(C#N)c(c1)C(F)(F)F Show InChI InChI=1S/C18H14F4N2O4S/c1-17(26,10-29(27,28)14-6-3-12(19)4-7-14)16(25)24-13-5-2-11(9-23)15(8-13)18(20,21)22/h2-8,26H,10H2,1H3,(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372098

(CHEMBL255367)Show SMILES Cc1cc(ccc1Cl)-n1c(O)c2C3CCC(O3)c2c1O |THB:18:17:16:14.13,9:11:16:14.13| Show InChI InChI=1S/C15H14ClNO3/c1-7-6-8(2-3-9(7)16)17-14(18)12-10-4-5-11(20-10)13(12)15(17)19/h2-3,6,10-11,18-19H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372101
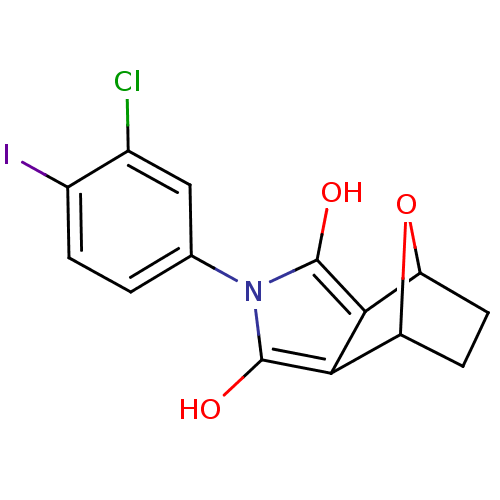
(CHEMBL270586)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc(I)c(Cl)c1 |THB:9:8:7:5.4,1:2:7:5.4| Show InChI InChI=1S/C14H11ClINO3/c15-7-5-6(1-2-8(7)16)17-13(18)11-9-3-4-10(20-9)12(11)14(17)19/h1-2,5,9-10,18-19H,3-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 221 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50158073
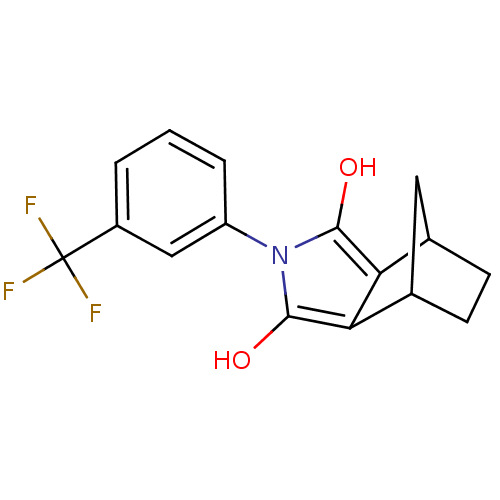
((2R,6S)-4-[3-(trifluoromethyl)phenyl]-4-azatricycl...)Show SMILES Oc1c2C3CCC(C3)c2c(O)n1-c1cccc(c1)C(F)(F)F |TLB:1:2:7:5.4,THB:9:8:7:5.4| Show InChI InChI=1S/C16H14F3NO2/c17-16(18,19)10-2-1-3-11(7-10)20-14(21)12-8-4-5-9(6-8)13(12)15(20)22/h1-3,7-9,21-22H,4-6H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372100

(CHEMBL270450)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc(Cl)c(Cl)c1 |THB:9:8:7:5.4,1:2:7:5.4| Show InChI InChI=1S/C14H11Cl2NO3/c15-7-2-1-6(5-8(7)16)17-13(18)11-9-3-4-10(20-9)12(11)14(17)19/h1-2,5,9-10,18-19H,3-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372093
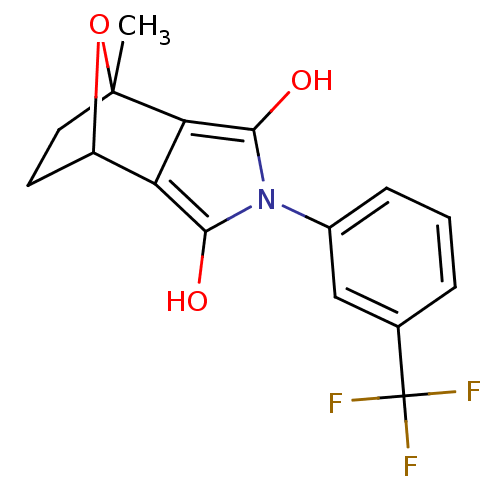
(CHEMBL404506)Show SMILES CC12CCC(O1)c1c(O)n(c(O)c21)-c1cccc(c1)C(F)(F)F |THB:10:12:5:2.3,7:6:5:2.3| Show InChI InChI=1S/C16H14F3NO3/c1-15-6-5-10(23-15)11-12(15)14(22)20(13(11)21)9-4-2-3-8(7-9)16(17,18)19/h2-4,7,10,21-22H,5-6H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372099

