 Found 168 hits with Last Name = 'galli' and Initial = 'f'
Found 168 hits with Last Name = 'galli' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4
(Homo sapiens (Human)) | BDBM50170601
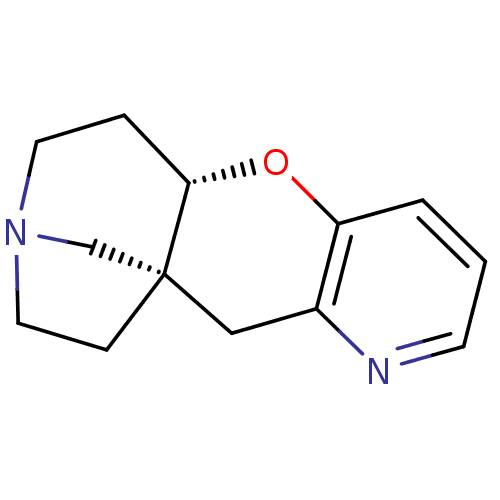
((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50004108

((+-)-nicotine | (R,S)-nicotine | (RS)-nicotine | 3...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 187 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-3
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit alpha
(Homo sapiens (Human)) | BDBM50170601

((1R,10S)-9-oxa-4,13-diazatetracyclo[11.2.1.0^{1,10...)Show InChI InChI=1S/C13H16N2O/c1-2-11-10(14-5-1)8-13-4-7-15(9-13)6-3-12(13)16-11/h1-2,5,12H,3-4,6-9H2/t12-,13+/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Research
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 306: 407-20 (2003)
Article DOI: 10.1124/jpet.103.049262
BindingDB Entry DOI: 10.7270/Q2GM85WX |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267760
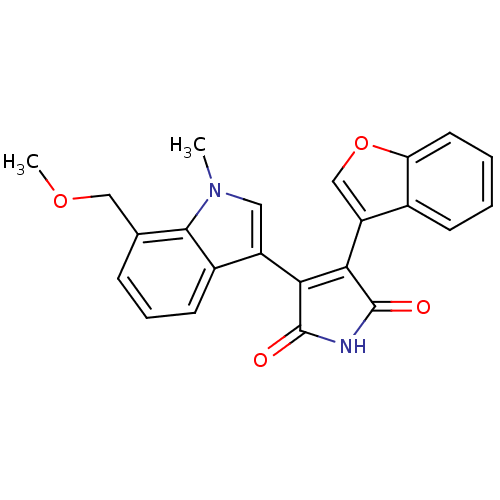
(3-Benzofuran-3-yl-4-(7-methoxymethyl-1-methyl-1H-i...)Show SMILES COCc1cccc2c(cn(C)c12)C1=C(C(=O)NC1=O)c1coc2ccccc12 |t:15| Show InChI InChI=1S/C23H18N2O4/c1-25-10-16(15-8-5-6-13(11-28-2)21(15)25)19-20(23(27)24-22(19)26)17-12-29-18-9-4-3-7-14(17)18/h3-10,12H,11H2,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267461
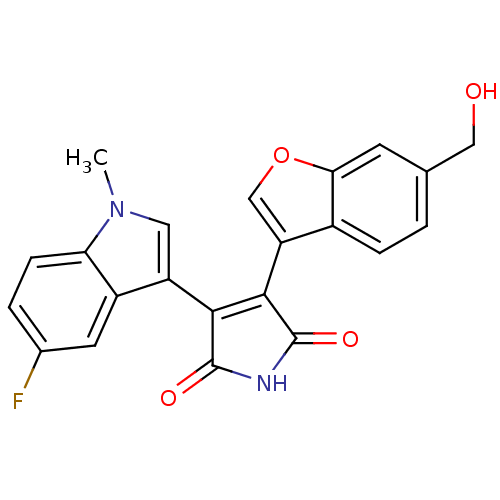
(3-(5-Fluoro-1-methyl-1H-indol-3-yl)-4-(6-hydroxyme...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(F)ccc12 |t:4| Show InChI InChI=1S/C22H15FN2O4/c1-25-8-15(14-7-12(23)3-5-17(14)25)19-20(22(28)24-21(19)27)16-10-29-18-6-11(9-26)2-4-13(16)18/h2-8,10,26H,9H2,1H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase Chk1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | <0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CHK1 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267607

