

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379268 (CHEMBL3216556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant AChE by Lineweaver-Burk plot analysis | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271142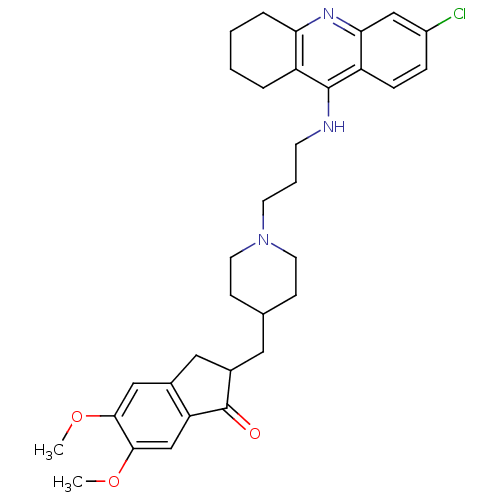 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271142 (6-Chloro-9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271140 (9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)methyl]pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10590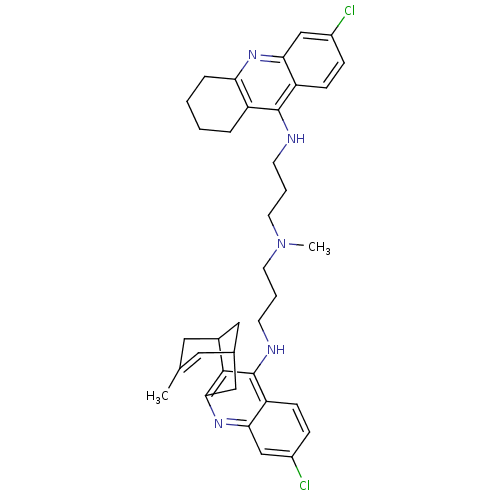 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10586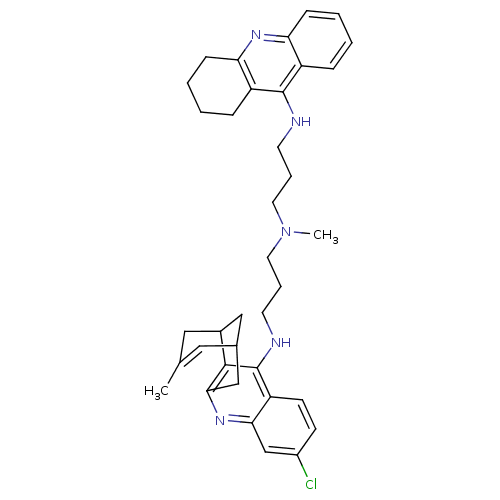 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{3-{N-{...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50379273 (CHEMBL1994202 | US9238626, (-)-Huprine Y HCl) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271141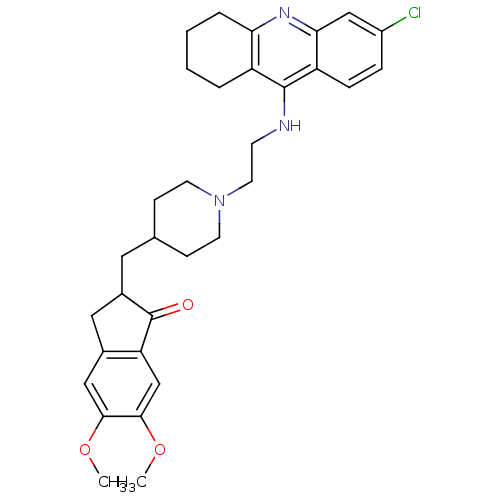 (6-Chloro-9-[(2-{4-[(5,6-dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM9063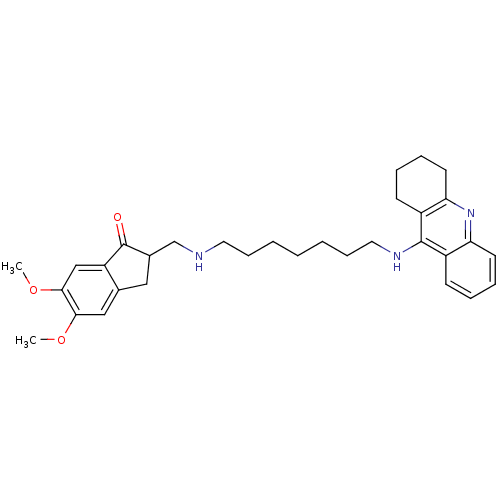 (5,6-Dimethoxy-2-{[7-(1,2,3,4-tetrahydro-acridin-9-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271141 (6-Chloro-9-[(2-{4-[(5,6-dimethoxy-1-oxoindan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50200340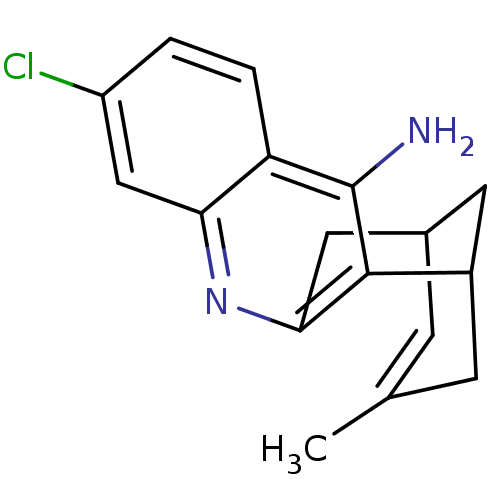 ((+/-)-huprine Y-HCl | (+/-)-huprineY hydrochloride...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10592 (7-chloro-15-methyl-10-azatetracyclo[11.3.1.0^{2,11...