(CHEMBL437214)Show SMILES Cc1ccc(cc1Cl)-n1c(O)c2C3CCC(O3)c2c1O |THB:18:17:16:14.13,9:11:16:14.13| Show InChI InChI=1S/C15H14ClNO3/c1-7-2-3-8(6-9(7)16)17-14(18)12-10-4-5-11(20-10)13(12)15(17)19/h2-3,6,10-11,18-19H,4-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 486 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372097
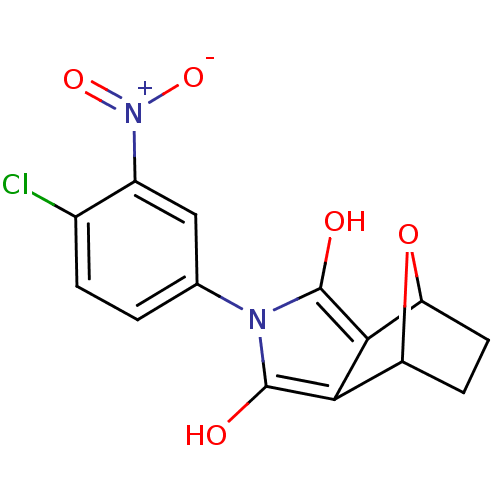
(CHEMBL271924)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc(Cl)c(c1)[N+]([O-])=O |THB:9:8:7:5.4,1:2:7:5.4| Show InChI InChI=1S/C14H11ClN2O5/c15-7-2-1-6(5-8(7)17(20)21)16-13(18)11-9-3-4-10(22-9)12(11)14(16)19/h1-2,5,9-10,18-19H,3-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 692 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372096
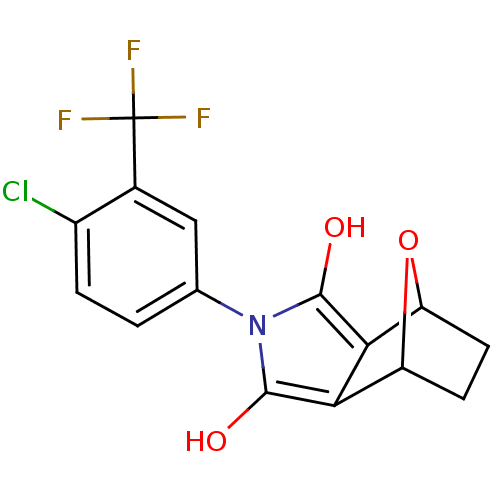
(CHEMBL271925)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc(Cl)c(c1)C(F)(F)F |THB:9:8:7:5.4,1:2:7:5.4| Show InChI InChI=1S/C15H11ClF3NO3/c16-8-2-1-6(5-7(8)15(17,18)19)20-13(21)11-9-3-4-10(23-9)12(11)14(20)22/h1-2,5,9-10,21-22H,3-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372095
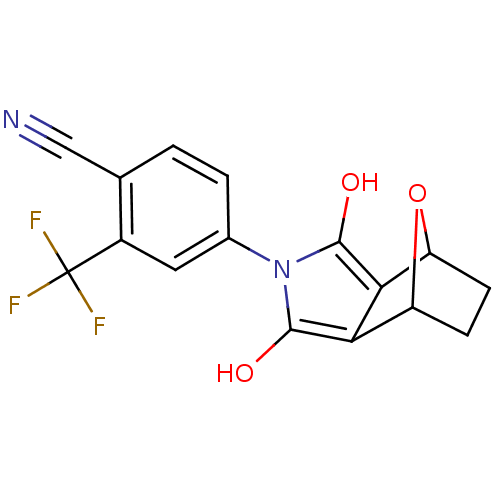
(CHEMBL427721)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1ccc(C#N)c(c1)C(F)(F)F |THB:9:8:7:5.4,1:2:7:5.4| Show InChI InChI=1S/C16H11F3N2O3/c17-16(18,19)9-5-8(2-1-7(9)6-20)21-14(22)12-10-3-4-11(24-10)13(12)15(21)23/h1-2,5,10-11,22-23H,3-4H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372094

