

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM21190 (4-(2-{[5-amino-2-(furan-2-yl)-[1,2,4]triazolo[1,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Binding affinity towards human Adenosine A2a receptor expressed in HEK293 cells using 6 nM [3H]CGS-21680 | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395423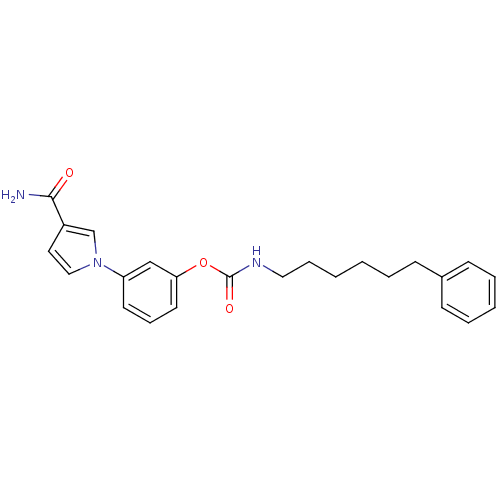 (CHEMBL2165084) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395424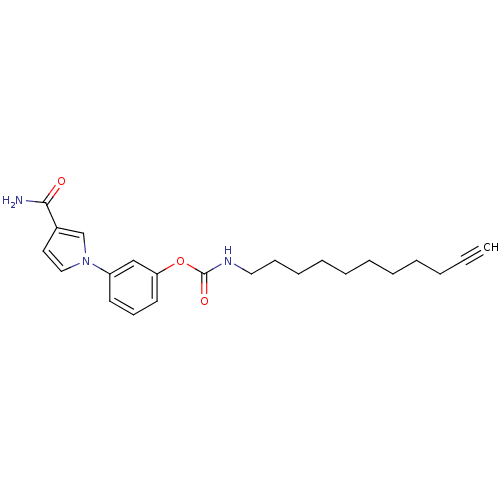 (CHEMBL2165083) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139771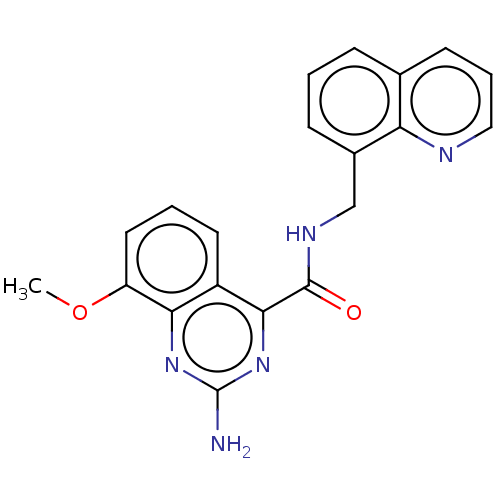 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139773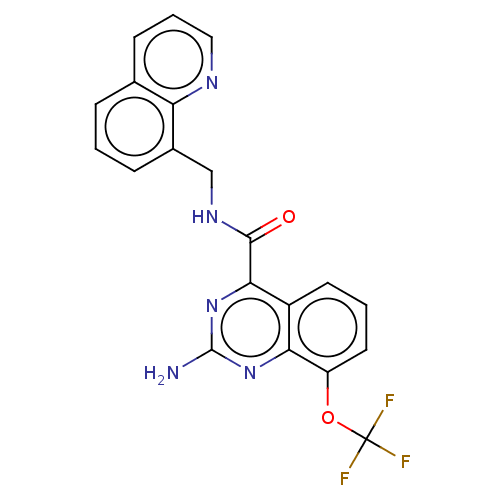 (CHEMBL3765379 | US10138212, Example 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139748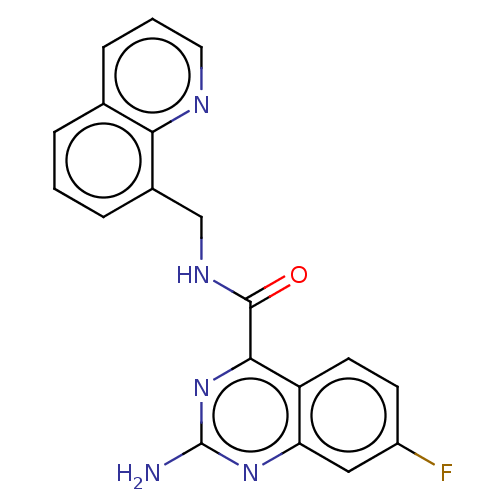 (CHEMBL3763717) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50176057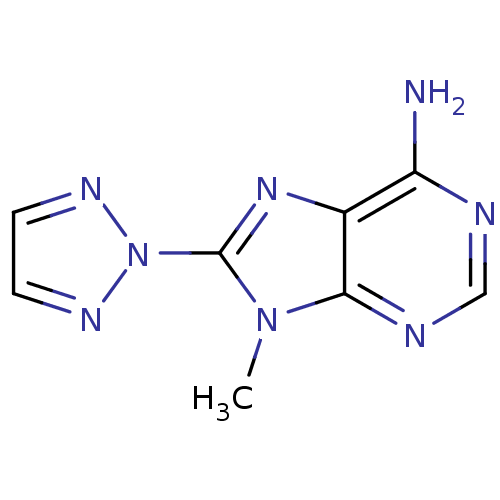 (9-Methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Binding affinity towards human Adenosine A1 receptor expressed in CHO cells using 1 nM [3H]DPCPX | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395421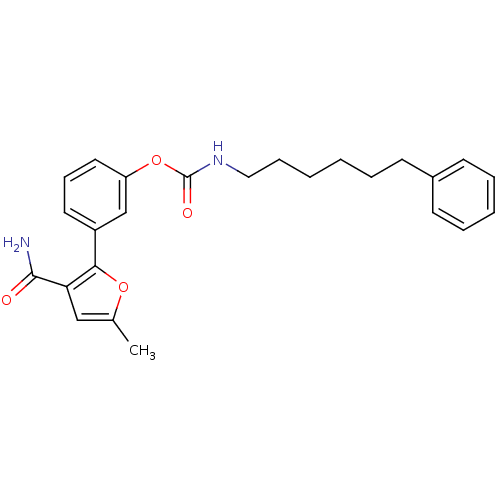 (CHEMBL2165094) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139655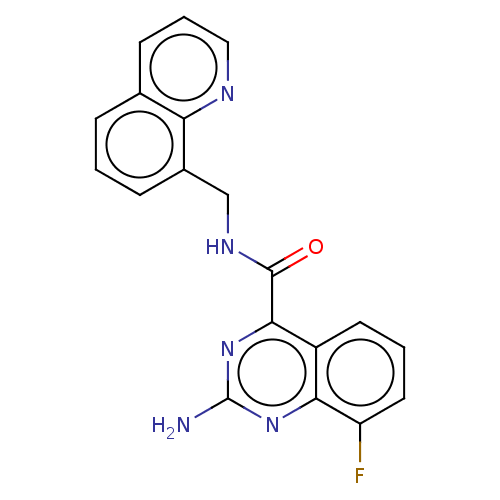 (CHEMBL3764083) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM50395420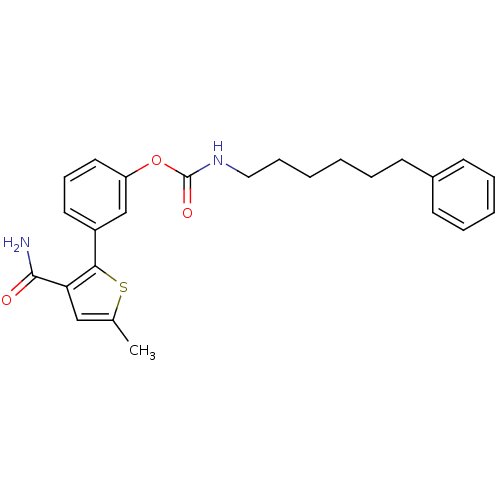 (CHEMBL2165070) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289804 (Methyl (4-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.600 | -52.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50139771 (CHEMBL3765580 | US10138212, Example 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289851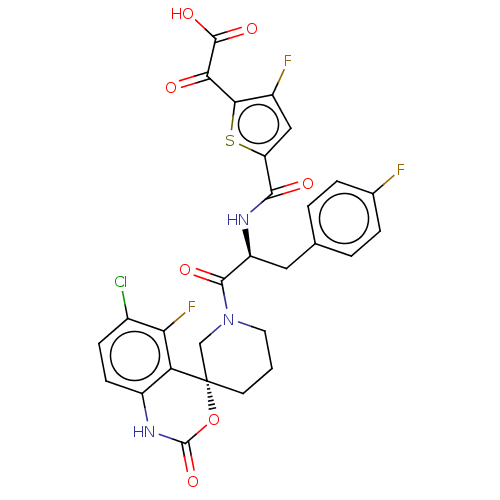 (2-(5-(((S)-1-((R)-6-chloro-5-fluoro-2-oxo-1,2-dihy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | -52.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139765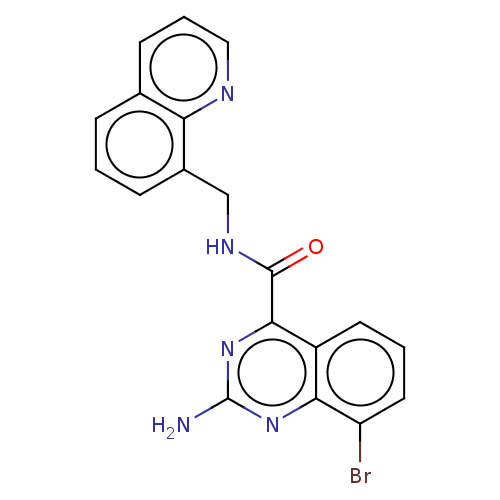 (CHEMBL3763830 | US10138212, Example 96) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508614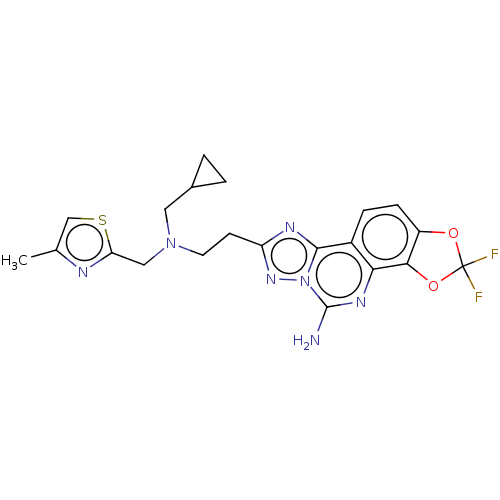 (8-(2-{(cyclopropylmethyl)[(4- methyl-1,3-thiazol-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM289807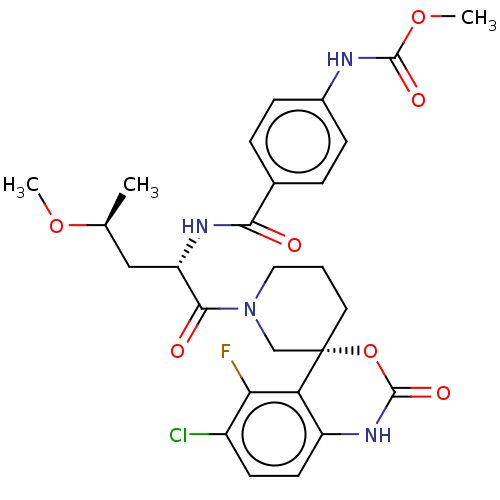 (US10093683, Example 118A | US10093683, Example 118...