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10587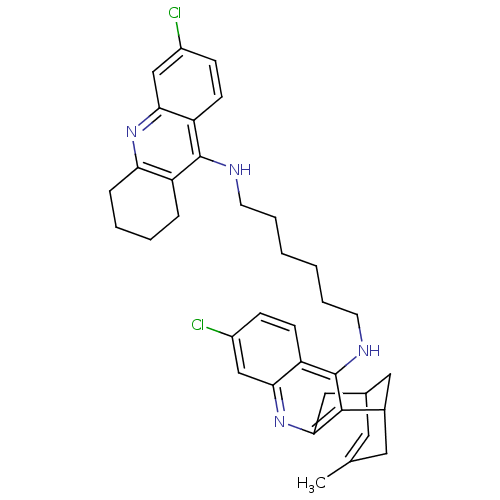 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271190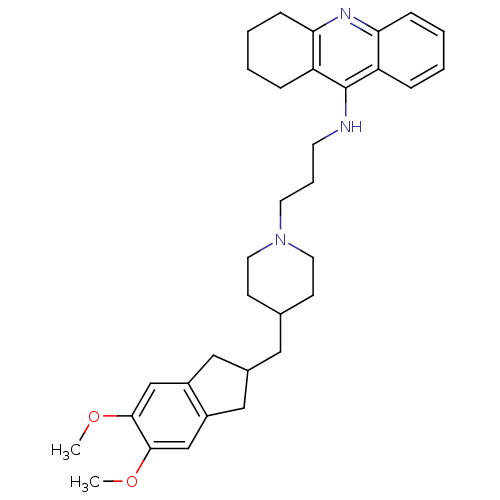 (9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271192 (6-Chloro-9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271140 (9-[(3-{4-[(5,6-Dimethoxy-1-oxoindan-2-yl)methyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10583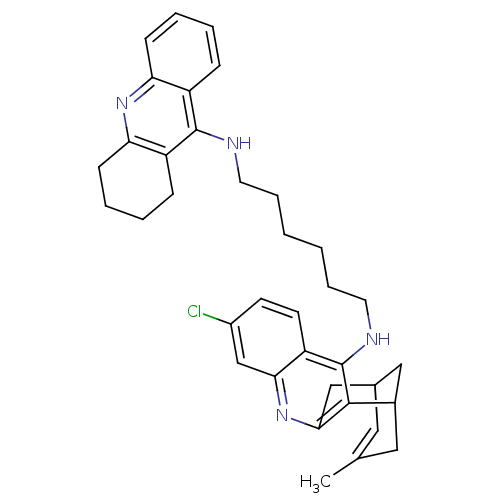 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{6-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271192 (6-Chloro-9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10589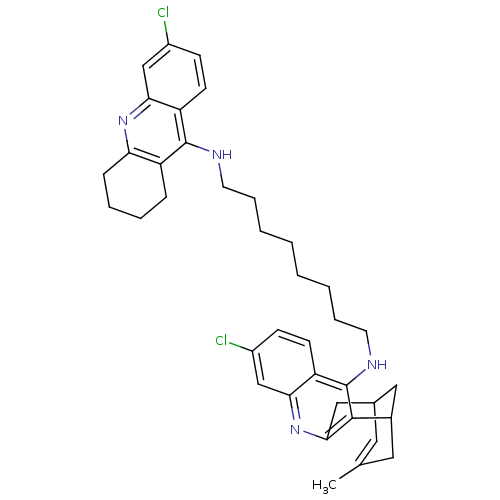 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10585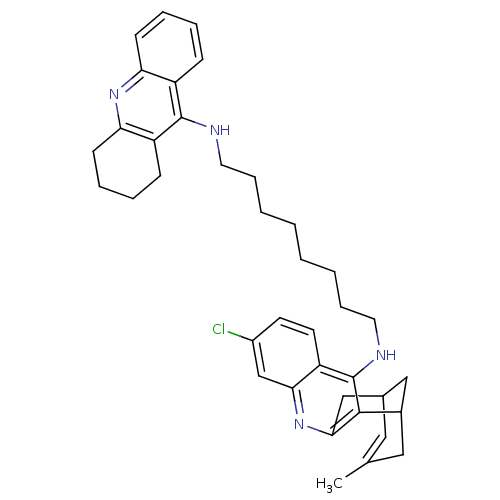 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{8-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108994 (CHEMBL3600555) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50377921 (SODIUM NITROPRUSSIDE) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271191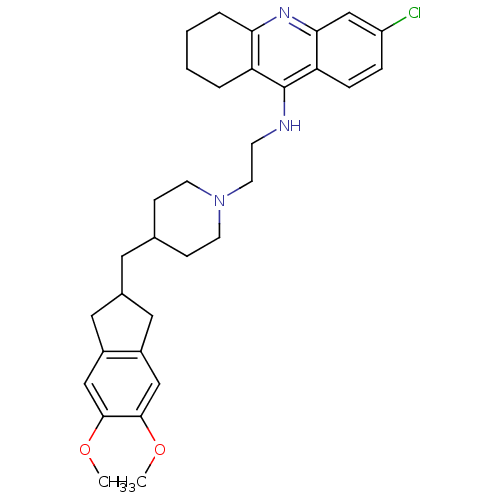 (6-Chloro-9-[(2-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108990 (CHEMBL3600551) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10584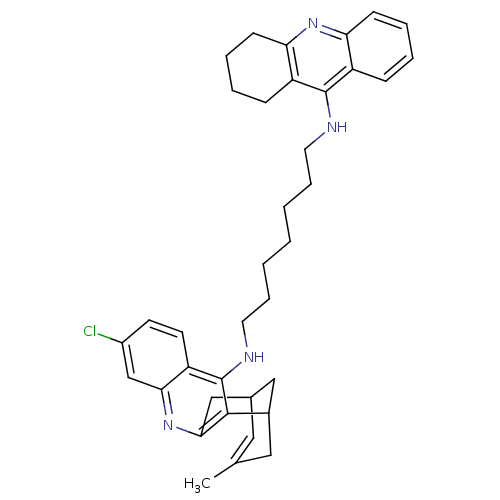 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(1,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271190 (9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.16 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM50271189 (9-[(2-{4-[(5,6-Dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.28 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271191 (6-Chloro-9-[(2-{4-[(5,6-dimethoxyindan-2-yl)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50027470 (CHEMBL3216778) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108991 (CHEMBL3600552) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108995 (CHEMBL3600556) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10588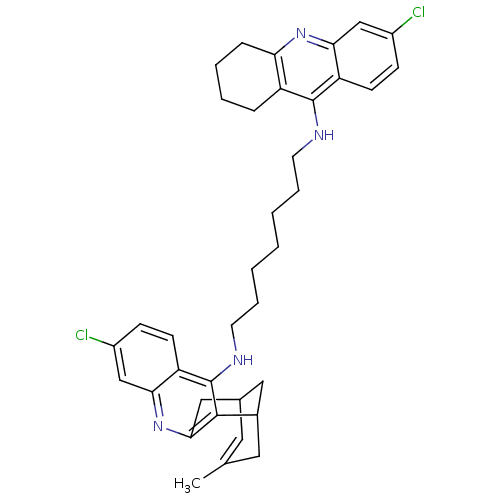 (3-Chloro-6,7,10,11-tetrahydro-9-methyl-12-{{7-[(6-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50377921 (SODIUM NITROPRUSSIDE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.04 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50027471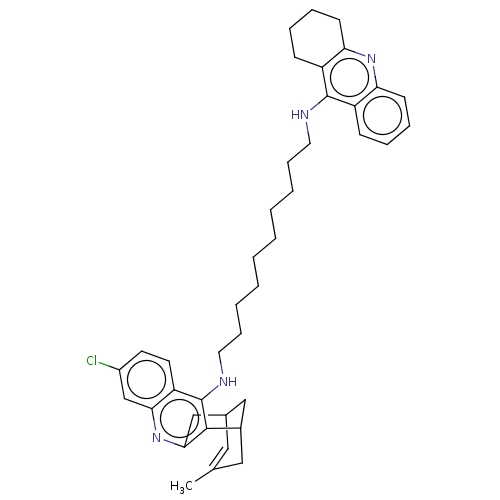 (CHEMBL3216554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins prior substrate addition measured after 5 m... | J Med Chem 55: 661-9 (2012) Article DOI: 10.1021/jm200840c BindingDB Entry DOI: 10.7270/Q26974KH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50271189 (9-[(2-{4-[(5,6-Dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.73 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.73 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM8979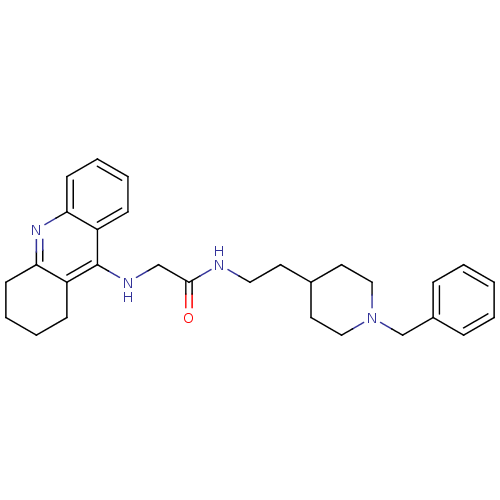 (CHEMBL486901 | N-[2-(1-benzylpiperidin-4-yl)ethyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of