(CHEMBL255157)Show SMILES Oc1c2C3CCC(O3)c2c(O)n1-c1cccc(c1)C(F)(F)F |THB:9:8:7:5.4,1:2:7:5.4| Show InChI InChI=1S/C15H12F3NO3/c16-15(17,18)7-2-1-3-8(6-7)19-13(20)11-9-4-5-10(22-9)12(11)14(19)21/h1-3,6,9-10,20-21H,4-5H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [3H]DHT from human androgen receptor in MDA453 cells |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM28569
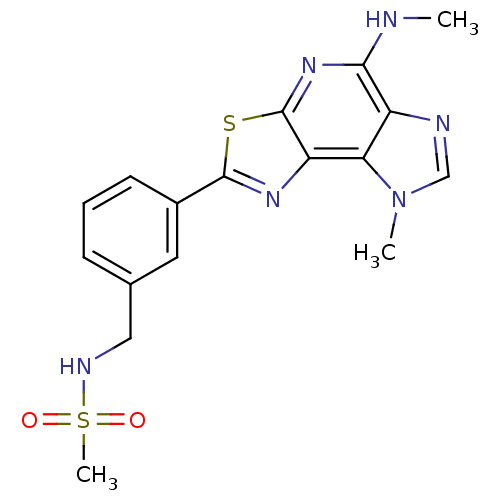
(N-({3-[12-methyl-8-(methylamino)-5-thia-3,7,10,12-...)Show SMILES CNc1nc2sc(nc2c2n(C)cnc12)-c1cccc(CNS(C)(=O)=O)c1 Show InChI InChI=1S/C17H18N6O2S2/c1-18-15-12-14(23(2)9-19-12)13-17(22-15)26-16(21-13)11-6-4-5-10(7-11)8-20-27(3,24)25/h4-7,9,20H,8H2,1-3H3,(H,18,22) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme catalyzed phosphorylation of GST-I-kappa-B-alpha were performed by adding enzyme at room temperature to substrate solutio... |
J Med Chem 52: 1994-2005 (2009)
Article DOI: 10.1021/jm8015816
BindingDB Entry DOI: 10.7270/Q2DB804M |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM28545
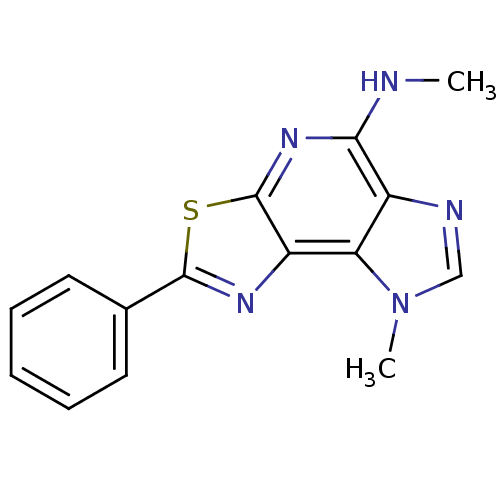
(N,12-dimethyl-4-phenyl-5-thia-3,7,10,12-tetraazatr...)Show InChI InChI=1S/C15H13N5S/c1-16-13-10-12(20(2)8-17-10)11-15(19-13)21-14(18-11)9-6-4-3-5-7-9/h3-8H,1-2H3,(H,16,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme catalyzed phosphorylation of GST-I-kappa-B-alpha were performed by adding enzyme at room temperature to substrate solutio... |
J Med Chem 52: 1994-2005 (2009)
Article DOI: 10.1021/jm8015816
BindingDB Entry DOI: 10.7270/Q2DB804M |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186457