(3-(5-Bromo-1-methyl-1H-indol-3-yl)-4-(6-hydroxymet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(Br)ccc12 |t:4| Show InChI InChI=1S/C22H15BrN2O4/c1-25-8-15(14-7-12(23)3-5-17(14)25)19-20(22(28)24-21(19)27)16-10-29-18-6-11(9-26)2-4-13(16)18/h2-8,10,26H,9H2,1H3,(H,24,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
RAC-gamma serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of AKT3 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267800
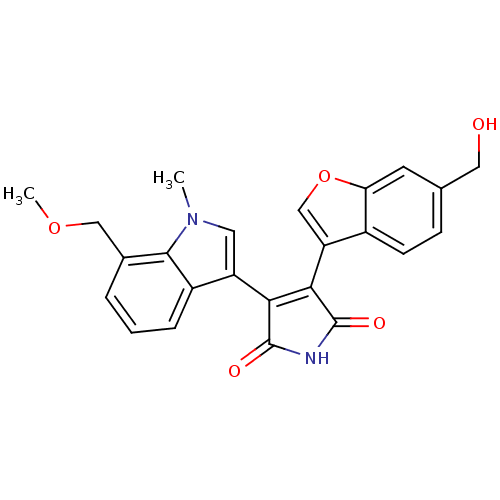
(3-(6-Hydroxymethylbenzofuran-3-yl)-4-(7-methoxymet...)Show SMILES COCc1cccc2c(cn(C)c12)C1=C(C(=O)NC1=O)c1coc2cc(CO)ccc12 |t:15| Show InChI InChI=1S/C24H20N2O5/c1-26-9-17(16-5-3-4-14(11-30-2)22(16)26)20-21(24(29)25-23(20)28)18-12-31-19-8-13(10-27)6-7-15(18)19/h3-9,12,27H,10-11H2,1-2H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267523
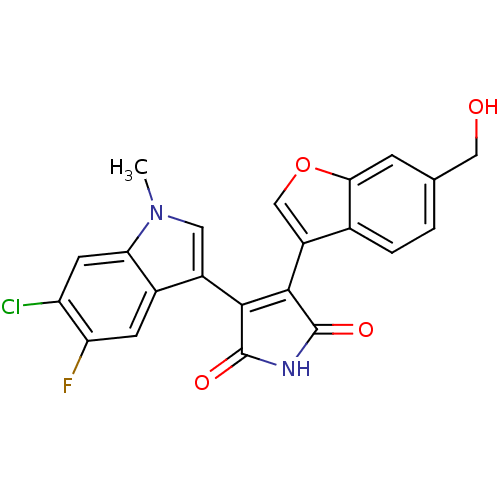
(3-(6-Chloro-5-fluoro-1-methyl-1H-indol-3-yl)-4-(6-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(F)c(Cl)cc12 |t:4| Show InChI InChI=1S/C22H14ClFN2O4/c1-26-7-13(12-5-16(24)15(23)6-17(12)26)19-20(22(29)25-21(19)28)14-9-30-18-4-10(8-27)2-3-11(14)18/h2-7,9,27H,8H2,1H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267802
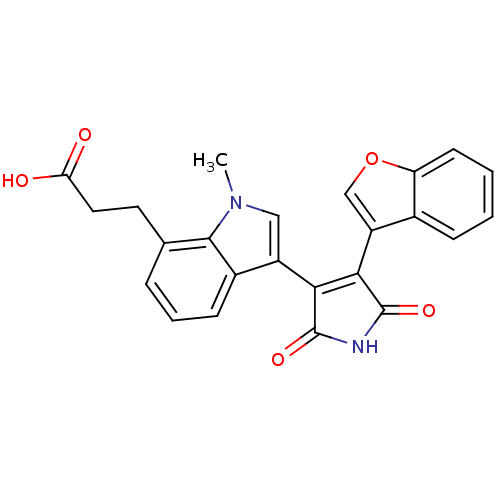
