

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398985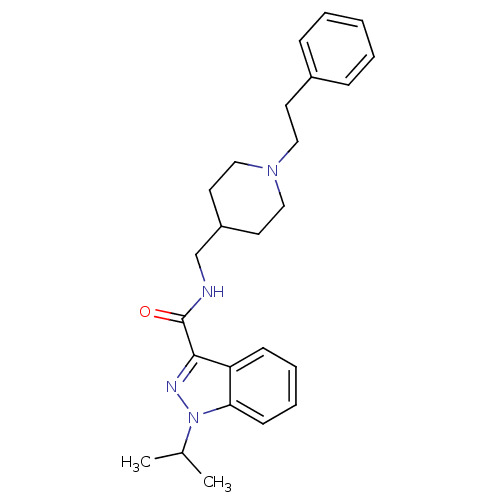 (CHEMBL2177130) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398983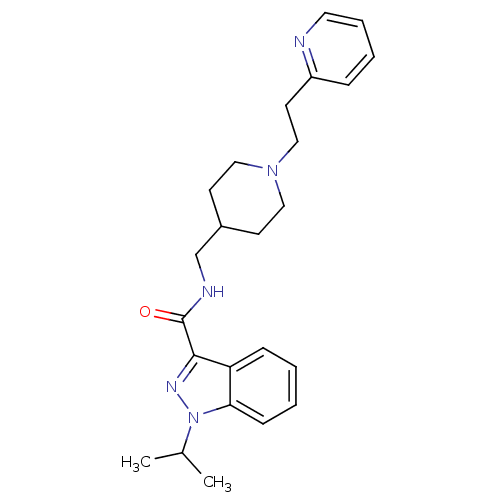 (CHEMBL2179697) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398981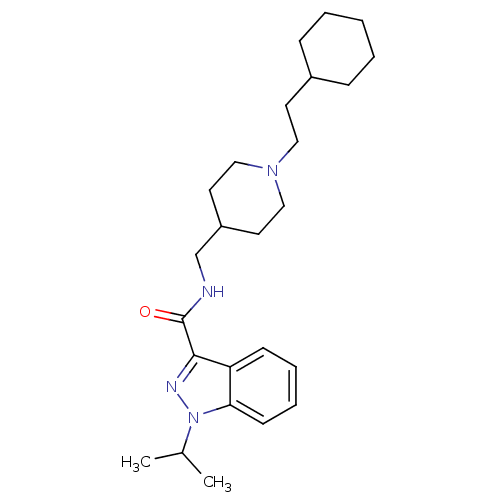 (CHEMBL2179699) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM85026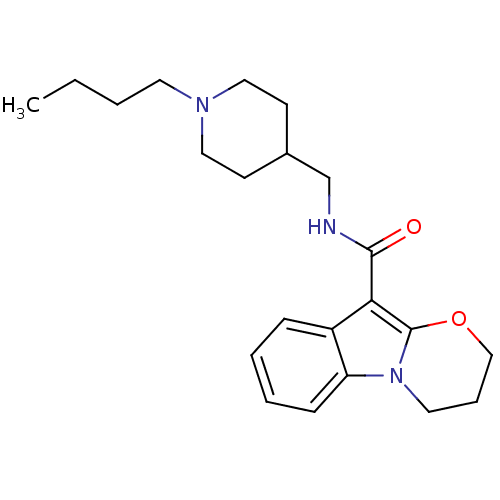 (N-(1-Butylpiperidine-4-ylmethyl)-1,2-(trimethylene...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398975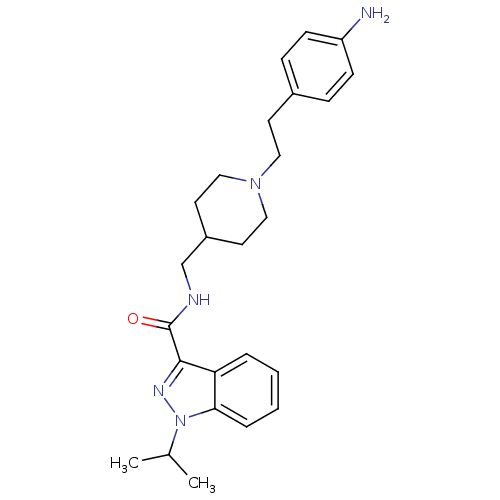 (CHEMBL2179706) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398978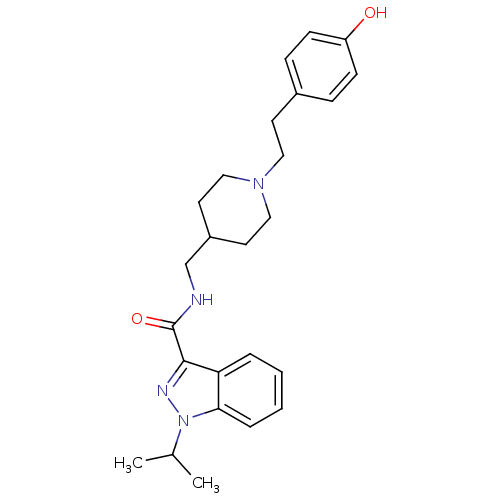 (CHEMBL2179704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398976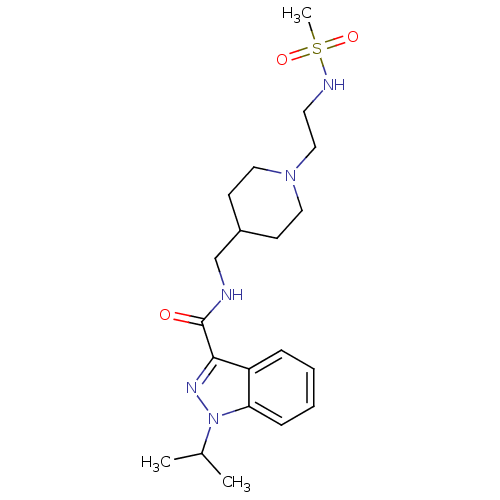 (CHEMBL2179702) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398980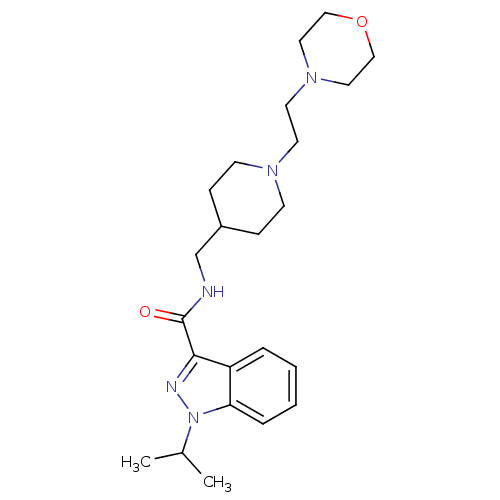 (CHEMBL2179700) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398986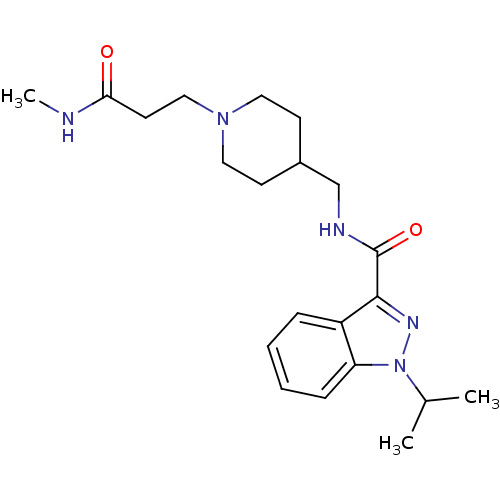 (CHEMBL2179703) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398984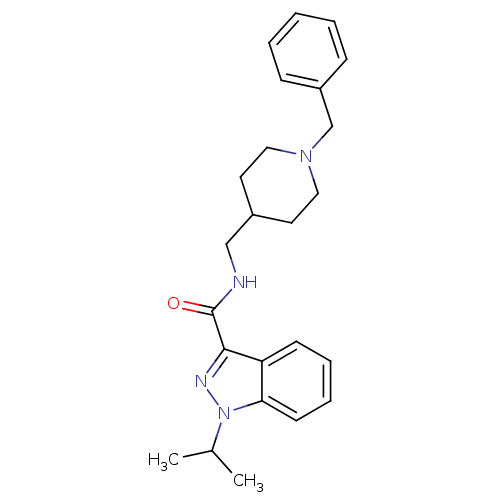 (CHEMBL2179696) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398964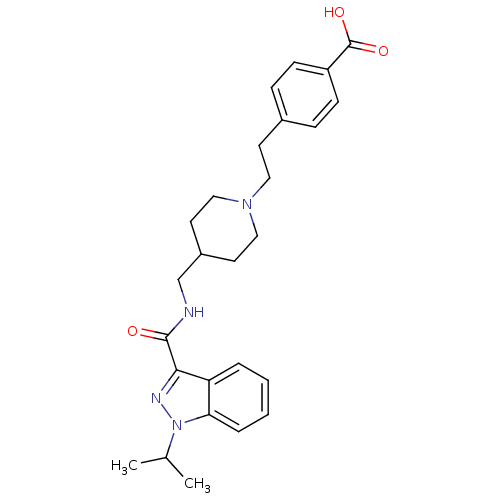 (CHEMBL2179707) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398979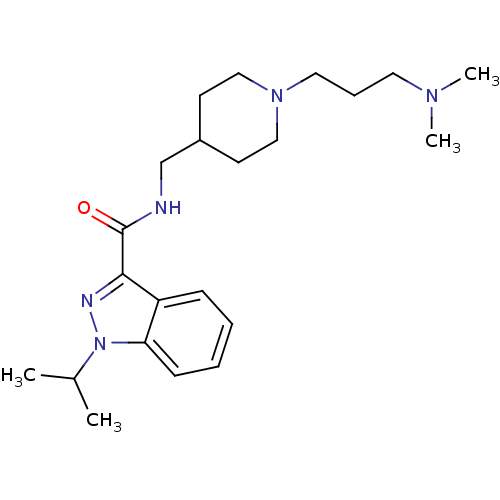 (CHEMBL2179701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398977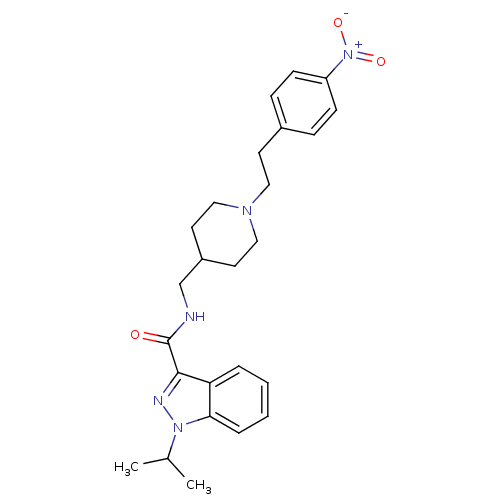 (CHEMBL2179705) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50031942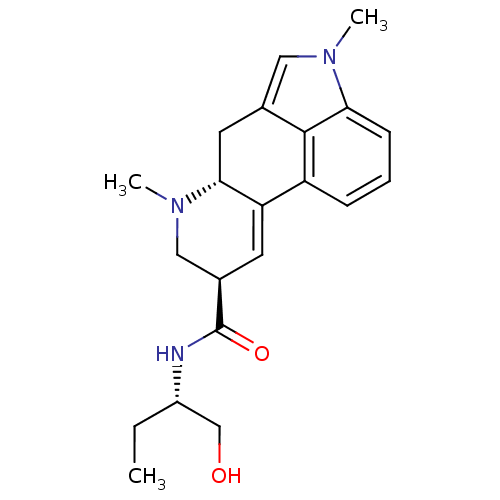 ((6aR,9R)-4,6a,7-Trimethyl-4,6,6a,7,8,9-hexahydro-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398963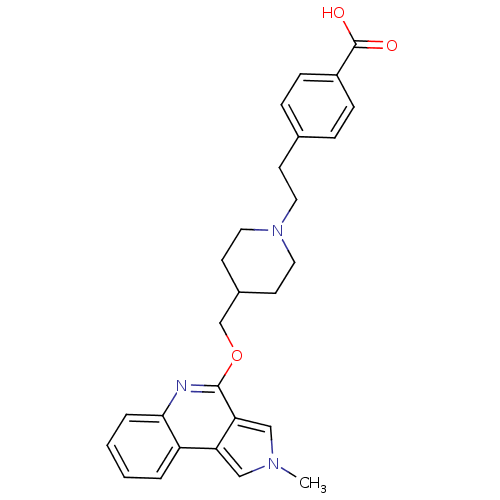 (CHEMBL2179674) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398965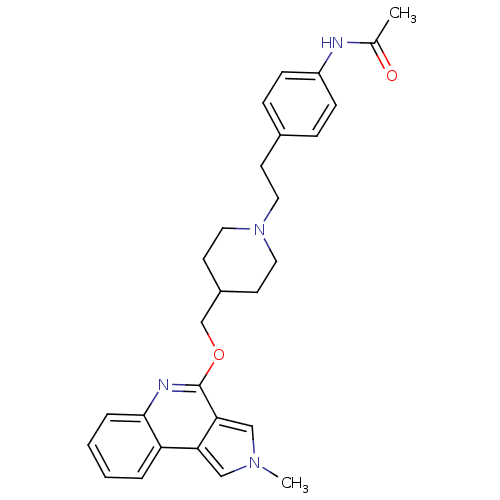 (CHEMBL2179670) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398972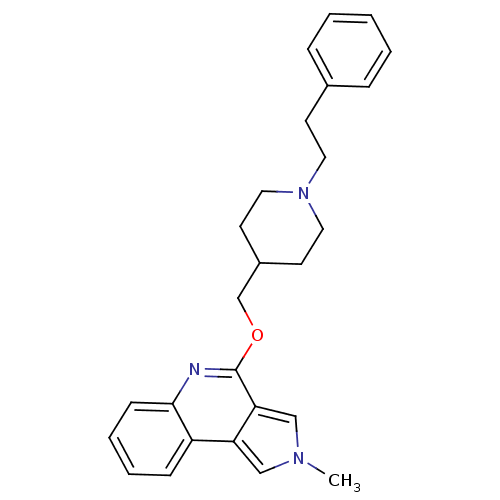 (CHEMBL2179678) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398969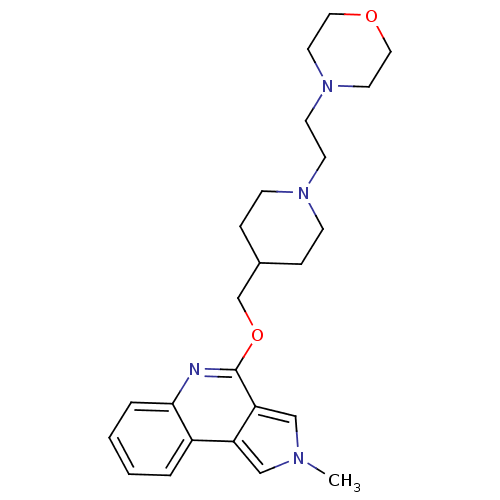 (CHEMBL2179676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398968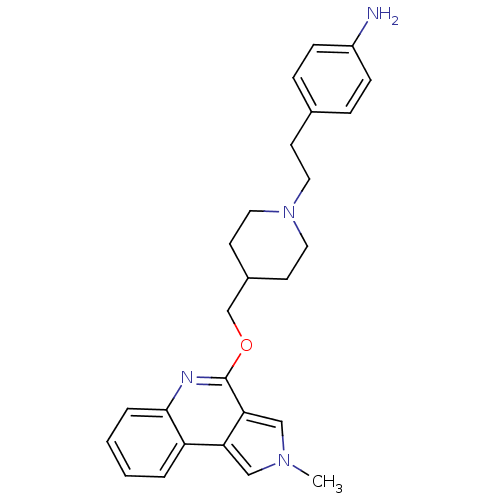 (CHEMBL2179675) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398973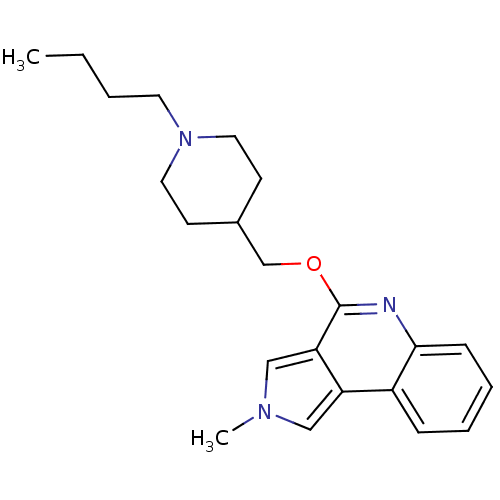 (CHEMBL2179679) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398967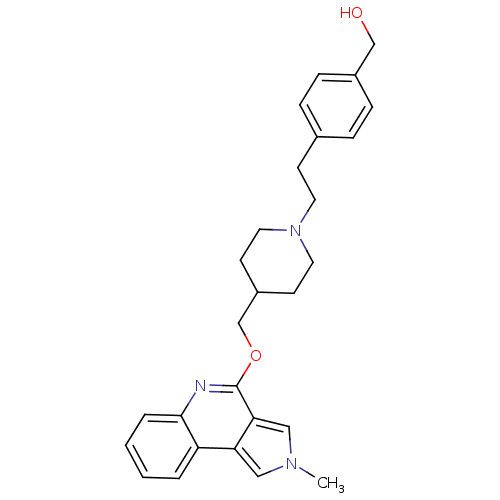 (CHEMBL2179672) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398978 (CHEMBL2179704) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398966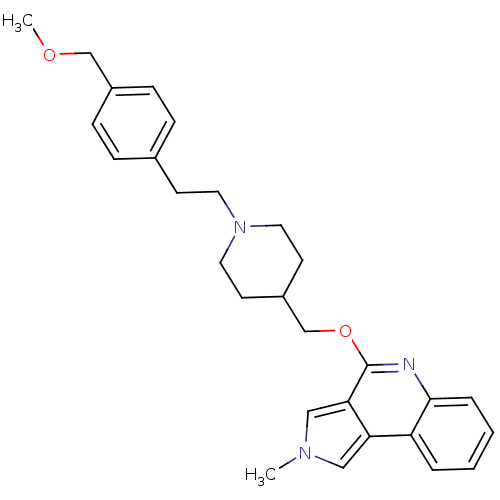 (CHEMBL2179671) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50267626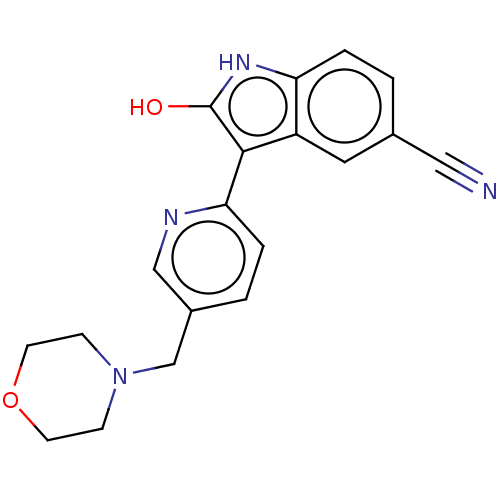 (AZ-11548415 | AZD-1080) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Pharma S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant full length human GSK3beta expressed in Sf21 insect cells using biotinylated-AAEELDSRAGS(PO3H2)PQL peptide as substrate