(1-(4-(5-(2-(4,6-dimethylpyridin-2-ylamino)thiazol-...)Show SMILES COc1cc(C)c(Sc2cnc(Nc3cc(C)cc(C)n3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C25H29N5O3S2/c1-15-10-17(3)27-22(11-15)28-25-26-14-23(35-25)34-21-13-19(20(33-5)12-16(21)2)24(32)30-8-6-29(7-9-30)18(4)31/h10-14H,6-9H2,1-5H3,(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186478
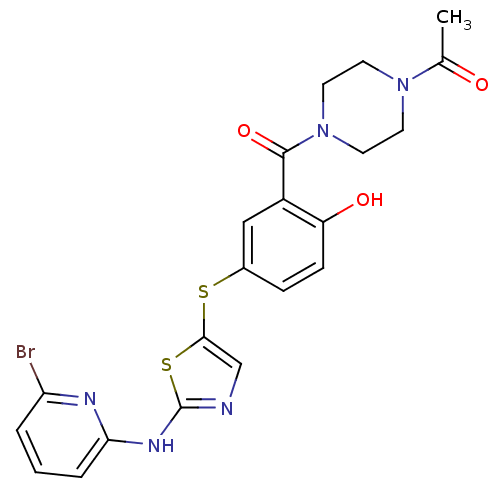
(1-(4-(5-(2-(6-bromopyridin-2-ylamino)thiazol-5-ylt...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cccc(Br)n3)s2)ccc1O Show InChI InChI=1S/C21H20BrN5O3S2/c1-13(28)26-7-9-27(10-8-26)20(30)15-11-14(5-6-16(15)29)31-19-12-23-21(32-19)25-18-4-2-3-17(22)24-18/h2-6,11-12,29H,7-10H2,1H3,(H,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372091

(CHEMBL403669)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(c1)C(F)(F)F |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C18H15F3N2O3/c1-16-5-6-17(2,26-16)13-12(16)14(24)23(15(13)25)10-4-3-9(8-22)11(7-10)18(19,20)21/h3-4,7,24-25H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human androgen receptor in MDA453 cells by alkaline phosphatase reporter gene assay |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372090

(CHEMBL403668)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H15IN2O3/c1-16-5-6-17(2,23-16)13-12(16)14(21)20(15(13)22)10-4-3-9(8-19)11(18)7-10/h3-4,7,21-22H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human androgen receptor in MDA453 cells by alkaline phosphatase reporter gene assay |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186487

(CHEMBL209881 | N-(5-(4-methoxy-2-methyl-5-(morphol...)Show SMILES CNCc1ccc([nH]1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCOCC3)c(OC)cc2C)s1 Show InChI InChI=1S/C23H27N5O4S2/c1-14-10-18(31-3)16(22(30)28-6-8-32-9-7-28)11-19(14)33-20-13-25-23(34-20)27-21(29)17-5-4-15(26-17)12-24-2/h4-5,10-11,13,24,26H,6-9,12H2,1-3H3,(H,25,27,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186484

(1-(4-(5-(2-(4,6-dimethylpyridin-2-ylamino)thiazol-...)Show SMILES CC(=O)N1CCN(CC1)C(=O)c1cc(Sc2cnc(Nc3cc(C)cc(C)n3)s2)ccc1O Show InChI InChI=1S/C23H25N5O3S2/c1-14-10-15(2)25-20(11-14)26-23-24-13-21(33-23)32-17-4-5-19(30)18(12-17)22(31)28-8-6-27(7-9-28)16(3)29/h4-5,10-13,30H,6-9H2,1-3H3,(H,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186459

(CHEMBL211842 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)C3CC3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C22H26N4O4S2/c1-13-10-17(30-3)16(21(29)26-8-6-25(7-9-26)14(2)27)11-18(13)31-19-12-23-22(32-19)24-20(28)15-4-5-15/h10-12,15H,4-9H2,1-3H3,(H,23,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372079