(3-[3-(4-Benzofuran-3-yl-2,5-dioxo-2,5-dihydro-1H-p...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cccc(CCC(O)=O)c12 |t:4| Show InChI InChI=1S/C24H18N2O5/c1-26-11-16(15-7-4-5-13(22(15)26)9-10-19(27)28)20-21(24(30)25-23(20)29)17-12-31-18-8-3-2-6-14(17)18/h2-8,11-12H,9-10H2,1H3,(H,27,28)(H,25,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin A |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-dependent kinase 9
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK9/cyclin T1 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK1/Cyclin B |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267520
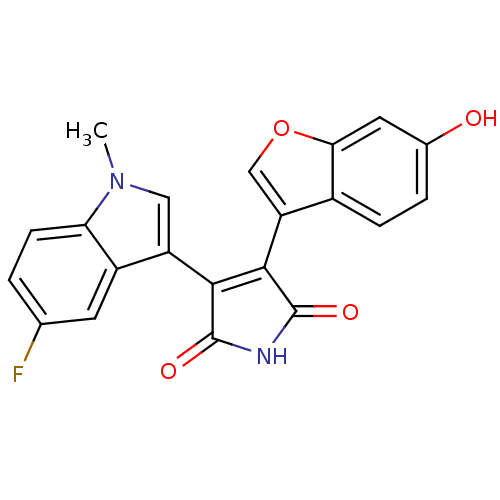
(3-(5-Fluoro-1-methyl-1H-indol-3-yl)-4-(6-hydroxybe...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(O)ccc23)c2cc(F)ccc12 |t:4| Show InChI InChI=1S/C21H13FN2O4/c1-24-8-14(13-6-10(22)2-5-16(13)24)18-19(21(27)23-20(18)26)15-9-28-17-7-11(25)3-4-12(15)17/h2-9,25H,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/P35 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Cyclin-dependent kinase 5
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/P25 (unknown origin) |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267759
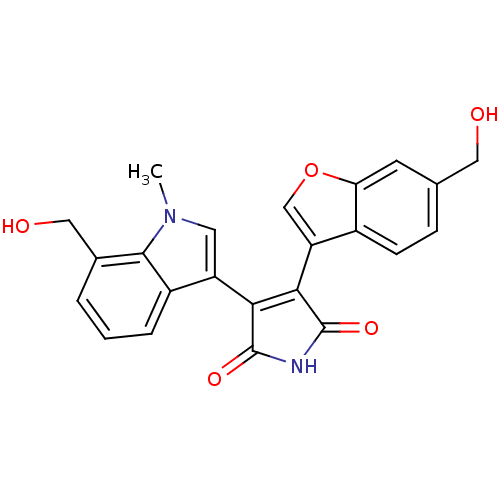
(3-(6-Hydroxymethylbenzofuran-3-yl)-4-(7-hydroxymet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cccc(CO)c12 |t:4| Show InChI InChI=1S/C23H18N2O5/c1-25-8-16(15-4-2-3-13(10-27)21(15)25)19-20(23(29)24-22(19)28)17-11-30-18-7-12(9-26)5-6-14(17)18/h2-8,11,26-27H,9-10H2,1H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267758

