

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120151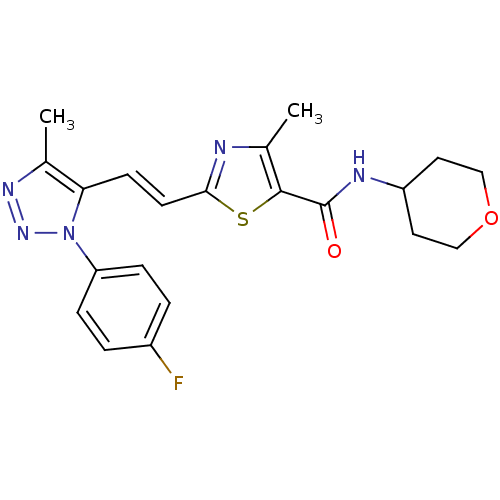 (US8697696, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.60 | -45.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120150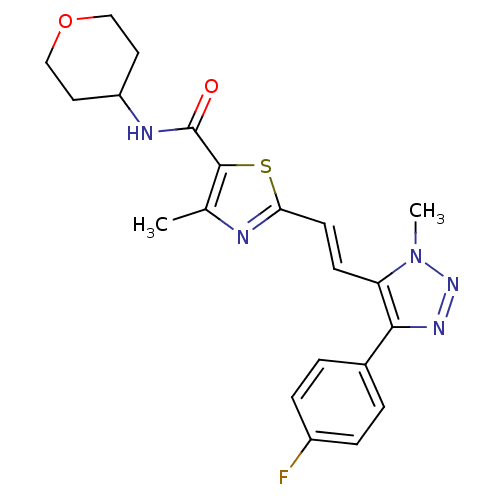 (US8697696, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.20 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120152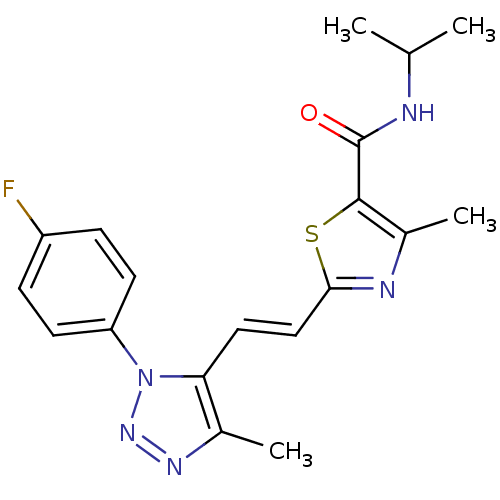 (US8697696, 9) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 6.80 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120149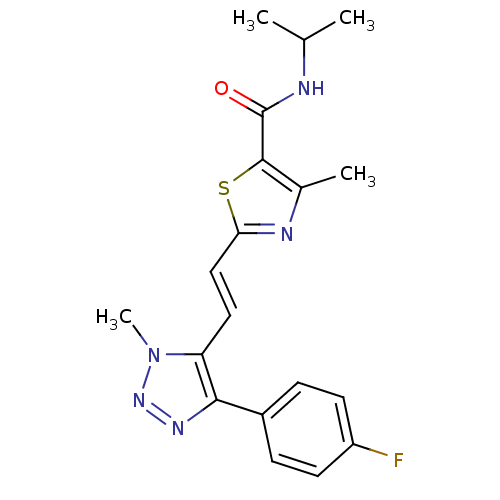 (US8697696, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 11.3 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120154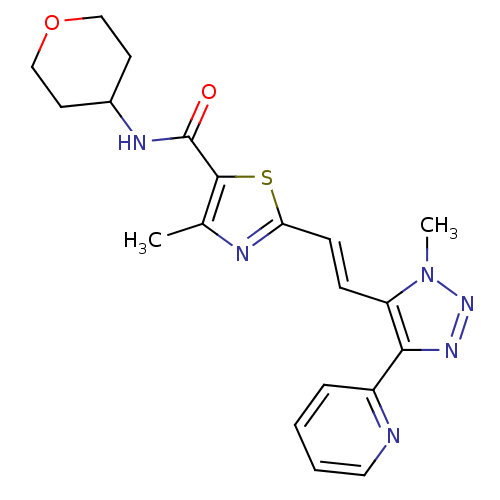 (US8697696, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 12.7 | -41.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120146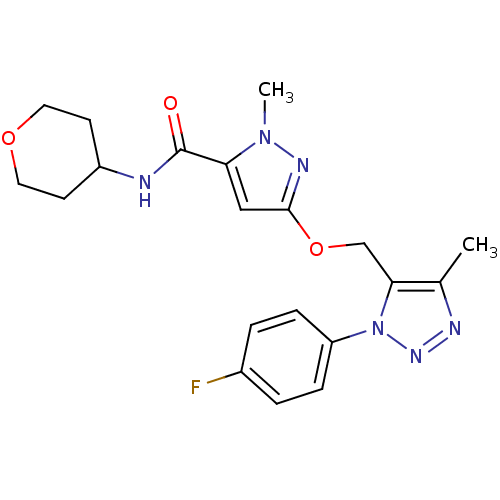 (US8697696, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 15.7 | -41.