

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172038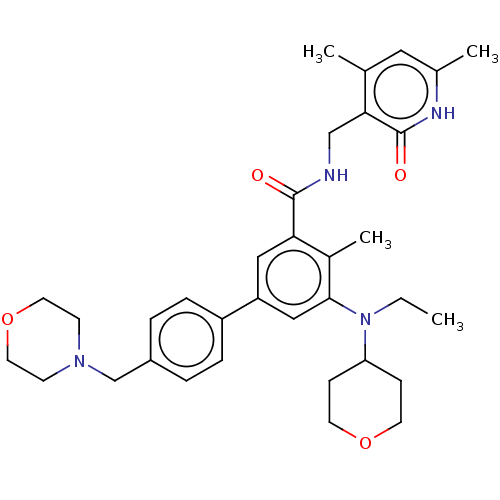 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110357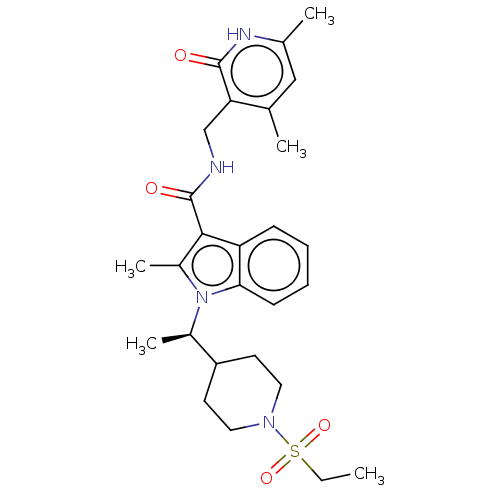 (CHEMBL3605455) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM172038 (US10155002, Compound 44 | US10647700, Compound EPZ...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110359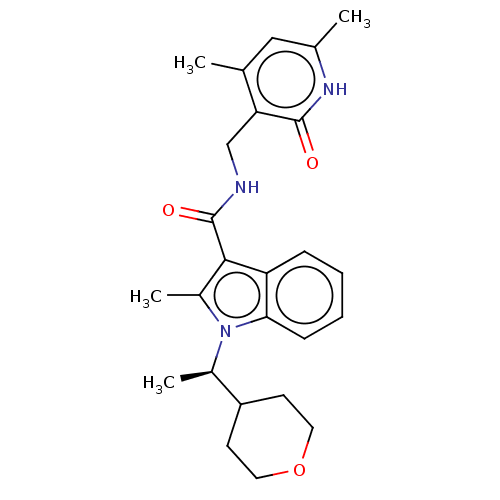 (CHEMBL3605453) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27595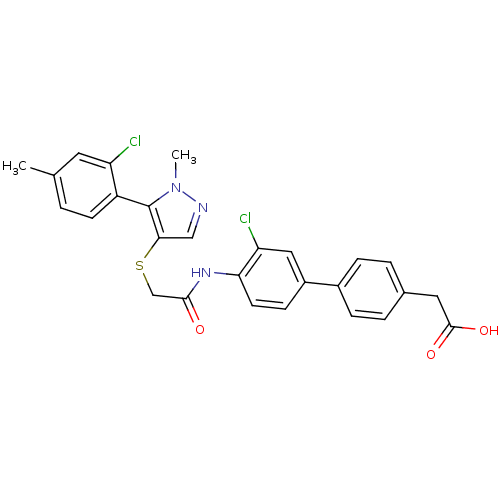 (2-{4-[3-chloro-4-(2-{[5-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27583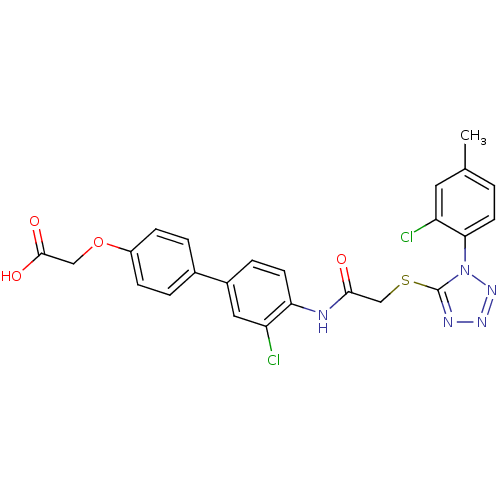 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27599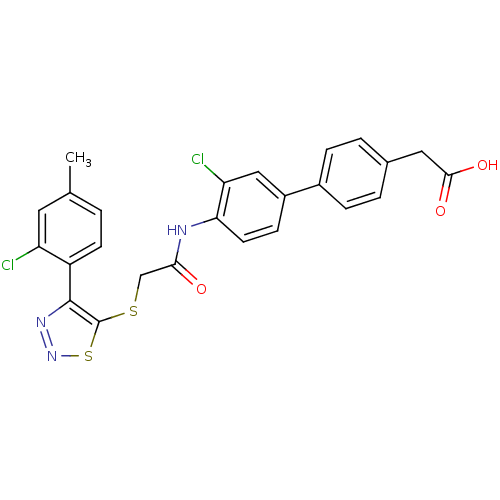 (2-{4-[3-chloro-4-(2-{[4-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110355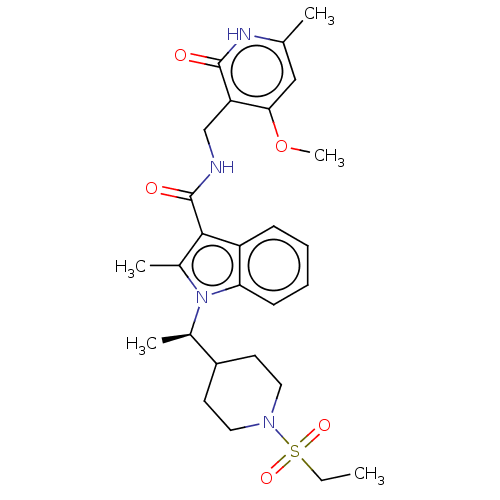 (CHEMBL3605457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50017293 (CHEMBL3287735 | US10647700, Compound GSK126) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110358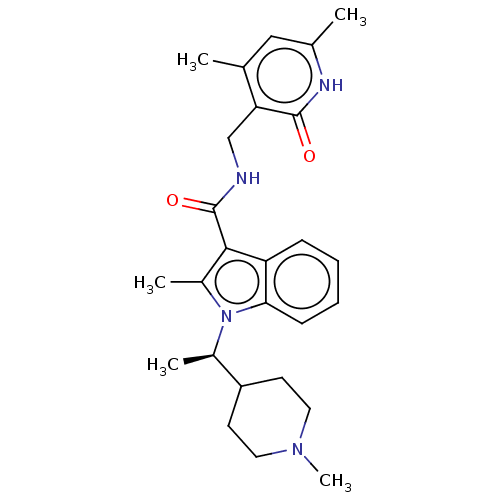 (CHEMBL3605454) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476896 (CHEMBL232576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110356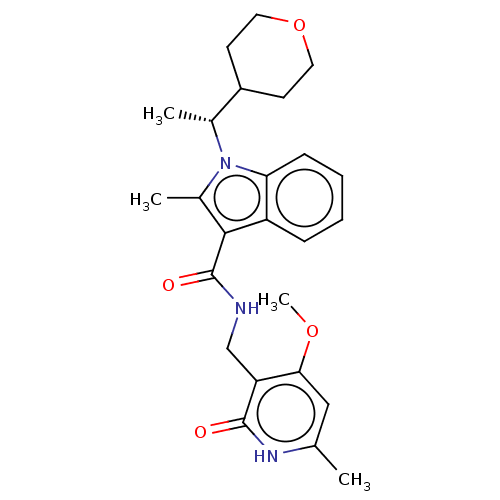 (CHEMBL3605456) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27591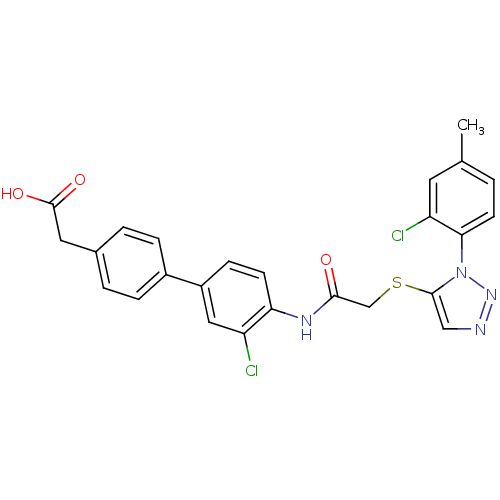 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27584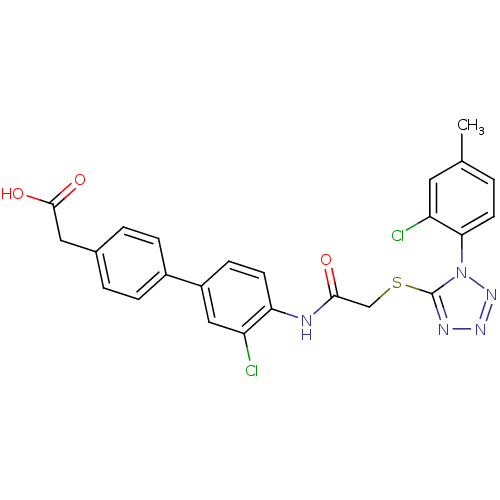 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476895 (CHEMBL232379) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110355 (CHEMBL3605457) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110357 (CHEMBL3605455) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476903 (CHEMBL230188) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27607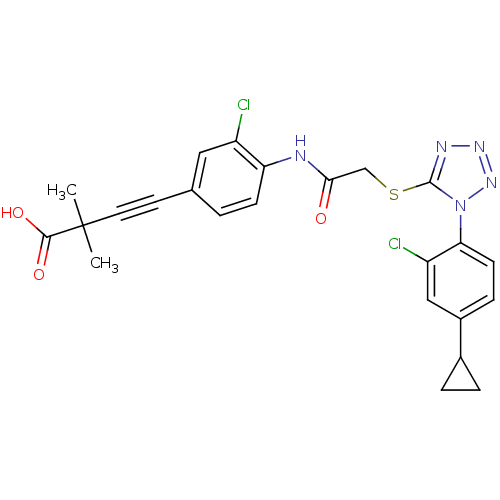 (4-[3-chloro-4-(2-{[1-(2-chloro-4-cyclopropylphenyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110359 (CHEMBL3605453) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110356 (CHEMBL3605456) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476898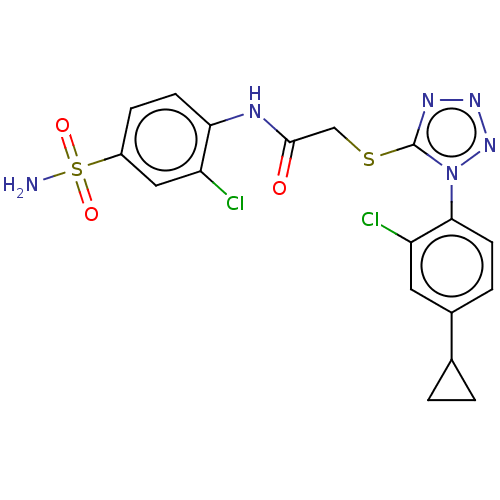 (CHEMBL232377) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110354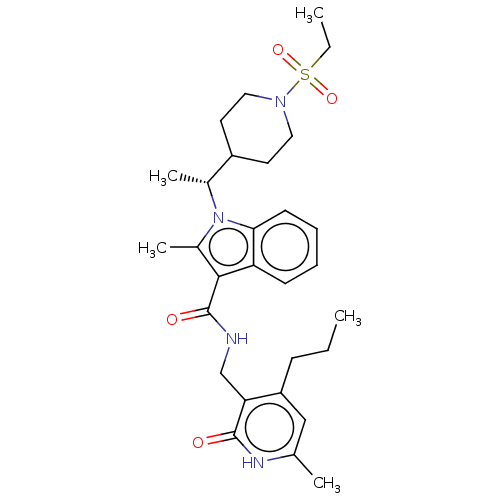 (CHEMBL3605458) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110360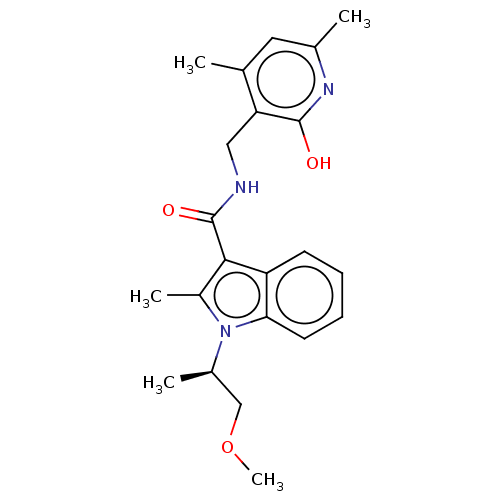 (CHEMBL3605452) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476897 (CHEMBL232178) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476904 (CHEMBL232575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476885 (CHEMBL231030) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110350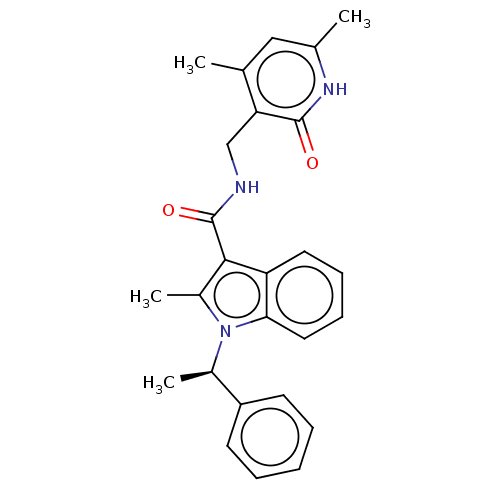 (CHEMBL3605446) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110358 (CHEMBL3605454) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27590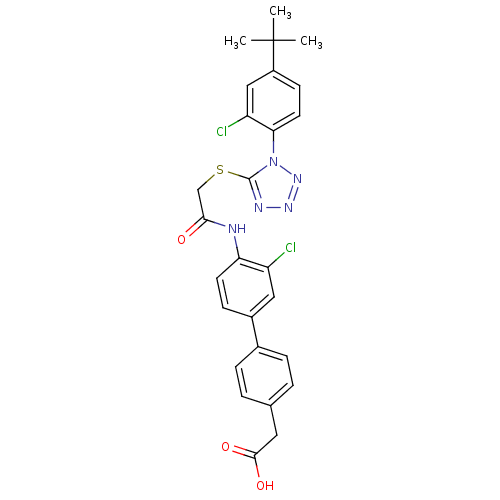 (2-{4-[4-(2-{[1-(4-tert-butyl-2-chlorophenyl)-1H-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476894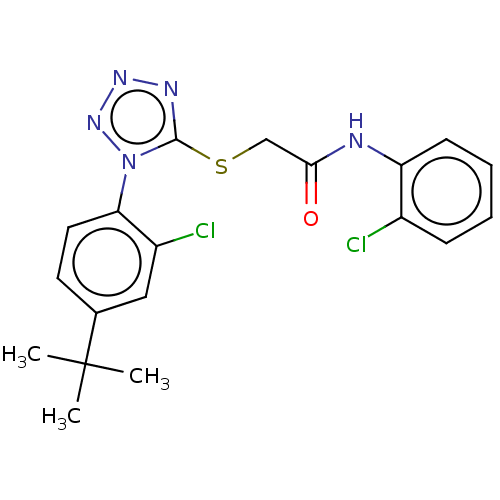 (CHEMBL230187) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476899 (CHEMBL232963) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27592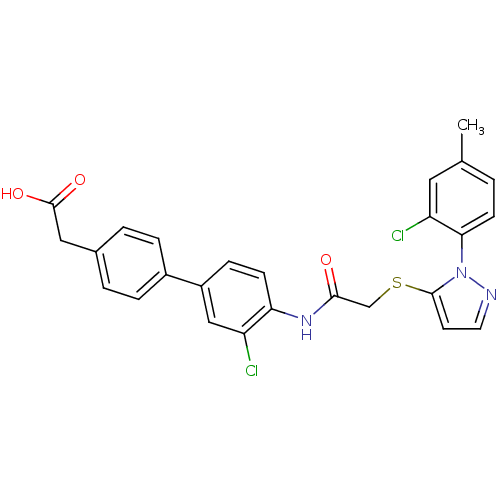 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27589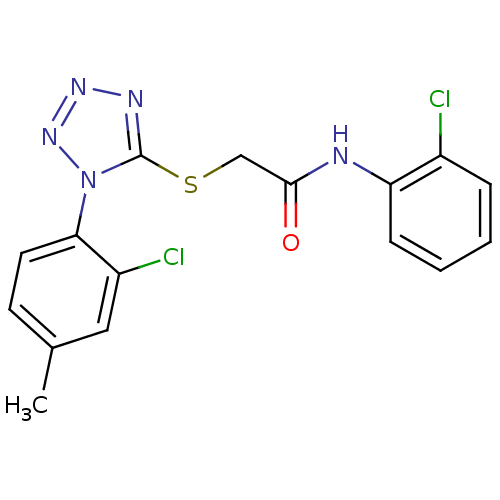 (2-{[1-(2-chloro-4-methylphenyl)-1H-1,2,3,4-tetrazo...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476896 (CHEMBL232576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27597 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM27606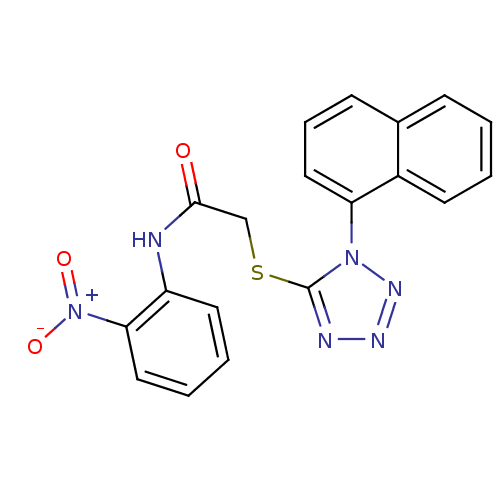 (2-{[1-(naphthalen-1-yl)-1H-1,2,3,4-tetrazol-5-yl]s...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27606 (2-{[1-(naphthalen-1-yl)-1H-1,2,3,4-tetrazol-5-yl]s...