(3-Benzofuran-3-yl-4-(7-hydroxymethyl-1-methyl-1H-i...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cccc(CO)c12 |t:4| Show InChI InChI=1S/C22H16N2O4/c1-24-9-15(14-7-4-5-12(10-25)20(14)24)18-19(22(27)23-21(18)26)16-11-28-17-8-3-2-6-13(16)17/h2-9,11,25H,10H2,1H3,(H,23,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK3/Cyclin E |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267605

(3-(benzofuran-3-yl)-4-(5-bromo-1-methyl-1H-indol-3...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cc(Br)ccc12 |t:4| Show InChI InChI=1S/C21H13BrN2O3/c1-24-9-14(13-8-11(22)6-7-16(13)24)18-19(21(26)23-20(18)25)15-10-27-17-5-3-2-4-12(15)17/h2-10H,1H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267606

(3-(5-Bromo-1-methyl-1H-indol-3-yl)-4-(7-methoxyben...)Show SMILES COc1cccc2c(coc12)C1=C(C(=O)NC1=O)c1cn(C)c2ccc(Br)cc12 |t:13| Show InChI InChI=1S/C22H15BrN2O4/c1-25-9-14(13-8-11(23)6-7-16(13)25)18-19(22(27)24-21(18)26)15-10-29-20-12(15)4-3-5-17(20)28-2/h3-10H,1-2H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267801
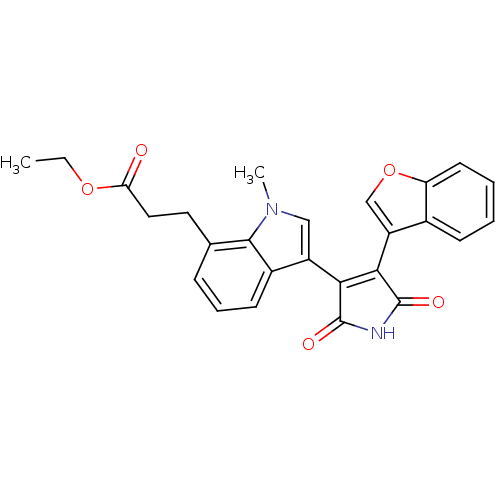
(3-[3-(4-Benzofuran-3-yl-2,5-dioxo-2,5-dihydro-1H-p...)Show SMILES CCOC(=O)CCc1cccc2c(cn(C)c12)C1=C(C(=O)NC1=O)c1coc2ccccc12 |t:19| Show InChI InChI=1S/C26H22N2O5/c1-3-32-21(29)12-11-15-7-6-9-17-18(13-28(2)24(15)17)22-23(26(31)27-25(22)30)19-14-33-20-10-5-4-8-16(19)20/h4-10,13-14H,3,11-12H2,1-2H3,(H,27,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267678

(3-[4-(6-Hydroxymethylbenzofuran-3-yl)-2,5-dioxo-2,...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cc(ccc12)C#N |t:4| Show InChI InChI=1S/C23H15N3O4/c1-26-9-16(15-6-12(8-24)3-5-18(15)26)20-21(23(29)25-22(20)28)17-11-30-19-7-13(10-27)2-4-14(17)19/h2-7,9,11,27H,10H2,1H3,(H,25,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267367

(3-(benzofuran-3-yl)-4-(6-hydroxy-1-methyl-1H-indol...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2ccc(O)cc12 |t:4| Show InChI InChI=1S/C21H14N2O4/c1-23-9-14(12-7-6-11(24)8-16(12)23)18-19(21(26)22-20(18)25)15-10-27-17-5-3-2-4-13(15)17/h2-10,24H,1H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267715

(3-(5-Cyclopropylethynyl-1-methyl-1H-indol-3-yl)-4-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccc(F)cc23)c2cc(ccc12)C#CC1CC1 |t:4| Show InChI InChI=1S/C26H17FN2O3/c1-29-12-19(17-10-15(6-8-21(17)29)5-4-14-2-3-14)23-24(26(31)28-25(23)30)20-13-32-22-9-7-16(27)11-18(20)22/h6-14H,2-3H2,1H3,(H,28,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Schistosoma mansoni) | BDBM50523223

(CHEMBL4540838)Show SMILES OC1=C(Nc2cccc(Cl)c2Cl)C(=O)c2ccccc2C1=O |c:1| Show InChI InChI=1S/C16H9Cl2NO3/c17-10-6-3-7-11(12(10)18)19-13-14(20)8-4-1-2-5-9(8)15(21)16(13)22/h1-7,19,22H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Schistosoma mansoni DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Schistosoma mansoni) | BDBM50384788