rat cortex AChE | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108992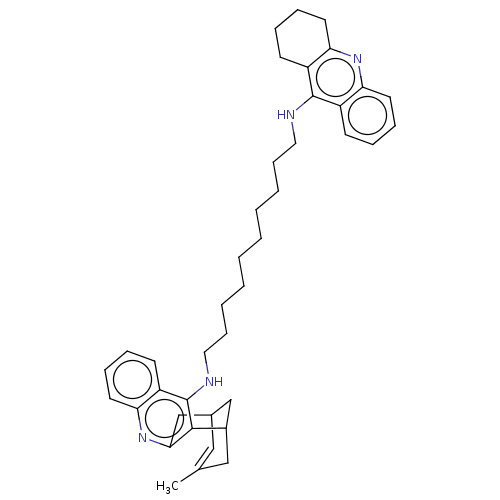 (CHEMBL3600553) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50023607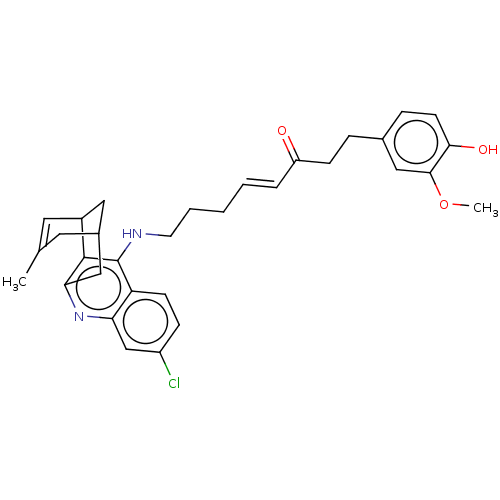 (CHEMBL3355579) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated for 20 mins before substrate addition by Ellman method | Bioorg Med Chem 22: 5298-307 (2014) Article DOI: 10.1016/j.bmc.2014.07.053 BindingDB Entry DOI: 10.7270/Q2M61MV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31895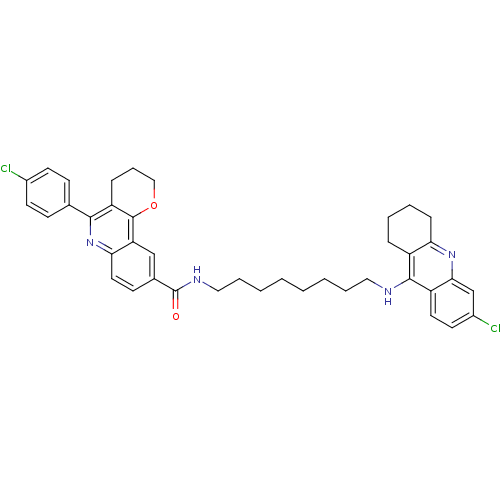 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 20....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.03 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50271190 (9-[(3-{4-[(5,6-dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50271189 (9-[(2-{4-[(5,6-Dimethoxyindan-2-yl)methyl]piperidi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.06 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human serum BChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 8.12 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of bovine erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8987 (6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.32 | n/a | n/a | n/a | n/a | n/a | n/a |