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476893 (CHEMBL393190) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476909 (CHEMBL232378) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476904 (CHEMBL232575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110367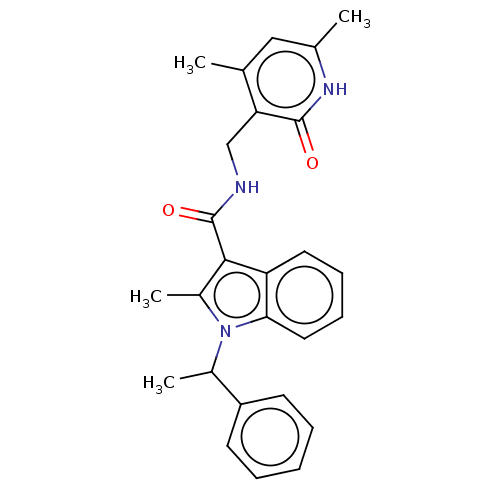 (CHEMBL3605445) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EZH2 (Homo sapiens (Human)) | BDBM50110362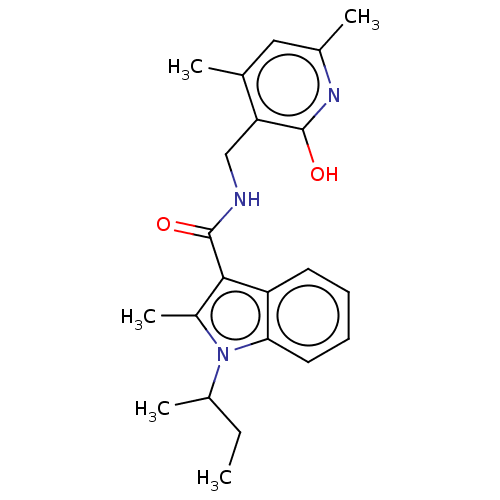 (CHEMBL3605451) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method | Bioorg Med Chem Lett 25: 3644-9 (2015) Article DOI: 10.1016/j.bmcl.2015.06.056 BindingDB Entry DOI: 10.7270/Q2Q24205 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27585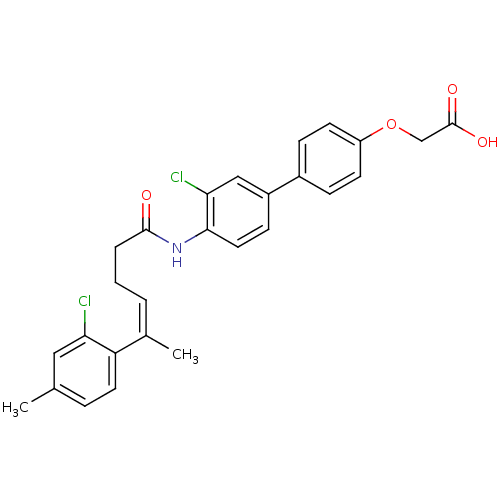 ((Z)-alkene compound, 8 | 2-(4-{3-chloro-4-[(4Z)-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476892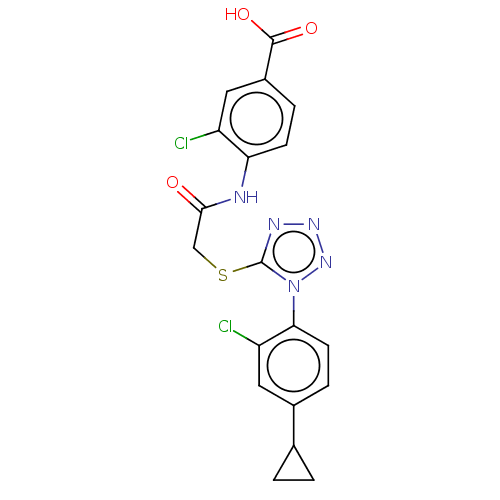 (CHEMBL391298) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nucleosome-remodeling factor subunit BPTF (Homo sapiens (Human)) | BDBM50571296 (CHEMBL4873093) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BPTF