

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278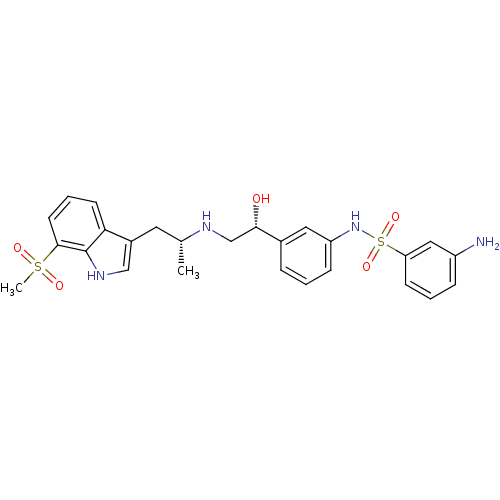 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267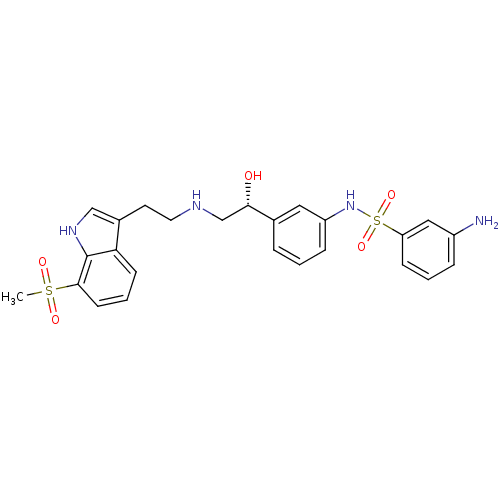 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Agonistic activity was determined by measuring cAMP accumulation in CHO cells expressing cloned human Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250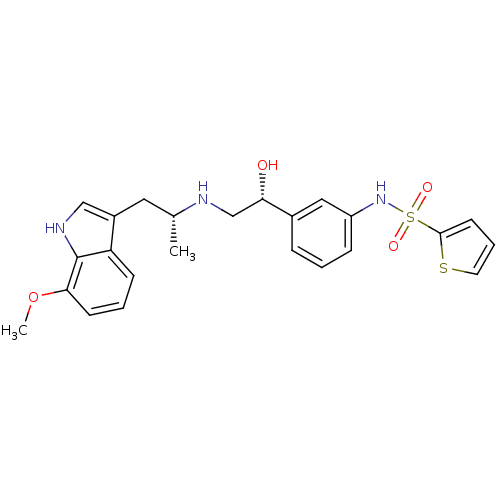 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257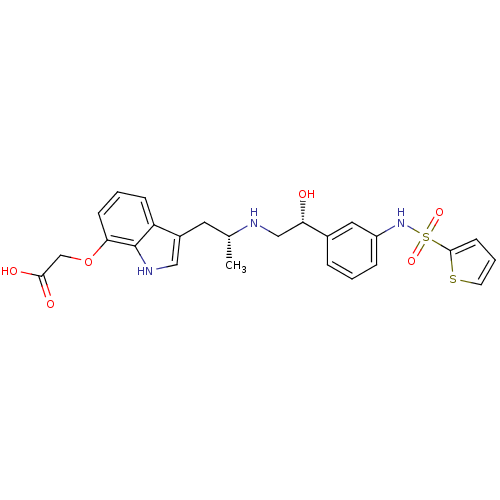 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252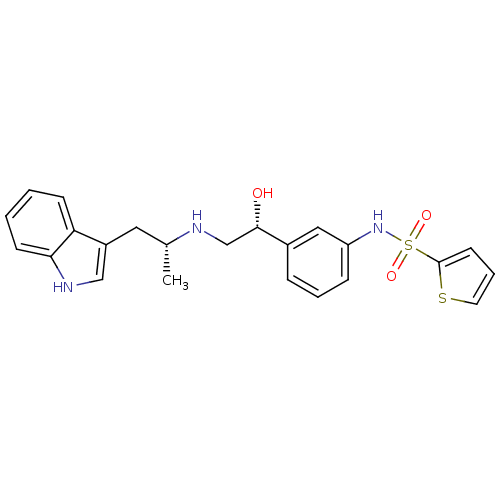 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-3 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270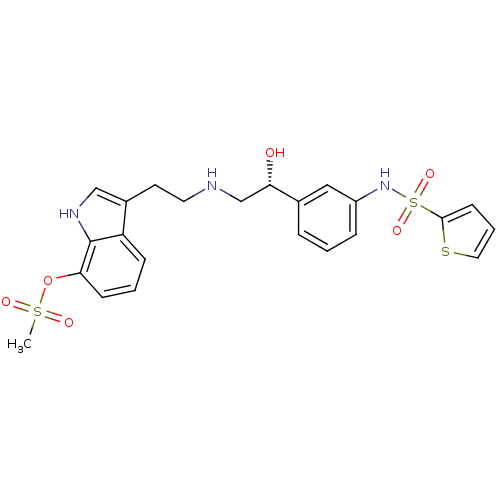 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-3 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156278 (3-Amino-N-(3-{(R)-1-hydroxy-2-[(R)-2-(7-methanesul...