(CHEMBL255331)Show SMILES CC12C[C@H](O)C(C)(O1)c1c(O)n(c(O)c21)-c1ccc(C#N)c(I)c1 |TLB:4:3:7:14.8,THB:9:8:7:3.2,12:14:7:3.2| Show InChI InChI=1S/C17H15IN2O4/c1-16-6-11(21)17(2,24-16)13-12(16)14(22)20(15(13)23)9-4-3-8(7-19)10(18)5-9/h3-5,11,21-23H,6H2,1-2H3/t11-,16?,17?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human androgen receptor in MDA453 cells by alkaline phosphatase reporter gene assay |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Androgen receptor
(Homo sapiens (Human)) | BDBM50372087

(CHEMBL403214)Show SMILES CC12CCC(C)(O1)c1c(O)n(c(O)c21)-c1ccc(c(c1)C(F)(F)F)[N+]([O-])=O |THB:11:13:6:2.3,8:7:6:2.3| Show InChI InChI=1S/C17H15F3N2O5/c1-15-5-6-16(2,27-15)12-11(15)13(23)21(14(12)24)8-3-4-10(22(25)26)9(7-8)17(18,19)20/h3-4,7,23-24H,5-6H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at human androgen receptor in MDA453 cells by alkaline phosphatase reporter gene assay |
Bioorg Med Chem Lett 18: 1910-5 (2008)
Article DOI: 10.1016/j.bmcl.2008.02.006
BindingDB Entry DOI: 10.7270/Q2DJ5GGT |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM28544
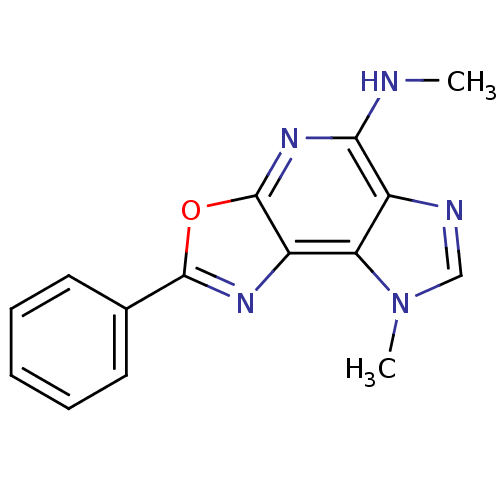
(N,12-dimethyl-4-phenyl-5-oxa-3,7,10,12-tetraazatri...)Show InChI InChI=1S/C15H13N5O/c1-16-13-10-12(20(2)8-17-10)11-15(19-13)21-14(18-11)9-6-4-3-5-7-9/h3-8H,1-2H3,(H,16,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme catalyzed phosphorylation of GST-I-kappa-B-alpha were performed by adding enzyme at room temperature to substrate solutio... |
J Med Chem 52: 1994-2005 (2009)
Article DOI: 10.1021/jm8015816
BindingDB Entry DOI: 10.7270/Q2DB804M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218834
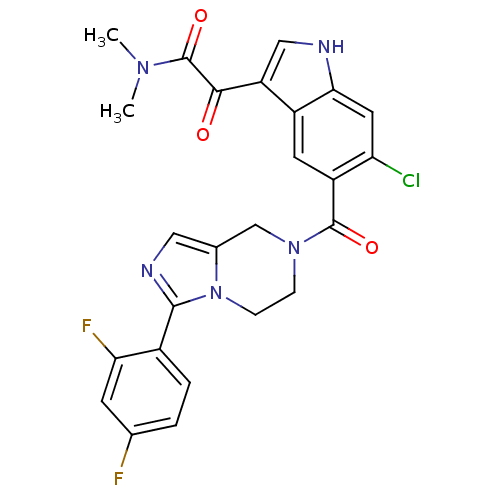
(2-(6-chloro-5-(3-(2,4-difluorophenyl)-5,6,7,8-tetr...)Show SMILES CN(C)C(=O)C(=O)c1c[nH]c2cc(Cl)c(cc12)C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C25H20ClF2N5O3/c1-31(2)25(36)22(34)18-11-29-21-9-19(26)17(8-16(18)21)24(35)32-5-6-33-14(12-32)10-30-23(33)15-4-3-13(27)7-20(15)28/h3-4,7-11,29H,5-6,12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186456