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120153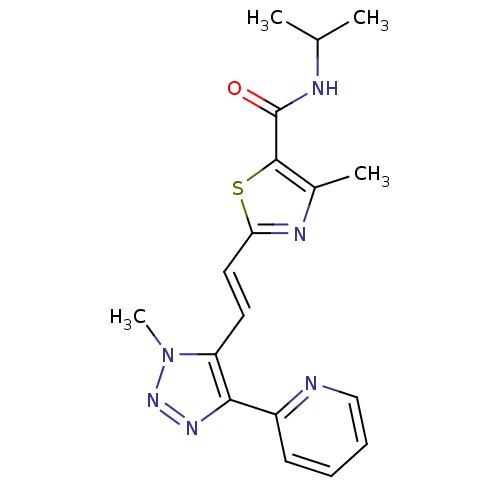 (US8697696, 10) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 20.7 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120147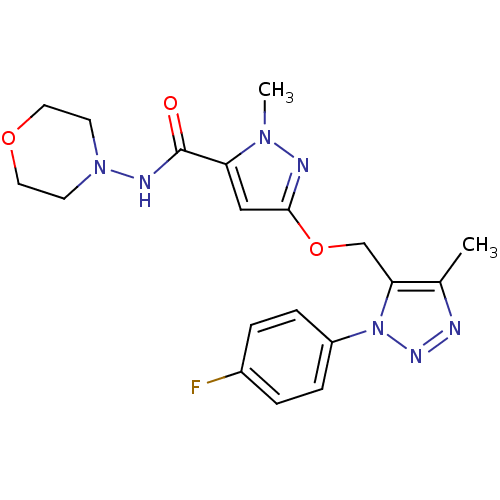 (US8697696, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 22.2 | -40.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120144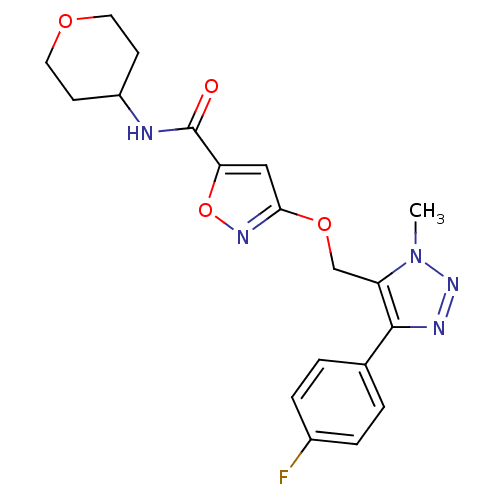 (US8697696, 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 28.8 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120148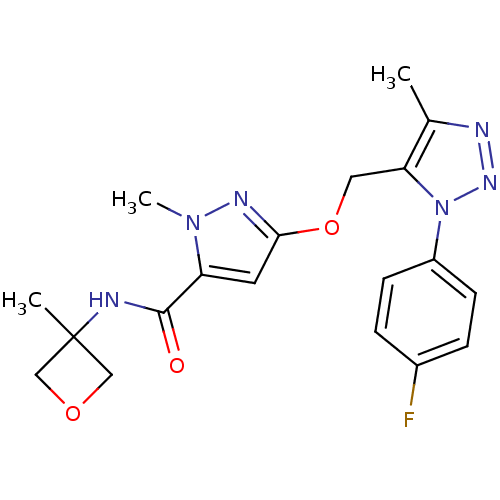 (US8697696, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43.2 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-5 (Homo sapiens (Human)) | BDBM120145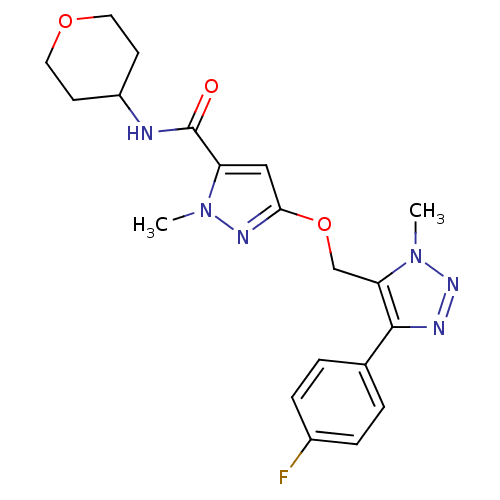 (US8697696, 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 45.3 | -39.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 4 |
Roche Palo Alto LLC US Patent | Assay Description Radioligand binding assays were carried out in a volume of 200 mL (96-well plates) which contained 100 mL of cell membranes, [3H]flumazenil at a conc... | US Patent US8697696 (2014) BindingDB Entry DOI: 