(LAPACHOL)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]-1-[#6](=O)-[#6](=O)-c2ccccc2-[#6]-1=O Show InChI InChI=1S/C15H14O3/c1-9(2)7-8-12-13(16)10-5-3-4-6-11(10)14(17)15(12)18/h3-7,12H,8H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Schistosoma mansoni DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Schistosoma mansoni) | BDBM50523217
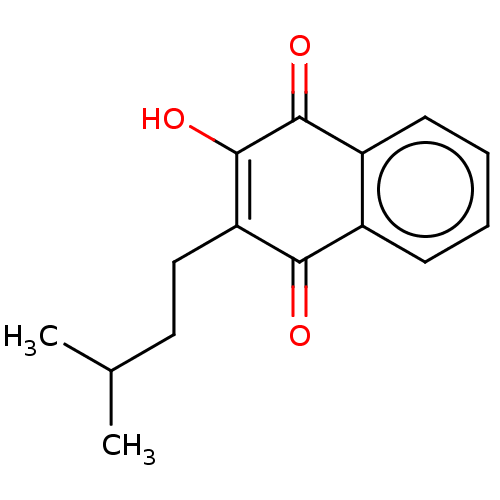
(CHEMBL155771)Show InChI InChI=1S/C15H16O3/c1-9(2)7-8-12-13(16)10-5-3-4-6-11(10)14(17)15(12)18/h3-6,9,18H,7-8H2,1-2H3 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Schistosoma mansoni DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267462
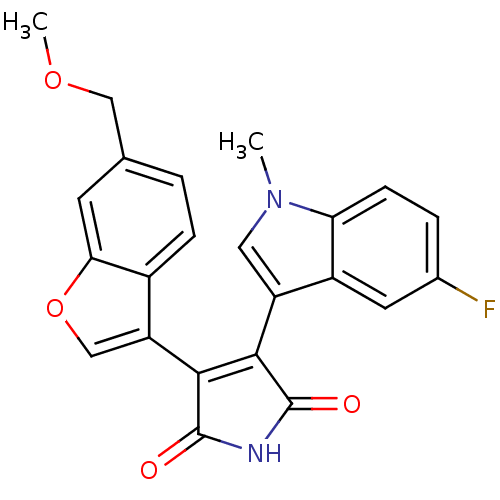
(3-(5-Fluoro-1-methyl-1H-indol-3-yl)-4-(6-methoxyme...)Show SMILES COCc1ccc2c(coc2c1)C1=C(C(=O)NC1=O)c1cn(C)c2ccc(F)cc12 |t:14| Show InChI InChI=1S/C23H17FN2O4/c1-26-9-16(15-8-13(24)4-6-18(15)26)20-21(23(28)25-22(20)27)17-11-30-19-7-12(10-29-2)3-5-14(17)19/h3-9,11H,10H2,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267639
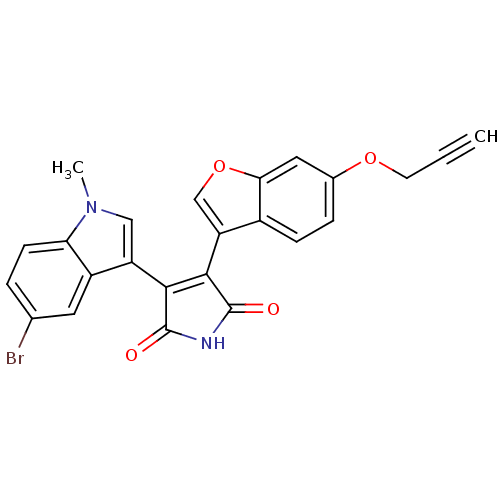
(3-(5-Bromo-1-methyl-1H-indol-3-yl)-4-(6-prop-2-yny...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(OCC#C)ccc23)c2cc(Br)ccc12 |t:4| Show InChI InChI=1S/C24H15BrN2O4/c1-3-8-30-14-5-6-15-18(12-31-20(15)10-14)22-21(23(28)26-24(22)29)17-11-27(2)19-7-4-13(25)9-16(17)19/h1,4-7,9-12H,8H2,2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2
(Homo sapiens (Human)) | BDBM50267759