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Merck Sharp & Dohme Corp. US Patent | Assay Description The effectiveness of a compound of the present invention as an inhibitor of Coagulation Factor XIa can be determined using a relevant purified serine... | US Patent US10093683 (2018) BindingDB Entry DOI: 10.7270/Q2RR2197 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508706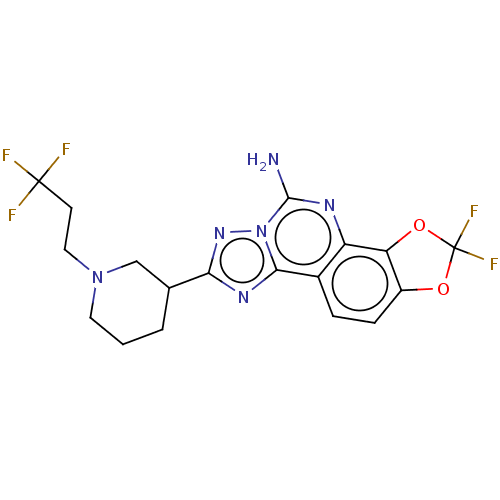 (US11046714, Example 140 | US11046714, Example 141) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508593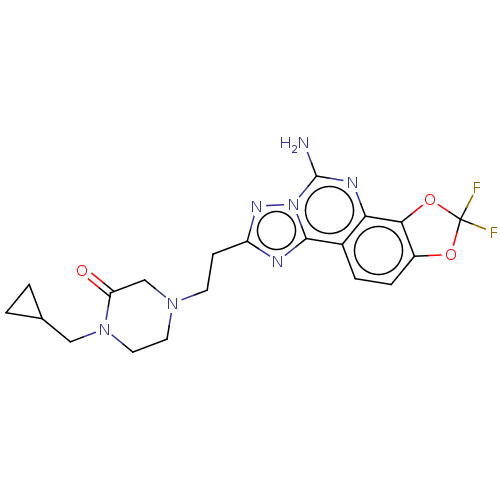 (4-[2-(5-amino-2,2-difluoro[1,3] dioxolo[4,5-h][1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50118812 ((2S,3S,4R,5R)-3,4-Dihydroxy-5-[6-(3-iodo-benzylami...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Urbino Carlo Bo Curated by ChEMBL | Assay Description Binding affinity towards human Adenosine A3 receptor expressed in HEK293 cells using 0.1 nM [3H]AB-MECA | J Med Chem 48: 6887-96 (2005) Article DOI: 10.1021/jm058018d BindingDB Entry DOI: 10.7270/Q28C9X1X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508630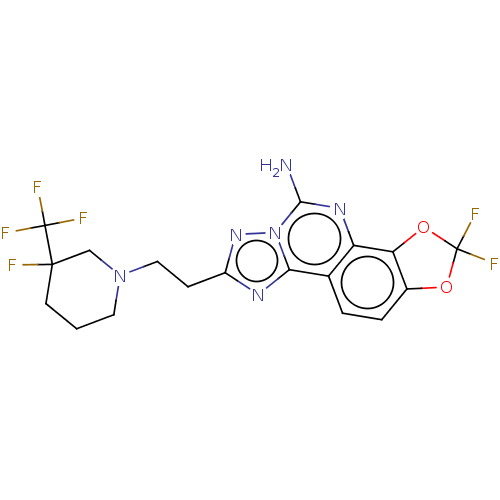 (US11046714, Example 72 | US11046714, Example 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508630 (US11046714, Example 72 | US11046714, Example 73) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508537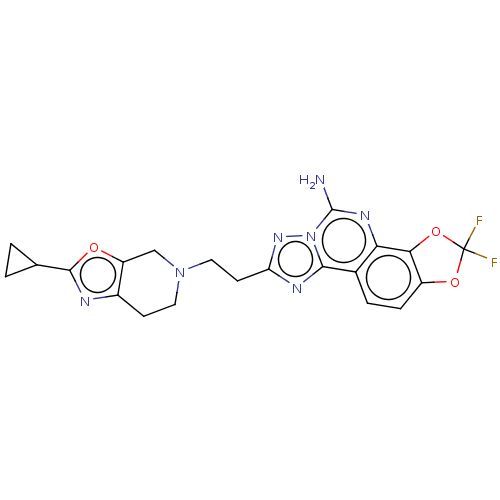 (8-[2-(2-cyclopropyl-6,7- dihydro[1,3]oxazolo[5,4- ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508704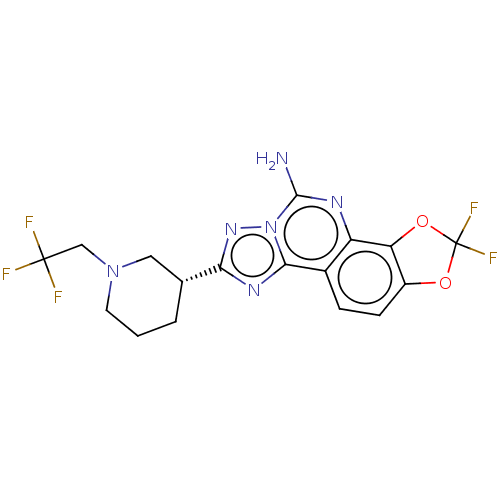 (US11046714, Example 138) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508693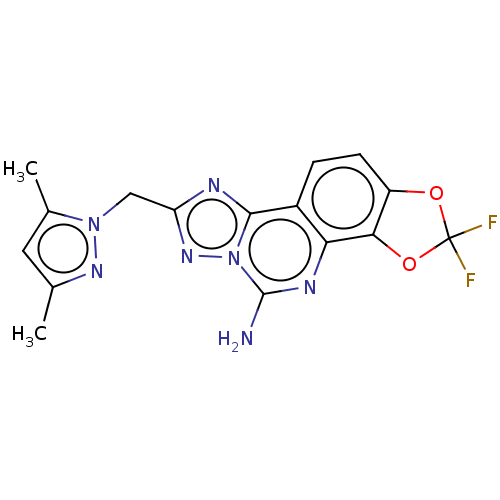 (8-[(3,5-dimethyl-1H-pyrazol-1-yl) methyl]-2,2-difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508611 (2,2-difluoro-8-(2-{[1- (trifluoromethyl)cyclobutyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50139653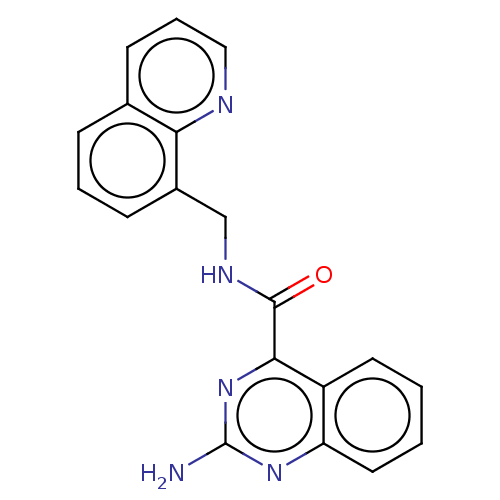 (CHEMBL3765818) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]SCH-58261 from human adenosine A2A receptor expressed in HEK cell membranes after 60 mins by microplate scintillation counting an... | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508689 (2,2-difluoro-8-({[1-(trifluoromethyl) cyclobutyl]a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508599 (4-[2-(5-amino-2,2-difluoro[1,3] dioxolo[4,5-h][1,2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508553 (8-{2-[(2R)-4,4-difluoro-2- methylpyrrolidin-1-yl]e...