10.7270/Q2DB80HJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319434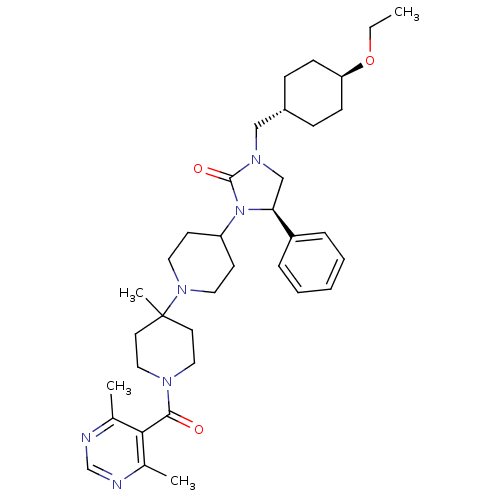 ((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310731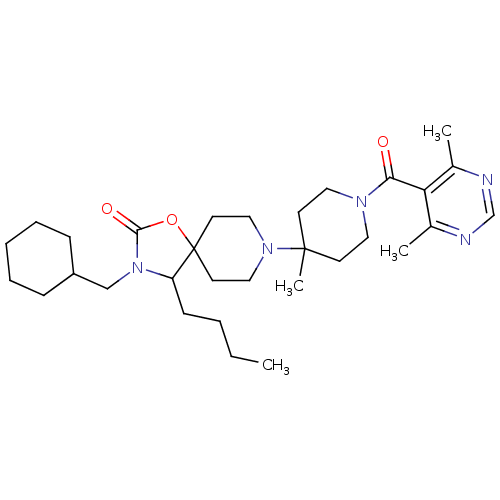 (4-butyl-3-(cyclohexylmethyl)-8-(1-(4,6-dimethylpyr...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319451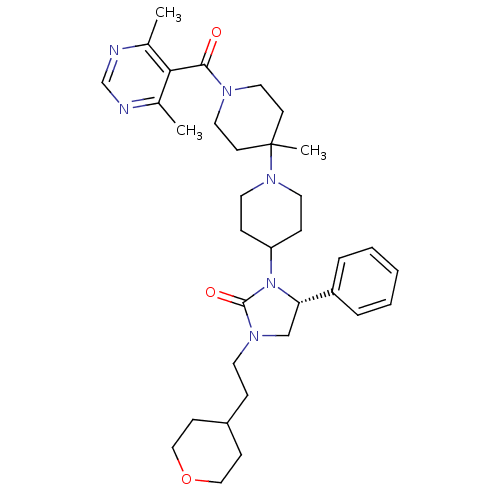 ((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310730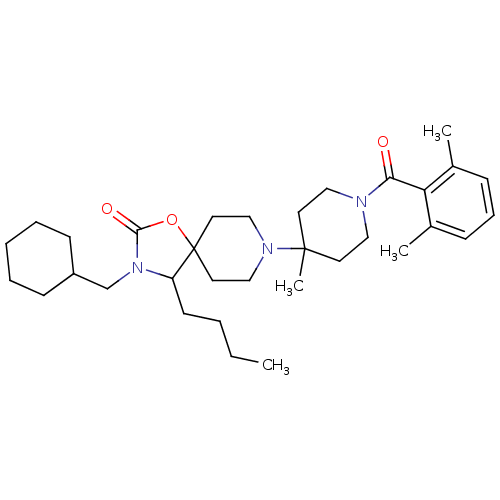 (4-butyl-3-(cyclohexylmethyl)-8-(1-(2,6-dimethylben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310747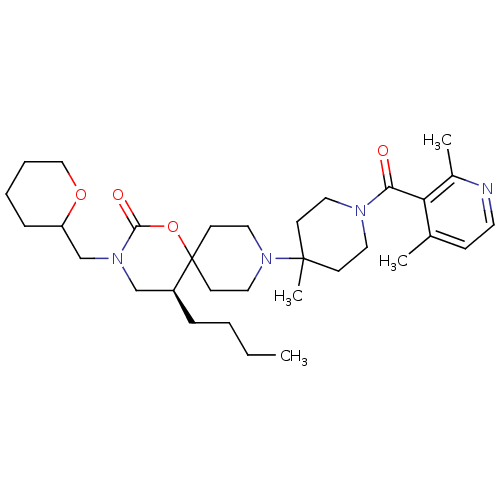 ((5S)-5-butyl-9-(1-(2,4-dimethylnicotinoyl)-4-methy...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254720 ((4-(7-butyl-9-(morpholinosulfonyl)-3,9-diazaspiro[...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310744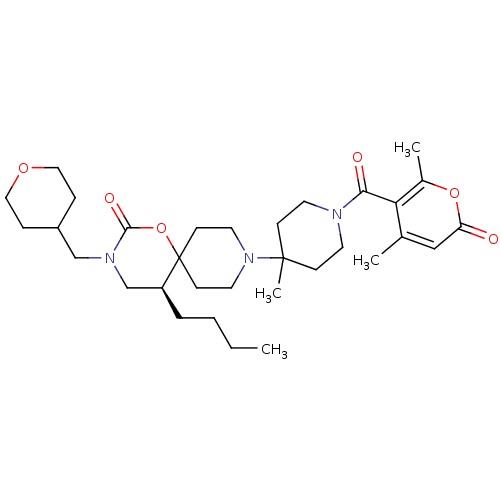 ((S)-5-butyl-9-(1-(4,6-dimethyl-2-oxo-2H-pyran-5-ca...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319447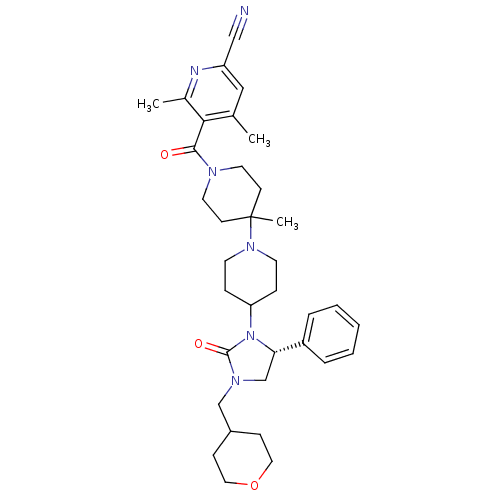 ((R)-4,6-dimethyl-5-(4'-methyl-4-(2-oxo-5-phenyl-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319442 ((R)-4,6-dimethyl-5-(4'-methyl-4-(2-oxo-5-(pyridin-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398095 (CHEMBL2182043 | US9321738, 2) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254677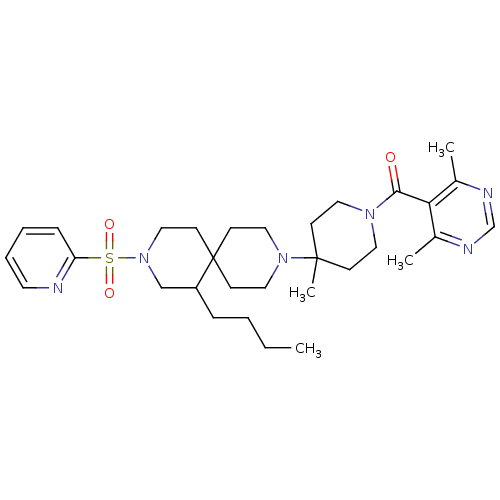 ((4-(7-butyl-9-(pyridin-2-ylsulfonyl)-3,9-diazaspir...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398093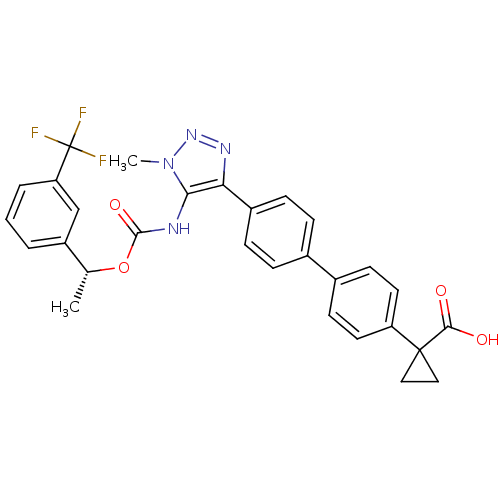 (CHEMBL2182046 | US9321738, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310726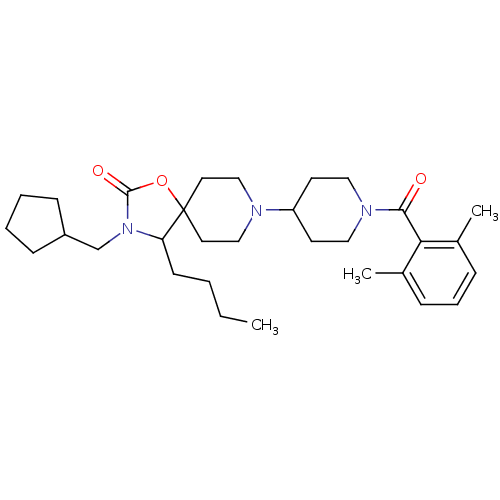 (4-butyl-3-(cyclopentylmethyl)-8-(1-(2,6-dimethylbe...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398094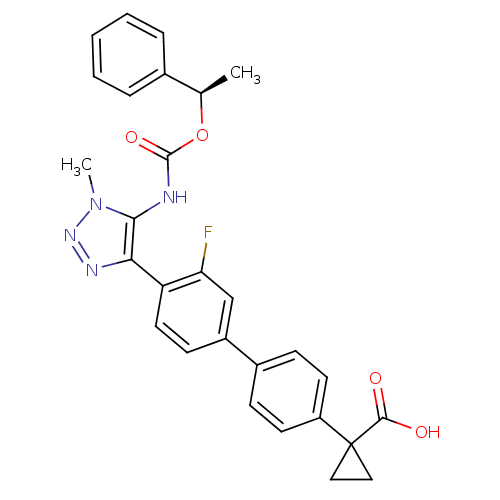 (CHEMBL2182044 | US9321738, 11) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398091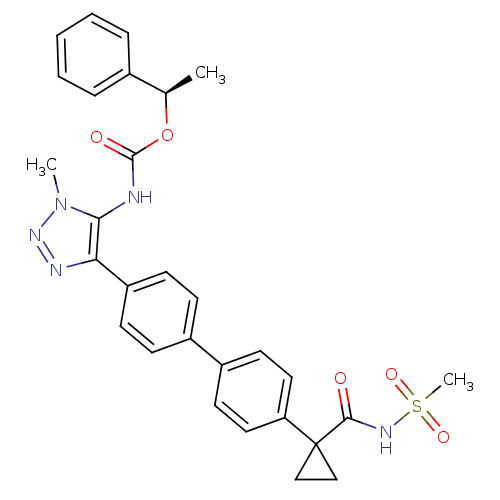 (CHEMBL2182049 | US9321738, 12) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 18.8 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254752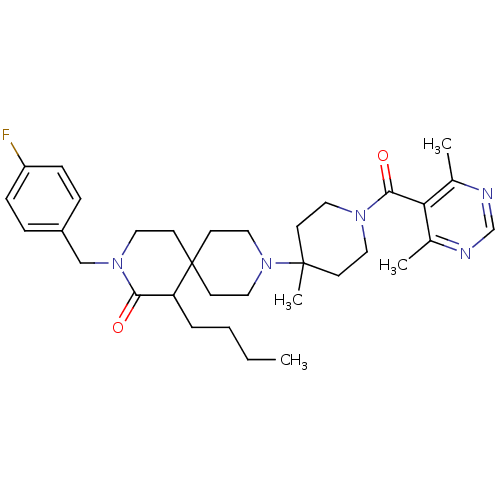 (1-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbonyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254748 ((R)-(4-(7-butyl-9-(2,2,2-trifluoroethylsulfonyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254675 ((4-(7-butyl-9-(phenylsulfonyl)-3,9-diazaspiro[5.5]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319453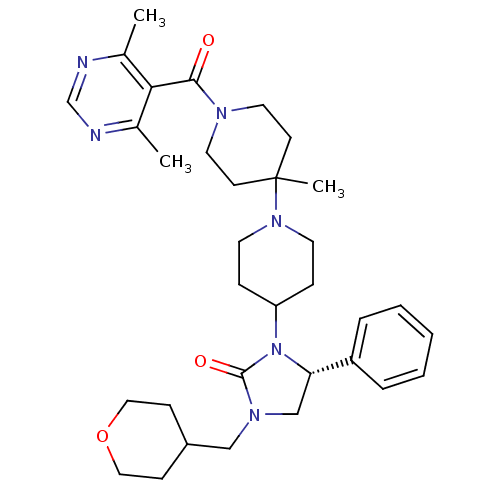 ((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319448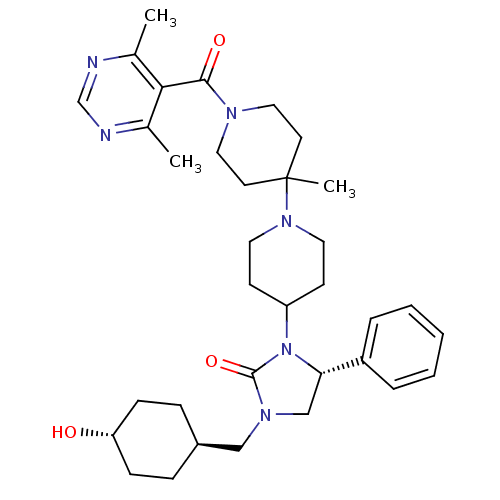 ((R)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319446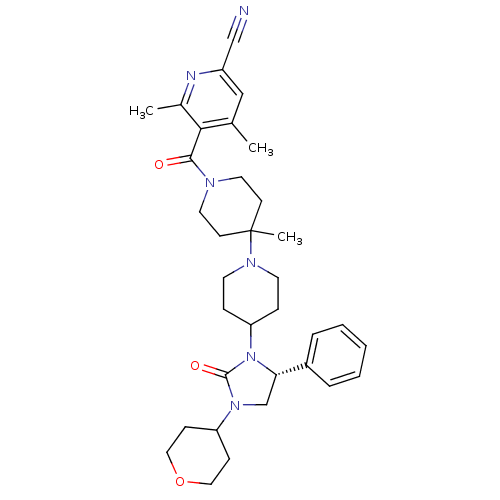 ((R)-4,6-dimethyl-5-(4'-methyl-4-(2-oxo-5-phenyl-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254718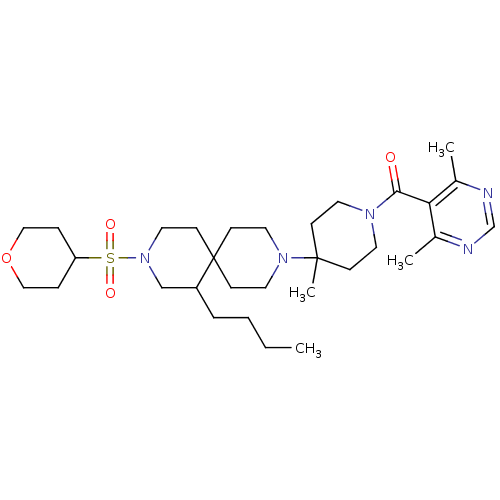 ((4-(7-butyl-9-(tetrahydro-2H-pyran-4-ylsulfonyl)-3...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254716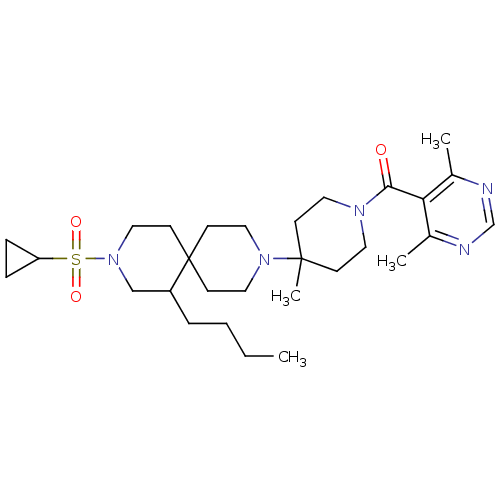 ((4-(7-butyl-9-(cyclopropylsulfonyl)-3,9-diazaspiro...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310743 ((S)-5-(4-(5-butyl-3-methyl-2-oxo-1-oxa-3,9-diazasp...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310727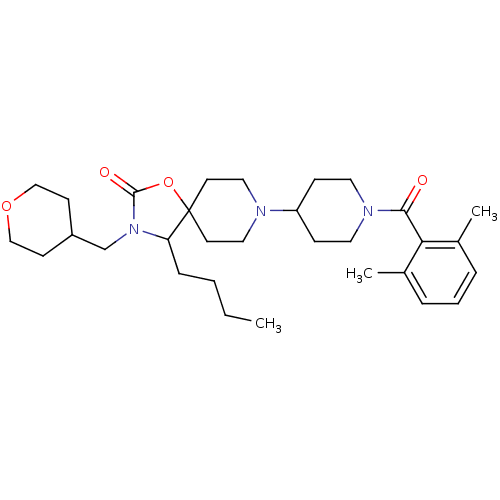 (4-butyl-8-(1-(2,6-dimethylbenzoyl)piperidin-4-yl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310742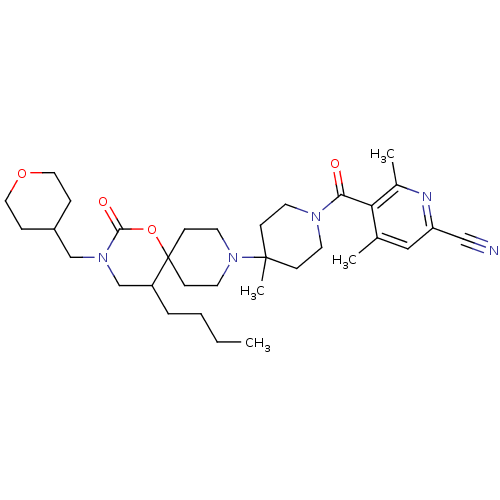 ((+/-)-5-(4-(5-butyl-2-oxo-3-((tetrahydro-2H-pyran-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319443 ((R)-4,6-dimethyl-5-(4'-methyl-4-(3-methyl-2-oxo-5-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysophosphatidic acid receptor 1 (Homo sapiens (Human)) | BDBM50398090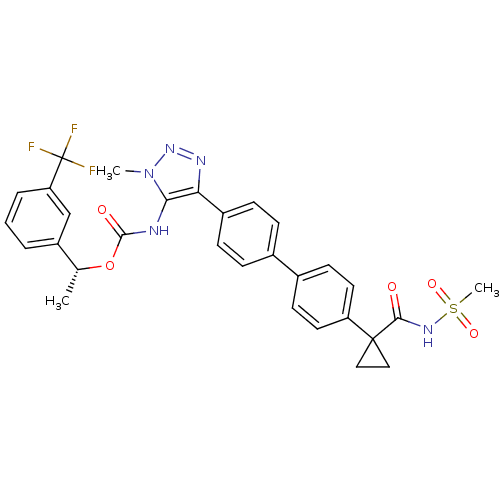 (CHEMBL2182050 | US9321738, 13) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 24.5 | n/a | n/a | n/a | n/a | n/a | 25 |
HOFFMAN-LA ROCHE INC. US Patent | Assay Description Test compounds were prepared by adding 90 μL of HBSS/20 mM HEPES/0.1% BSA buffer to 2 μL of serially diluted compounds. To prepare serial dilut... | US Patent US9321738 (2016) BindingDB Entry DOI: 10.7270/Q2B56HKJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254715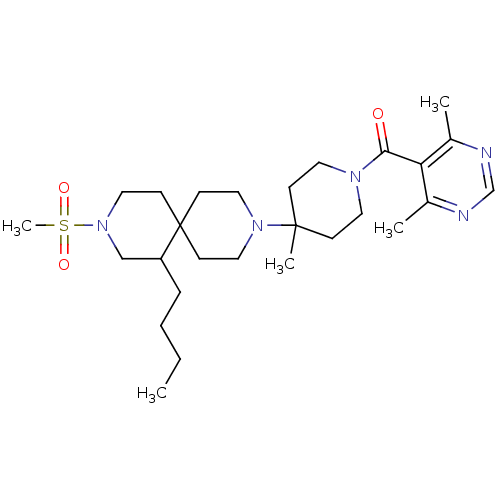 ((4-(7-butyl-9-(methylsulfonyl)-3,9-diazaspiro[5.5]...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319441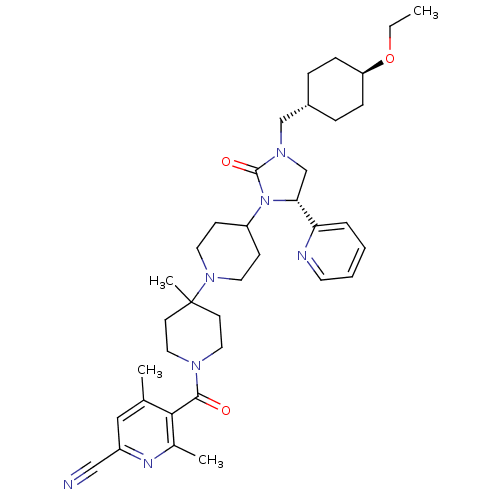 (5-(4-((R)-3-((trans-4-ethoxycyclohexyl)methyl)-2-o...