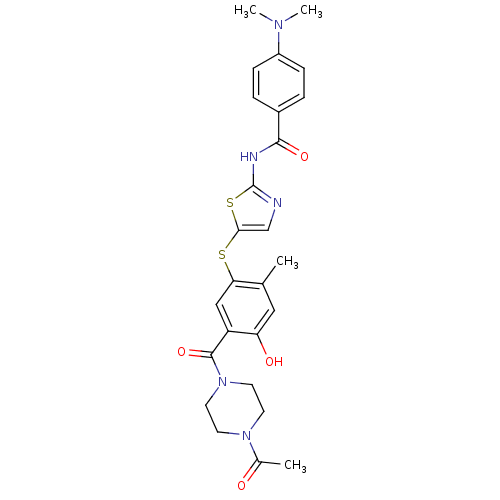
(CHEMBL211355 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES CN(C)c1ccc(cc1)C(=O)Nc1ncc(Sc2cc(C(=O)N3CCN(CC3)C(C)=O)c(O)cc2C)s1 Show InChI InChI=1S/C26H29N5O4S2/c1-16-13-21(33)20(25(35)31-11-9-30(10-12-31)17(2)32)14-22(16)36-23-15-27-26(37-23)28-24(34)18-5-7-19(8-6-18)29(3)4/h5-8,13-15,33H,9-12H2,1-4H3,(H,27,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218824
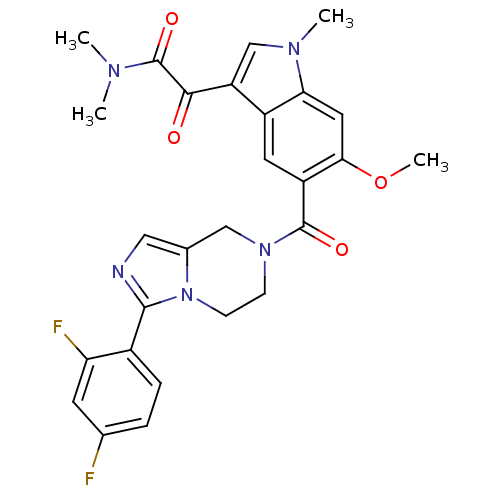
(2-(5-(3-(2,4-difluorophenyl)-5,6,7,8-tetrahydroimi...)Show SMILES COc1cc2n(C)cc(C(=O)C(=O)N(C)C)c2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C27H25F2N5O4/c1-31(2)27(37)24(35)20-14-32(3)22-11-23(38-4)19(10-18(20)22)26(36)33-7-8-34-16(13-33)12-30-25(34)17-6-5-15(28)9-21(17)29/h5-6,9-12,14H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218821
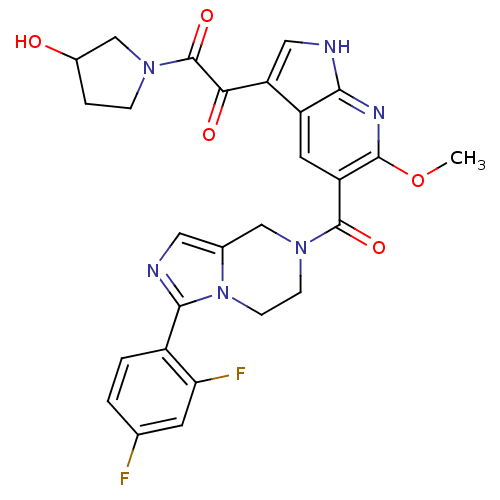
(1-(5-(3-(2,4-difluorophenyl)-5,6,7,8-tetrahydroimi...)Show SMILES COc1nc2[nH]cc(C(=O)C(=O)N3CCC(O)C3)c2cc1C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C27H24F2N6O5/c1-40-25-19(9-18-20(11-30-23(18)32-25)22(37)27(39)33-5-4-16(36)13-33)26(38)34-6-7-35-15(12-34)10-31-24(35)17-3-2-14(28)8-21(17)29/h2-3,8-11,16,36H,4-7,12-13H2,1H3,(H,30,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50218820
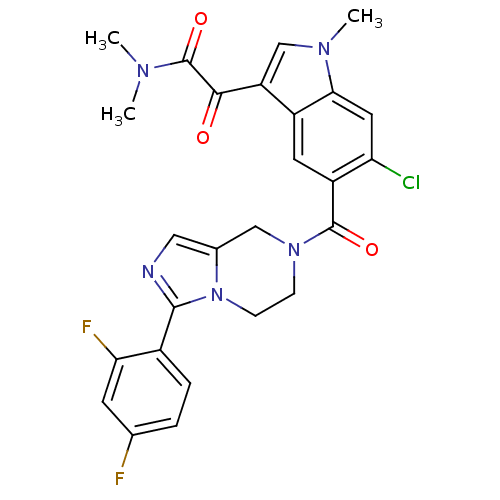
(2-(6-chloro-5-(3-(2,4-difluorophenyl)-5,6,7,8-tetr...)Show SMILES CN(C)C(=O)C(=O)c1cn(C)c2cc(Cl)c(cc12)C(=O)N1CCn2c(C1)cnc2-c1ccc(F)cc1F Show InChI InChI=1S/C26H22ClF2N5O3/c1-31(2)26(37)23(35)19-13-32(3)22-10-20(27)18(9-17(19)22)25(36)33-6-7-34-15(12-33)11-30-24(34)16-5-4-14(28)8-21(16)29/h4-5,8-11,13H,6-7,12H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha MAP kinase |
Bioorg Med Chem Lett 17: 5019-24 (2007)
Article DOI: 10.1016/j.bmcl.2007.07.029
BindingDB Entry DOI: 10.7270/Q2XW4JHT |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50186461