(3-(6-Hydroxymethylbenzofuran-3-yl)-4-(7-hydroxymet...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(CO)ccc23)c2cccc(CO)c12 |t:4| Show InChI InChI=1S/C23H18N2O5/c1-25-8-16(15-4-2-3-13(10-27)21(15)25)19-20(23(29)24-22(19)28)17-11-30-18-7-12(9-26)5-6-14(17)18/h2-8,11,26-27H,9-10H2,1H3,(H,24,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267676
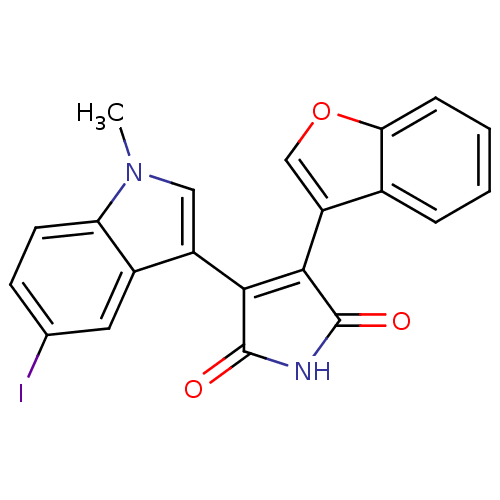
(3-Benzofuran-3-yl-4-(5-iodo-1-methyl-1H-indol-3-yl...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cc(I)ccc12 |t:4| Show InChI InChI=1S/C21H13IN2O3/c1-24-9-14(13-8-11(22)6-7-16(13)24)18-19(21(26)23-20(18)25)15-10-27-17-5-3-2-4-12(15)17/h2-10H,1H3,(H,23,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM2600
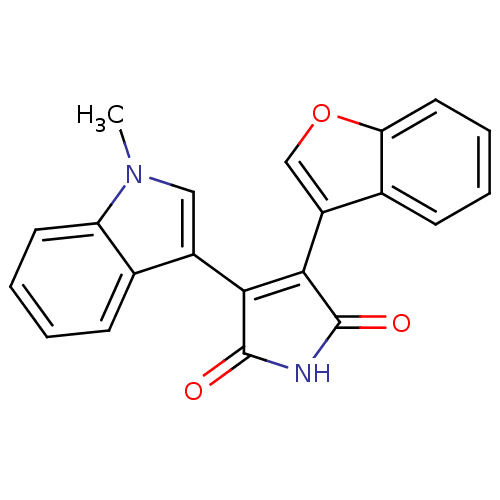
((Arylindolyl)maleimide deriv. 22 | 3-(1-benzofuran...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2ccccc12 |t:4| Show InChI InChI=1S/C21H14N2O3/c1-23-10-14(12-6-2-4-8-16(12)23)18-19(21(25)22-20(18)24)15-11-26-17-9-5-3-7-13(15)17/h2-11H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM15339
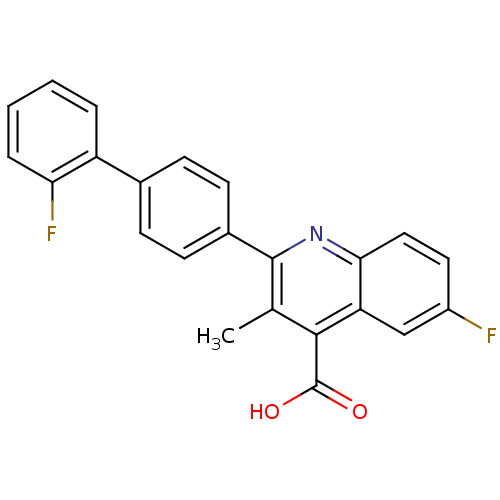
(6-fluoro-2-[4-(2-fluorophenyl)phenyl]-3-methyl-qui...)Show SMILES Cc1c(nc2ccc(F)cc2c1C(O)=O)-c1ccc(cc1)-c1ccccc1F Show InChI InChI=1S/C23H15F2NO2/c1-13-21(23(27)28)18-12-16(24)10-11-20(18)26-22(13)15-8-6-14(7-9-15)17-4-2-3-5-19(17)25/h2-12H,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of human DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267642
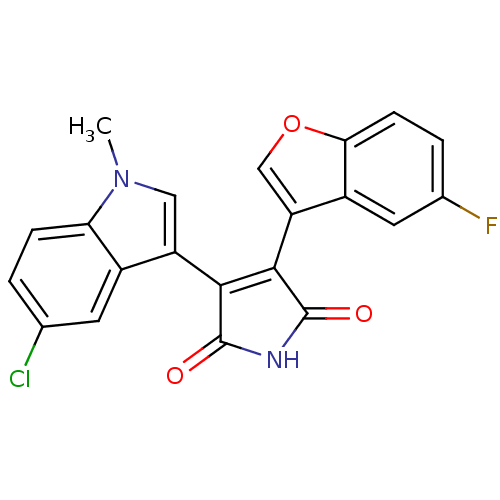
(3-(5-chloro-1-methyl-1H-indol-3-yl)-4-(5-fluoroben...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccc(F)cc23)c2cc(Cl)ccc12 |t:4| Show InChI InChI=1S/C21H12ClFN2O3/c1-25-8-14(12-6-10(22)2-4-16(12)25)18-19(21(27)24-20(18)26)15-9-28-17-5-3-11(23)7-13(15)17/h2-9H,1H3,(H,24,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50229962
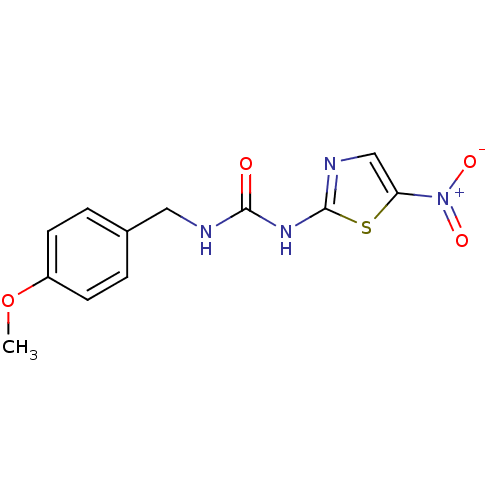
(1-(4-methoxybenzyl)-3-(5-nitrothiazol-2-yl)urea | ...)Show InChI InChI=1S/C12H12N4O4S/c1-20-9-4-2-8(3-5-9)6-13-11(17)15-12-14-7-10(21-12)16(18)19/h2-5,7H,6H2,1H3,(H2,13,14,15,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PDB
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267640