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310737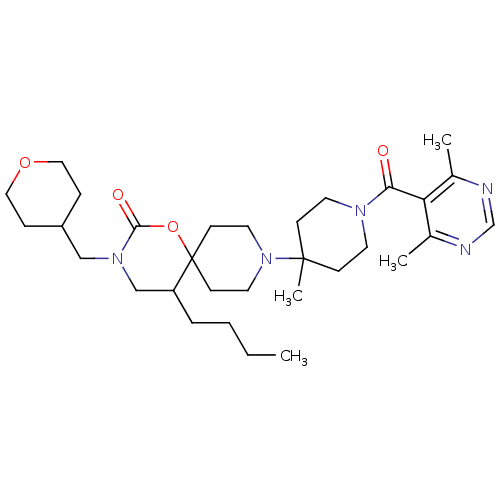 (5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbonyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310739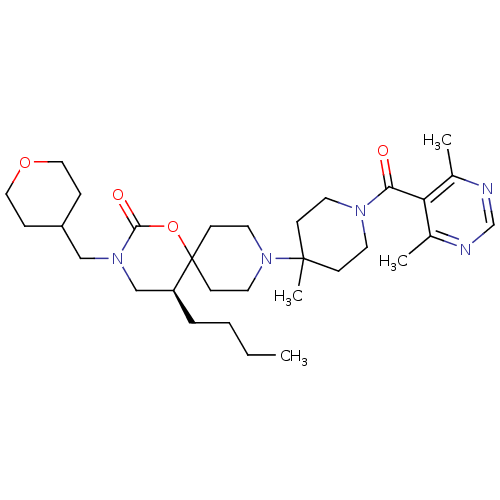 ((S)-5-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbony...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319445 ((R)-4-((3-(1'-(6-cyano-2,4-dimethylnicotinoyl)-4'-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319449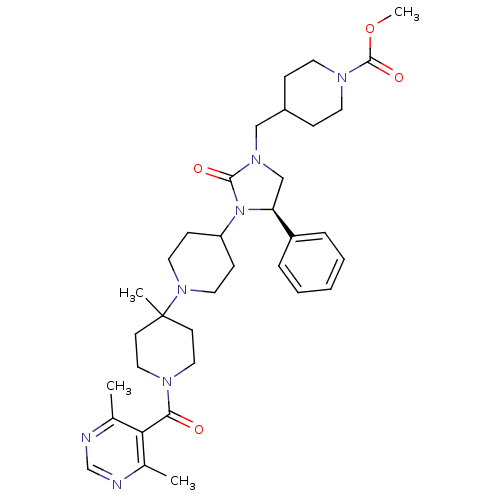 ((R)-methyl 4-((3-(1'-(4,6-dimethylpyrimidine-5-car...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254719 ((4-(7-butyl-9-(1-methyl-1H-imidazol-4-ylsulfonyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310746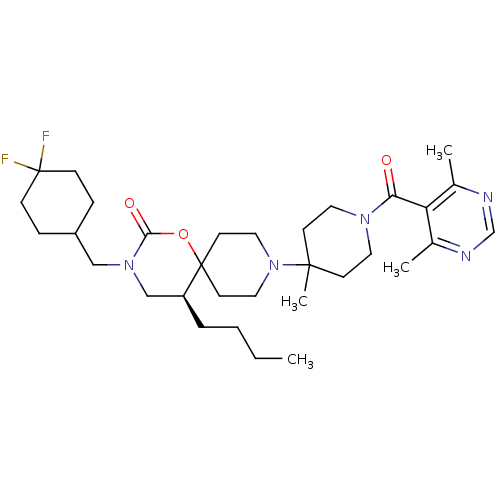 ((5S)-5-butyl-3-((4,4-difluorocyclohexyl)methyl)-9-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50319452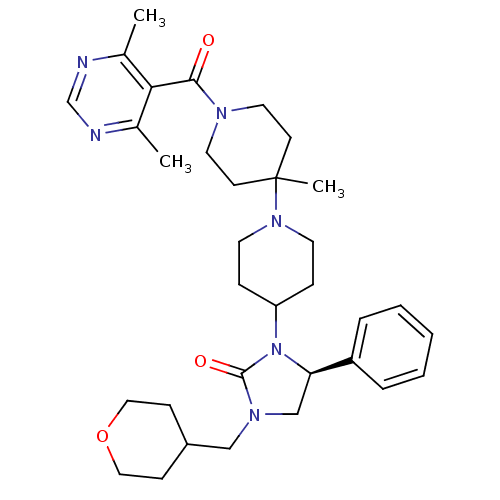 ((S)-3-(1'-(4,6-dimethylpyrimidine-5-carbonyl)-4'-m...