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156250 (CHEMBL361505 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 48 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156260 (CHEMBL184407 | Methanesulfonic acid 3-(2-{(R)-(R)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156267 (3-Amino-N-(3-{(R)-1-hydroxy-2-[2-(7-methanesulfony...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156252 (CHEMBL185836 | Thiophene-2-sulfonic acid (3-{(R)-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]iodocyanopindolol binding to Beta-2 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156270 (CHEMBL188622 | Methanesulfonic acid 3-(2-{2-hydrox...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5963-6 (2004) Article DOI: 10.1016/j.bmcl.2004.09.054 BindingDB Entry DOI: 10.7270/Q2BZ65HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-1 adrenergic receptor (Homo sapiens (Human)) | BDBM50156257 (CHEMBL188196 | [3-(2-{(R)-(R)-2-Hydroxy-2-[3-(thio...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Binding inhibition constant was determined by inhibition of [125I]-iodocyanopindolol binding to Beta-1 adrenergic receptor | Bioorg Med Chem Lett 14: 5959-62 (2004) Article DOI: 10.1016/j.bmcl.2004.10.035 BindingDB Entry DOI: 10.7270/Q2GQ6X7S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567336 (CHEMBL4876055) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567332 (CHEMBL4853586) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567334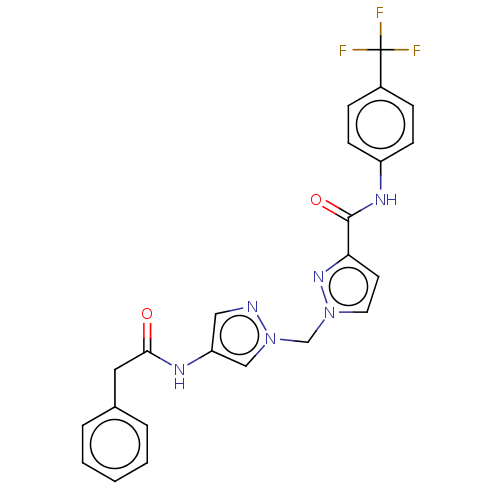 (CHEMBL4869045) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567333 (CHEMBL4865589) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567331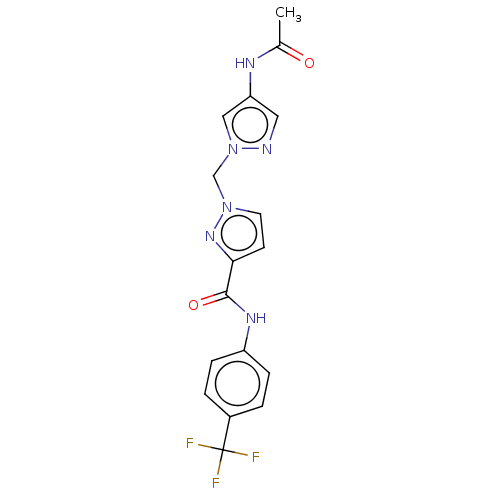 (CHEMBL4850885) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567335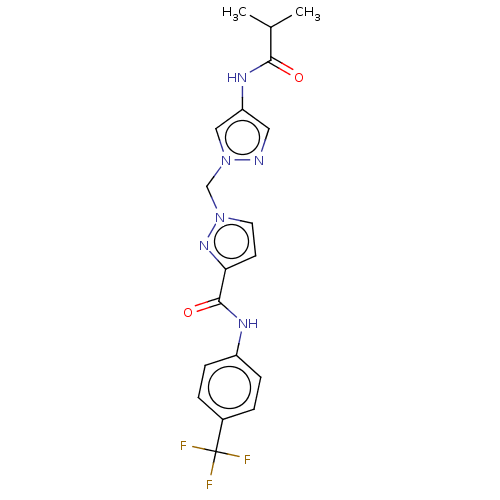 (CHEMBL4854814) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567321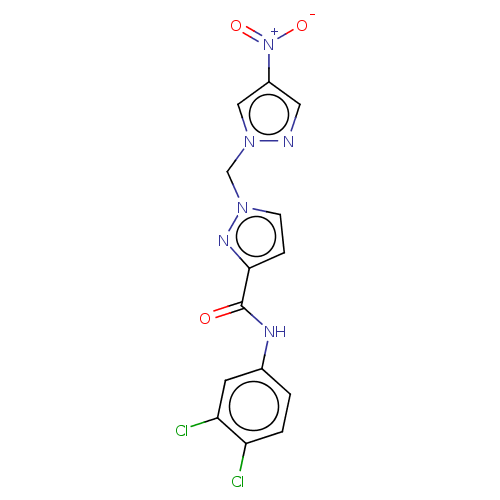 (CHEMBL4849248) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567330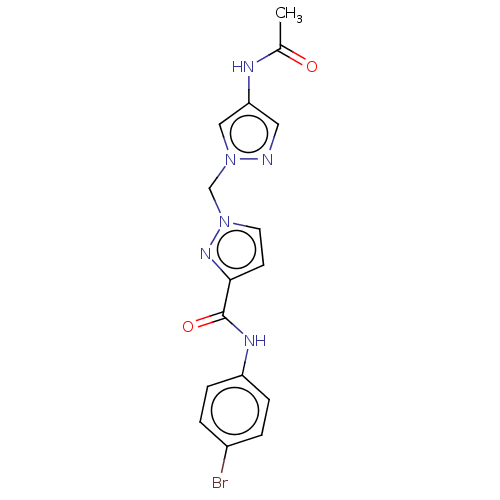 (CHEMBL4845740) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567311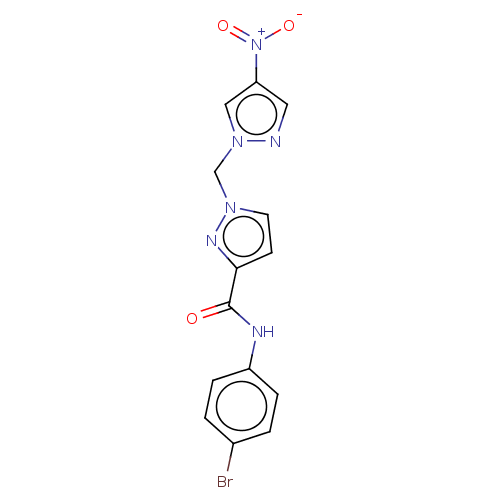 (CHEMBL4853734) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567314 (CHEMBL4867409) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567329 (CHEMBL4875814) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567317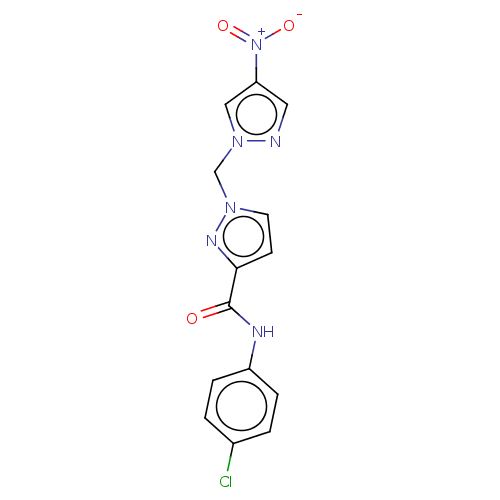 (CHEMBL4858182) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567319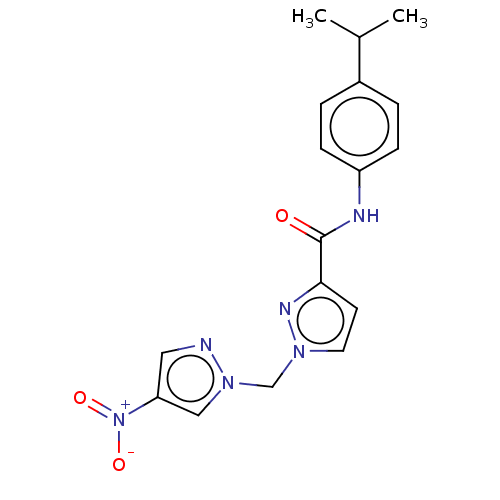 (CHEMBL4863040) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567328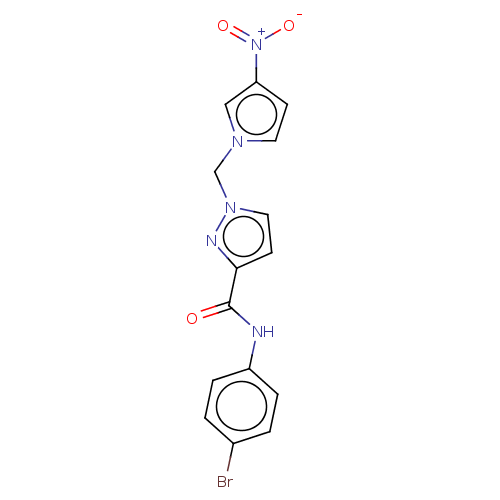 (CHEMBL4874770) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567315 (CHEMBL4849512) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567316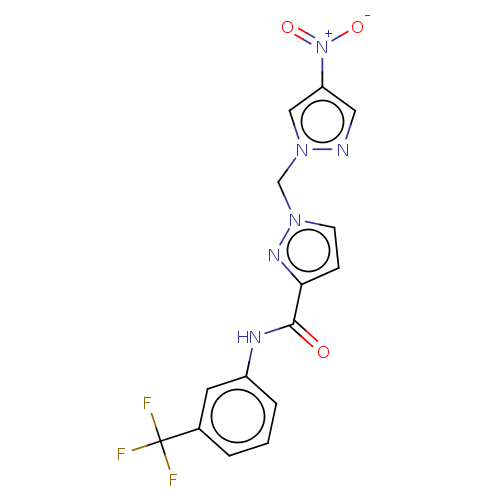 (CHEMBL4870109) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567327 (CHEMBL4878520) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567313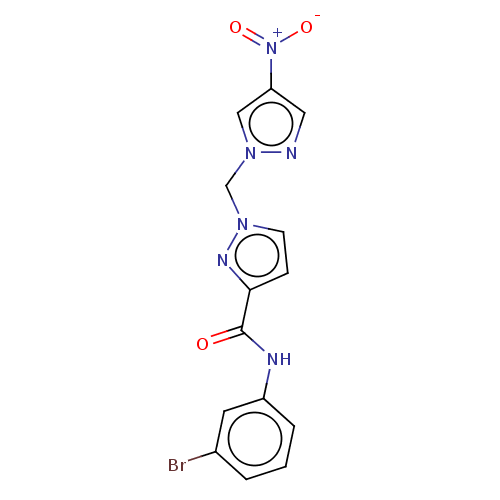 (CHEMBL4874103) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567323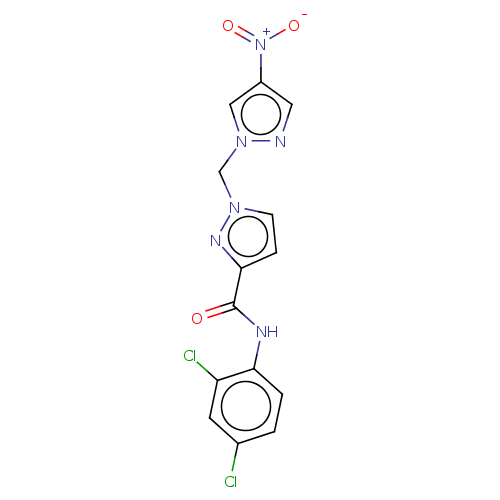 (CHEMBL4877296) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567312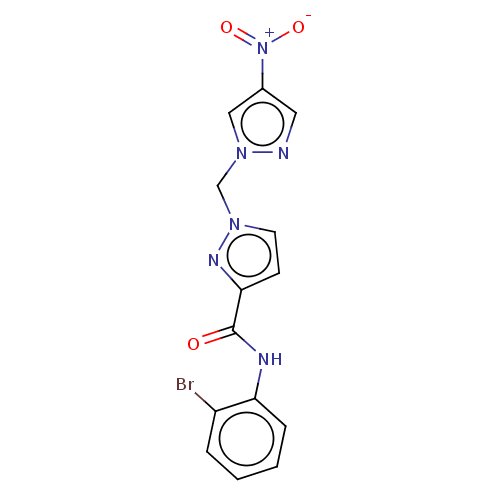 (CHEMBL4849180) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567326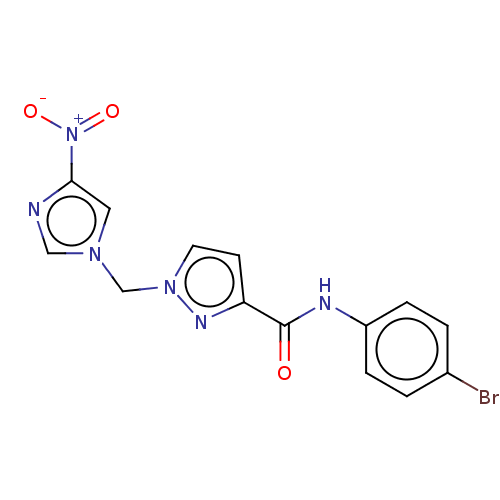 (CHEMBL4862722) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567325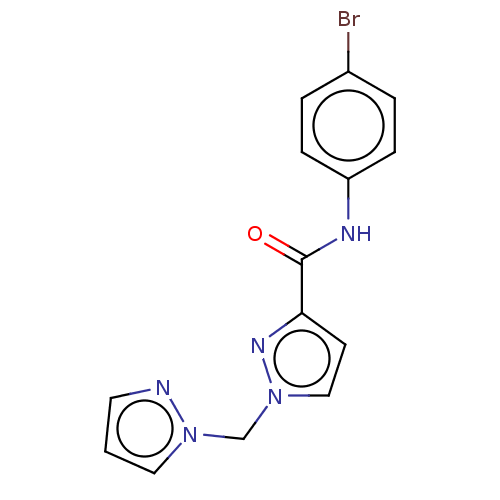 (CHEMBL4849932) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567324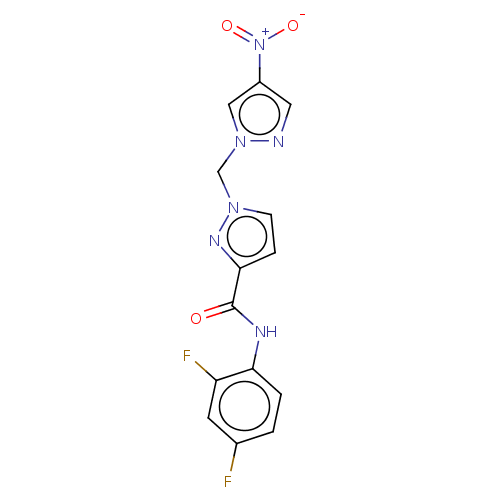 (CHEMBL4855767) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567322 (CHEMBL4860096) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567320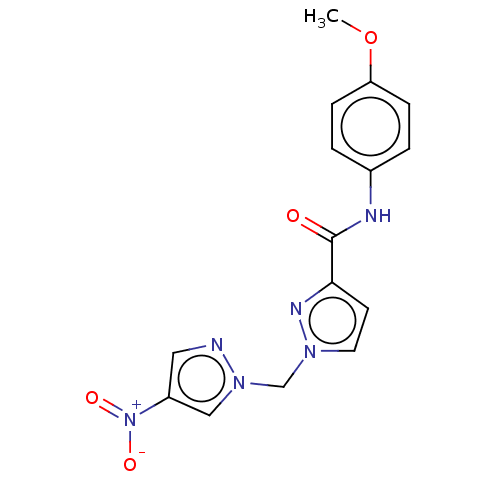 (CHEMBL4849924) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hypoxia-inducible factor 1-alpha (Homo sapiens (Human)) | BDBM50567318 (CHEMBL4849221) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hypoxia-induced HIF1 alpha transcriptional activity in human HeLa cells transfected with luciferase reporter containing of HRE assessed... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116375 BindingDB Entry DOI: 10.7270/Q2542S9V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 208 total ) | Next | Last >> |