(3-(6-Allyloxybenzofuran-3-yl)-4-(5-bromo-1-methyl-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3cc(OCC=C)ccc23)c2cc(Br)ccc12 |t:4| Show InChI InChI=1S/C24H17BrN2O4/c1-3-8-30-14-5-6-15-18(12-31-20(15)10-14)22-21(23(28)26-24(22)29)17-11-27(2)19-7-4-13(25)9-16(17)19/h3-7,9-12H,1,8H2,2H3,(H,26,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM8296
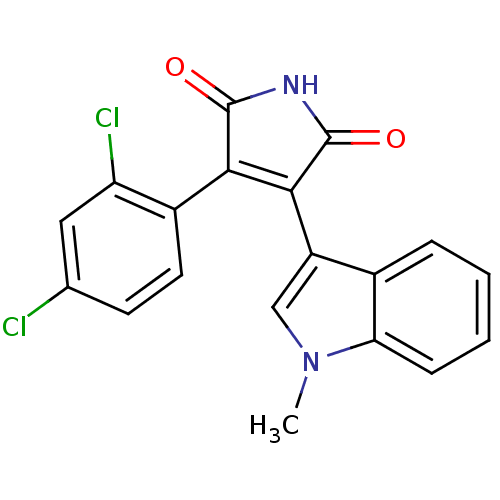
(3-(2,4-dichlorophenyl)-4-(1-methyl-1H-indol-3-yl)-...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2ccc(Cl)cc2Cl)c2ccccc12 |t:4| Show InChI InChI=1S/C19H12Cl2N2O2/c1-23-9-13(11-4-2-3-5-15(11)23)17-16(18(24)22-19(17)25)12-7-6-10(20)8-14(12)21/h2-9H,1H3,(H,22,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267803
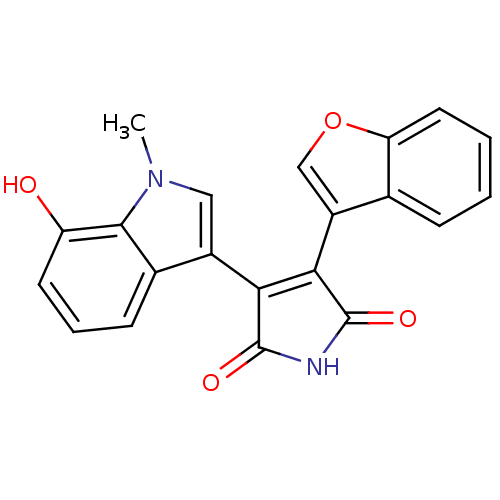
(3-(benzofuran-3-yl)-4-(7-hydroxy-1-methyl-1H-indol...)Show SMILES Cn1cc(C2=C(C(=O)NC2=O)c2coc3ccccc23)c2cccc(O)c12 |t:4| Show InChI InChI=1S/C21H14N2O4/c1-23-9-13(12-6-4-7-15(24)19(12)23)17-18(21(26)22-20(17)25)14-10-27-16-8-3-2-5-11(14)16/h2-10,24H,1H3,(H,22,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Schistosoma mansoni) | BDBM50523213
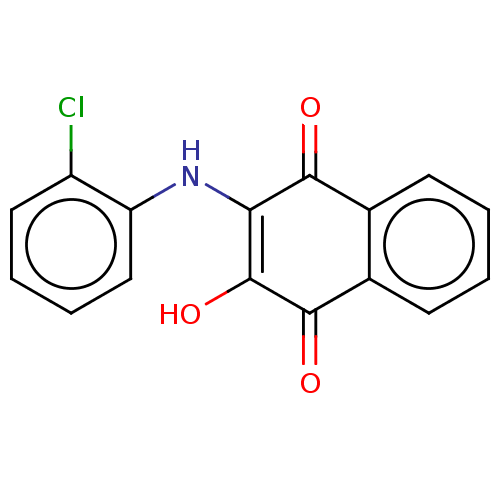
(CHEMBL4452960)Show InChI InChI=1S/C16H10ClNO3/c17-11-7-3-4-8-12(11)18-13-14(19)9-5-1-2-6-10(9)15(20)16(13)21/h1-8,18,21H | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of Schistosoma mansoni DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM50267675