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Binding affinity at CCR5 receptor by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 20: 3219-22 (2010) Article DOI: 10.1016/j.bmcl.2010.04.077 BindingDB Entry DOI: 10.7270/Q2FT8M63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50310733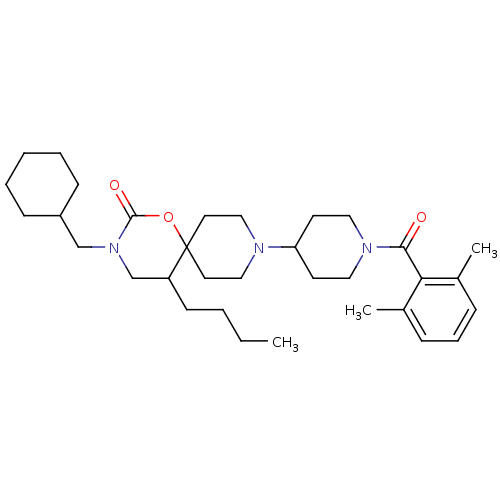 (5-butyl-3-(cyclohexylmethyl)-9-(1-(2,6-dimethylben...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Displacement of [125I]RANTES from human CCR5 receptor cotransfected with Galphai6 in CHO cells | Bioorg Med Chem Lett 19: 5401-6 (2009) Article DOI: 10.1016/j.bmcl.2009.07.122 BindingDB Entry DOI: 10.7270/Q25H7GD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-C chemokine receptor type 5 (Homo sapiens (Human)) | BDBM50254750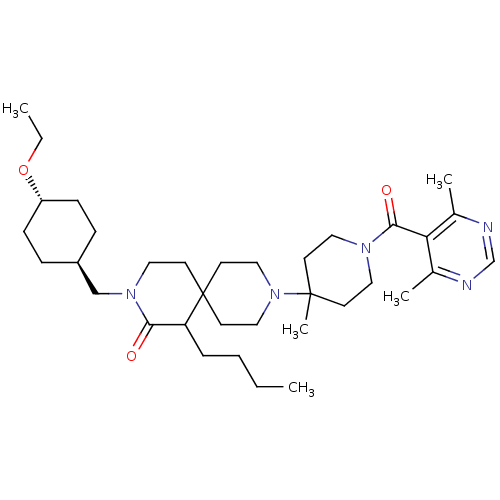 (1-butyl-9-(1-(4,6-dimethylpyrimidine-5-carbonyl)-4...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Palo Alto LLC Curated by ChEMBL | Assay Description Antagonist activity at CCR5 receptor (unknown origin) by radiolabelled RANTES binding assay | Bioorg Med Chem Lett 19: 209-13 (2008) Article DOI: 10.1016/j.bmcl.2008.10.115 BindingDB Entry DOI: 10.7270/Q2FQ9WGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 479 total ) | Next | Last >> |