(CHEMBL209163 | N-(5-(5-(1-acetylpiperazine-4-carbo...)Show SMILES COc1cc(C)c(Sc2cnc(NC(=O)c3ccc[nH]3)s2)cc1C(=O)N1CCN(CC1)C(C)=O Show InChI InChI=1S/C23H25N5O4S2/c1-14-11-18(32-3)16(22(31)28-9-7-27(8-10-28)15(2)29)12-19(14)33-20-13-25-23(34-20)26-21(30)17-5-4-6-24-17/h4-6,11-13,24H,7-10H2,1-3H3,(H,25,26,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of Itk |
Bioorg Med Chem Lett 16: 3706-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.060
BindingDB Entry DOI: 10.7270/Q20C4VBR |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit beta
(Homo sapiens (Human)) | BDBM28556
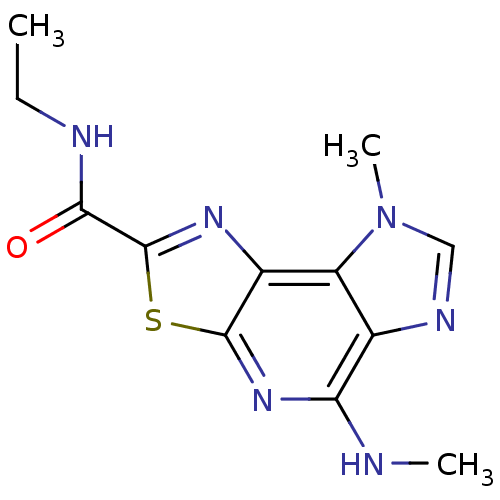
(N-ethyl-12-methyl-8-(methylamino)-5-thia-3,7,10,12...)Show InChI InChI=1S/C12H14N6OS/c1-4-14-10(19)12-16-7-8-6(15-5-18(8)3)9(13-2)17-11(7)20-12/h5H,4H2,1-3H3,(H,13,17)(H,14,19) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Bristol-Myers Squibb Company
| Assay Description
Assays measuring the enzyme catalyzed phosphorylation of GST-I-kappa-B-alpha were performed by adding enzyme at room temperature to substrate solutio... |
J Med Chem 52: 1994-2005 (2009)
Article DOI: 10.1021/jm8015816
BindingDB Entry DOI: 10.7270/Q2DB804M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data