Laboratori de Qu�mica Farmac�utica (Unitat Associada al CSIC) Curated by ChEMBL | Assay Description Inhibition of human erythrocyte AChE by Ellman's assay | J Med Chem 51: 3588-98 (2008) Article DOI: 10.1021/jm8001313 BindingDB Entry DOI: 10.7270/Q2F76DGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM31899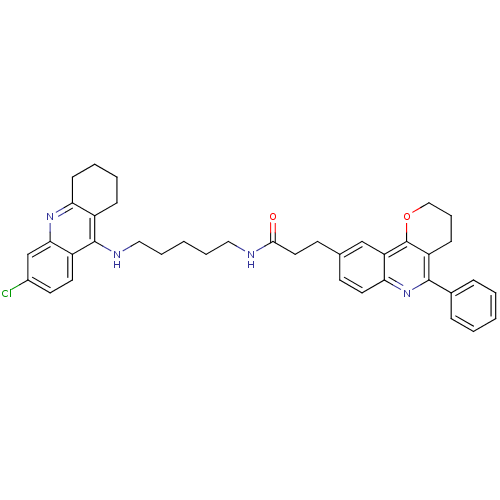 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 24....) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.64 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50108993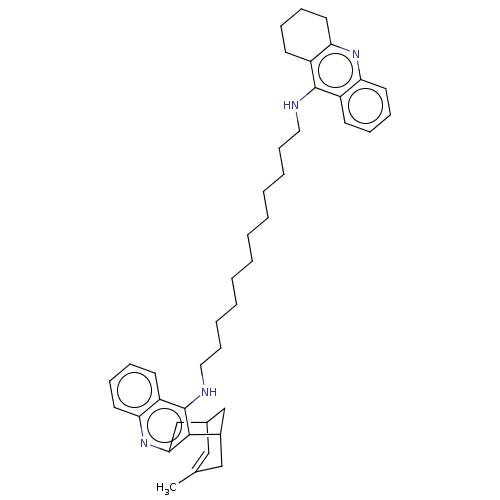 (CHEMBL3600554) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Universitat de Barcelona Curated by ChEMBL | Assay Description Inhibition of human recombinant AChE using acetylthiocholine iodide as substrate preincubated with enzyme for 20 mins prior to substrate addition by ... | Bioorg Med Chem 23: 5156-67 (2015) Article DOI: 10.1016/j.bmc.2015.01.031 BindingDB Entry DOI: 10.7270/Q2XS5X57 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Bos taurus (bovine)) | BDBM31894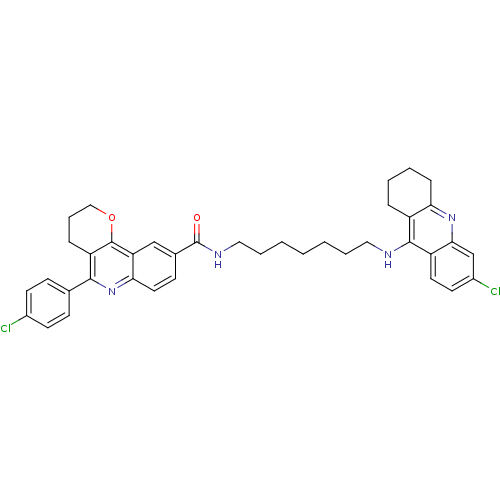 (Pyrano[3,2-c]quinoline-6-chlorotacrine hybrid, 19....) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | 8.0 | 25 |
Universitat de Barcelona | Assay Description AChE inhibitory activity was evaluated spectrophotometrically by the method of Ellman, using AChE from bovine or human erythrocytes and acetylthiocho... | J Med Chem 52: 5365-79 (2009) Article DOI: 10.1021/jm900859q BindingDB Entry DOI: 10.7270/Q2707ZSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 225 total ) | Next | Last >> |