(3-(5,7-Dibromo-1-methyl-1H-indol-3-yl)-4-(7-methox...)Show SMILES COc1cccc2c(coc12)C1=C(C(=O)NC1=O)c1cn(C)c2c(Br)cc(Br)cc12 |t:13| Show InChI InChI=1S/C22H14Br2N2O4/c1-26-8-13(12-6-10(23)7-15(24)19(12)26)17-18(22(28)25-21(17)27)14-9-30-20-11(14)4-3-5-16(20)29-2/h3-9H,1-2H3,(H,25,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Illinois at Chicago
Curated by ChEMBL
| Assay Description
Inhibition of human GSK3-beta by scintillation counting |
J Med Chem 52: 1853-63 (2009)
Article DOI: 10.1021/jm801317h
BindingDB Entry DOI: 10.7270/Q24M94FZ |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50384788

(LAPACHOL)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#6]-1-[#6](=O)-[#6](=O)-c2ccccc2-[#6]-1=O Show InChI InChI=1S/C15H14O3/c1-9(2)7-8-12-13(16)10-5-3-4-6-11(10)14(17)15(12)18/h3-7,12H,8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of human DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | |
Dihydroorotate dehydrogenase (quinone), mitochondrial
(Homo sapiens (Human)) | BDBM50523227
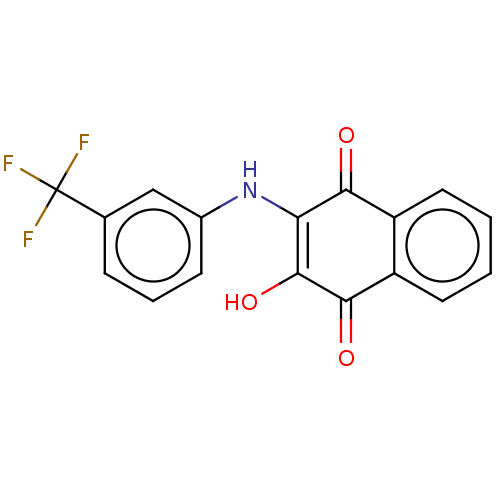
(CHEMBL4472078)Show SMILES OC1=C(Nc2cccc(c2)C(F)(F)F)C(=O)c2ccccc2C1=O |c:1| Show InChI InChI=1S/C17H10F3NO3/c18-17(19,20)9-4-3-5-10(8-9)21-13-14(22)11-6-1-2-7-12(11)15(23)16(13)24/h1-8,21,24H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade de S£o Paulo
Curated by ChEMBL
| Assay Description
Inhibition of human DHODH using DHO as substrate measured at 4 secs interval for 60 secs by DCIP reduction based indirect assay |
Eur J Med Chem 167: 357-366 (2019)
Article DOI: 10.1016/j.ejmech.2019.02.018
BindingDB Entry DOI: 10.7270/Q2XK8JZ2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data