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Mus musculus (mouse)) | BDBM26739 (3-(3-carbamoylphenyl)phenyl N-cyclohexylcarbamate ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Inhibition of mouse FAAH isolated from brain homogenate using [3H-ethanolamine]AEA as substrate incubated for 20 mins prior to substrate addition mea... | J Med Chem 55: 6898-915 (2012) Article DOI: 10.1021/jm300689c BindingDB Entry DOI: 10.7270/Q2ZK5HSK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508625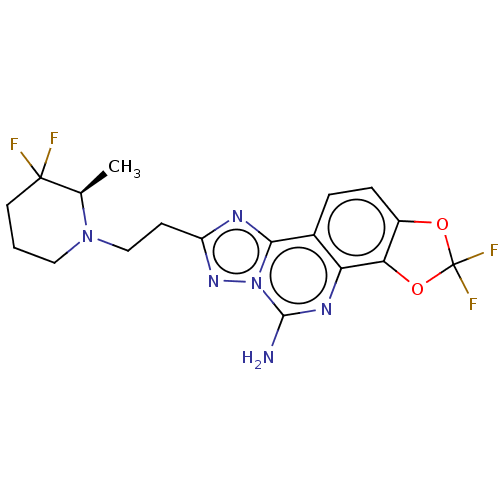 ((R or S)-8-[2-(3,3-difluoro-2- methylpiperidin-1-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508710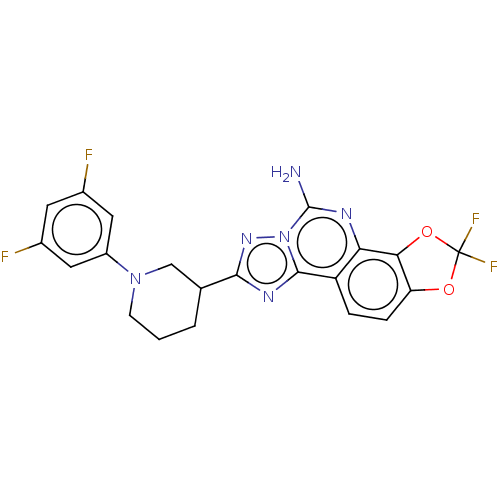 ((R or S)-8-[1-(3,5-difluorophenyl) piperidin-3-yl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508708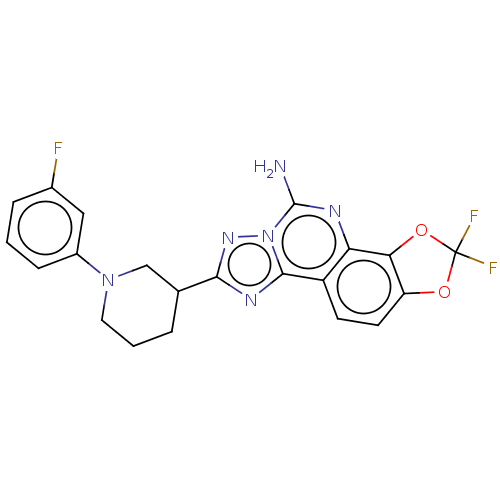 (US11046714, Example 142 | US11046714, Example 143) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508651 (8-[(2R or 2S)-2-(5,7-dihydro-6H- pyrrolo[3,4-b]pyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508638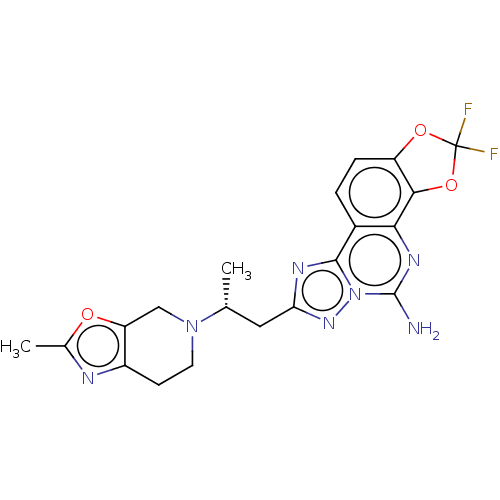 (2,2-difluoro-8-[(2R)-2-(2-methyl- 6,7-dihydro[1,3]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50139830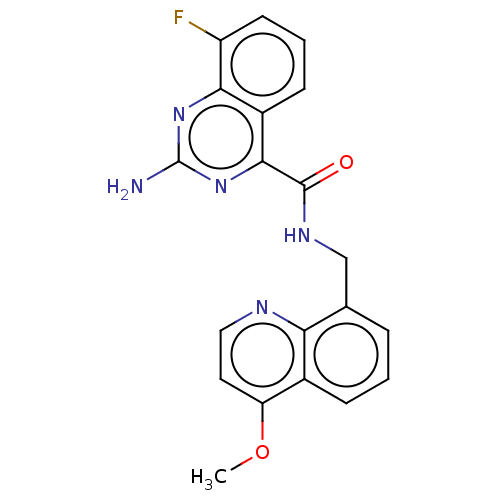 (CHEMBL3763374) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Rattus norvegicus (rat)) | BDBM50139655 (CHEMBL3764083) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to rat A2A adenosine receptor | Bioorg Med Chem Lett 26: 1348-54 (2016) Article DOI: 10.1016/j.bmcl.2015.11.048 BindingDB Entry DOI: 10.7270/Q2S46TT3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508636 (8-[(2R)-2-(5,8-dihydro-1,7- naphthyridin-7(6H)-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508691 (8-({(cyclopropylmethyl)[(4-methyl-1,3- thiazol-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508678 (2,2-difluoro-8-{[3-fluoro-3- (trifluoromethyl)azet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508675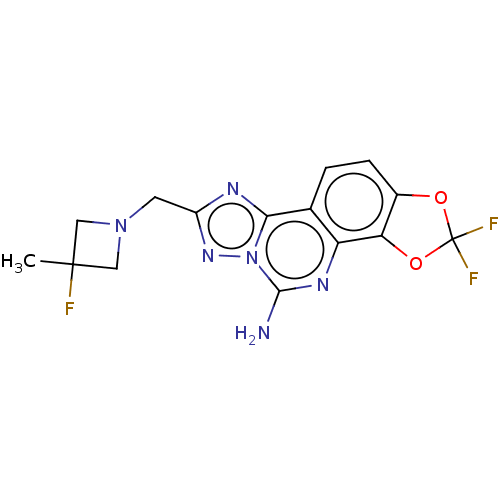 (2,2-difluoro-8-[(3-fluoro-3- methylazetidin-1-yl)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508552 (8-[2-(3,3-difluoropyrrolidin-1- yl)ethyl]-2,2-difl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508547 (2,2-difluoro-8-{2-[(2R,4S)-4- fluoro-2-methylpyrro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508616 (8-(2-{(cyclopropylmethyl)[(2- methyl-1,3-thiazol-5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508535 (2,2-difluoro-8-[2-(2-methyl-6,7- dihydro[1,3]oxazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508587 (2,2-difluoro-8-{2-[(2R)-2- methyl-4-(2-methyl-1,3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508705 (US11046714, Example 139) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508642 (2,2-difluoro-8-[(2R)-2-(2-methyl- 6,7-dihydro[1,3]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508542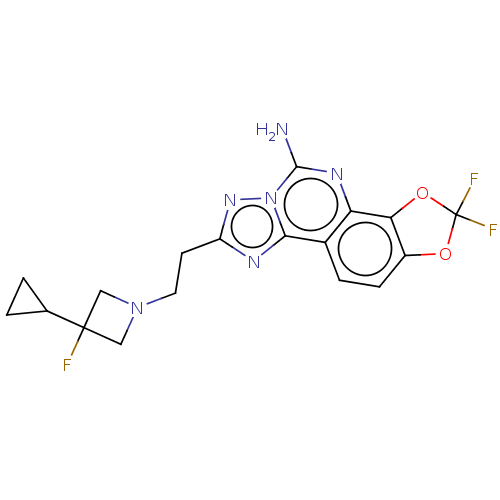 (8-[2-(3-cyclopropyl-3- fluoroazetidin-1-yl)ethyl]-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM508551 (8-{2-[(3S,4S)-3,4- difluoropyrrolidin-1-yl]ethyl}-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The following table shows representative data for the compounds of the Examples as A2a receptor antagonists as determined by a competition binding as... | Citation and Details BindingDB Entry DOI: 10.7270/Q28S4T2C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 785 total ) | Next | Last >> |