pre... | ACS Med Chem Lett 11: 825-831 (2020) Article DOI: 10.1021/acsmedchemlett.9b00633 BindingDB Entry DOI: 10.7270/Q2930XQ1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398982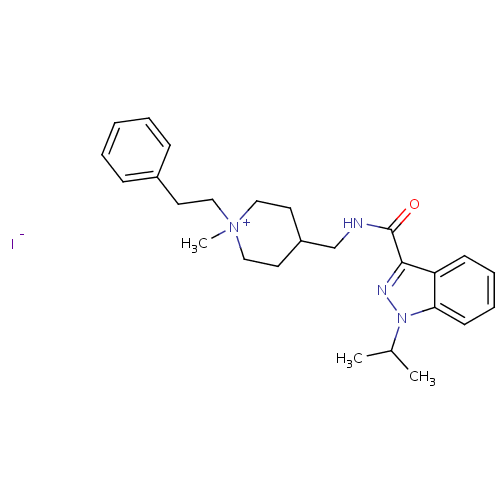 (CHEMBL2179698) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398967 (CHEMBL2179672) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398985 (CHEMBL2177130) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398972 (CHEMBL2179678) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398975 (CHEMBL2179706) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 79.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398971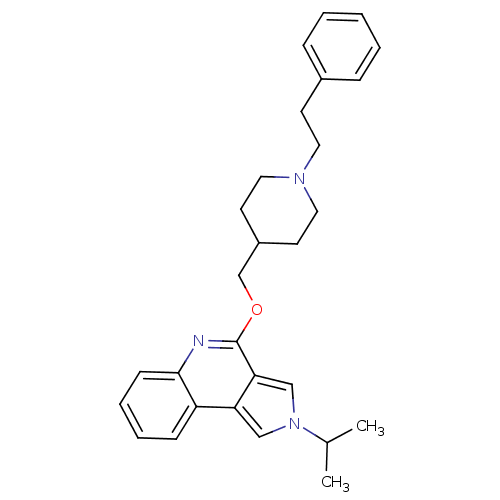 (CHEMBL2179677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398983 (CHEMBL2179697) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398971 (CHEMBL2179677) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398970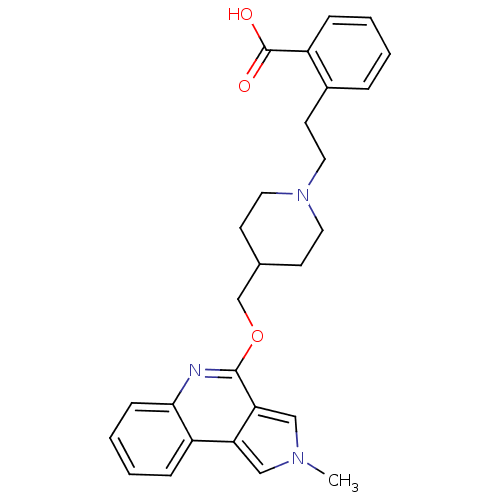 (CHEMBL2179673) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398966 (CHEMBL2179671) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398968 (CHEMBL2179675) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM50398974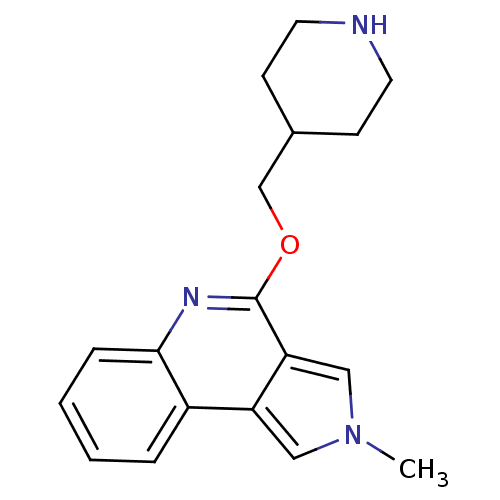 (CHEMBL2179680) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H](1-(2-(methylsulfonamido)ethyl)piperidin-4-yl)methyl 1-methyl-1H-indole-3-carboxylate from human 5HT4R expressed in HEK293 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398970 (CHEMBL2179673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398979 (CHEMBL2179701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398976 (CHEMBL2179702) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398977 (CHEMBL2179705) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398973 (CHEMBL2179679) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398980 (CHEMBL2179700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398984 (CHEMBL2179696) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398982 (CHEMBL2179698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398981 (CHEMBL2179699) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398965 (CHEMBL2179670) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398963 (CHEMBL2179674) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398964 (CHEMBL2179707) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50398969 (CHEMBL2179676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from human 5HT2AR expressed in CHOK1 cells | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 4 (Homo sapiens (Human)) | BDBM29525 (3H-GR113808 | CHEMBL518682 | GR 113808 | [3H] GR 1...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Angelini Santa Palomba Research Center Curated by ChEMBL | Assay Description Antagonist activity at human 5HT4ER expressed in CHO cells assessed as reduction in cAMP levels | J Med Chem 55: 9446-66 (2012) Article DOI: 10.1021/jm300573d BindingDB Entry DOI: 10.7270/Q27082JT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 413 total ) | Next | Last >> |