in human HEK293 cells by NanoBRET assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128208 BindingDB Entry DOI: 10.7270/Q2M0497K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476889 (CHEMBL232765) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476891 (CHEMBL233167) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027] (Human immunodeficiency virus type 1 group M subtyp...) | BDBM27594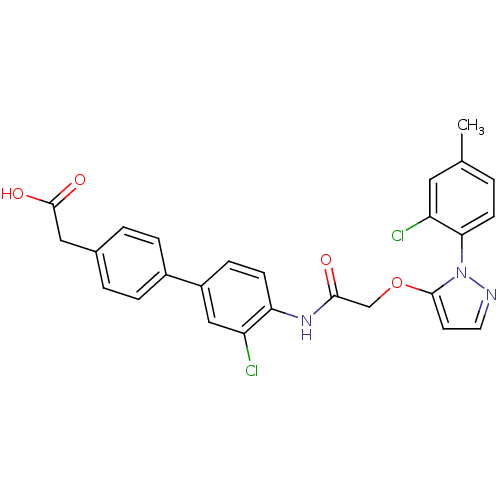 (2-{4-[3-chloro-4-(2-{[1-(2-chloro-4-methylphenyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Boehringer Ingelheim (Canada) Ltd. | Assay Description IC50 values for wild-type and mutant RTs were obtained from a scintillation proximity assay using poly rC/biotin-dG15 and 3H-dGTP. Each value represe... | Bioorg Med Chem Lett 19: 1199-205 (2009) Article DOI: 10.1016/j.bmcl.2008.12.074 BindingDB Entry DOI: 10.7270/Q2ZG6QKQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 250 total ) | Next | Last >> |