 Found 109 hits with Last Name = 'galvin' and Initial = 'km'
Found 109 hits with Last Name = 'galvin' and Initial = 'km' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RET |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Abl1 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139157

(CHEMBL3764588)Show SMILES Cl.Cn1nccc1-c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C30H26F3N5O3.ClH/c1-38-25(8-11-35-38)19-12-20(14-21(13-19)30(31,32)33)29(40)36-22-4-2-17-3-5-23(16-18(17)15-22)41-26-9-10-34-28-24(26)6-7-27(39)37-28;/h3,5,8-14,16,22H,2,4,6-7,15H2,1H3,(H,36,40)(H,34,37,39);1H/t22-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of RET |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139150
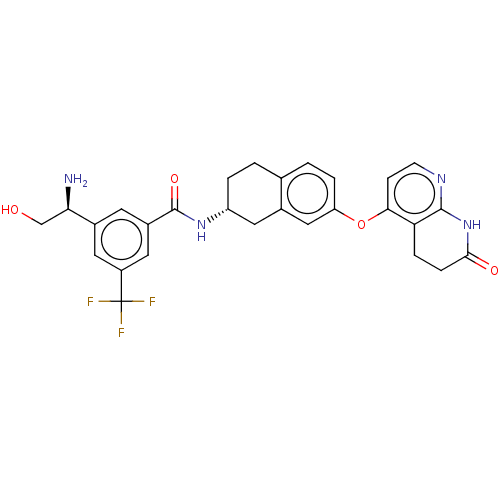
(CHEMBL3763999)Show SMILES Cl.N[C@H](CO)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C28H27F3N4O4.ClH/c29-28(30,31)19-10-17(23(32)14-36)9-18(11-19)27(38)34-20-3-1-15-2-4-21(13-16(15)12-20)39-24-7-8-33-26-22(24)5-6-25(37)35-26;/h2,4,7-11,13,20,23,36H,1,3,5-6,12,14,32H2,(H,34,38)(H,33,35,37);1H/t20-,23-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139149

(CHEMBL3765012)Show SMILES Cl.Cl.NC1(CCC1)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C30H29F3N4O3.2ClH/c31-30(32,33)21-13-19(12-20(16-21)29(34)9-1-10-29)28(39)36-22-4-2-17-3-5-23(15-18(17)14-22)40-25-8-11-35-27-24(25)6-7-26(38)37-27;;/h3,5,8,11-13,15-16,22H,1-2,4,6-7,9-10,14,34H2,(H,36,39)(H,35,37,38);2*1H/t22-;;/m1../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139147
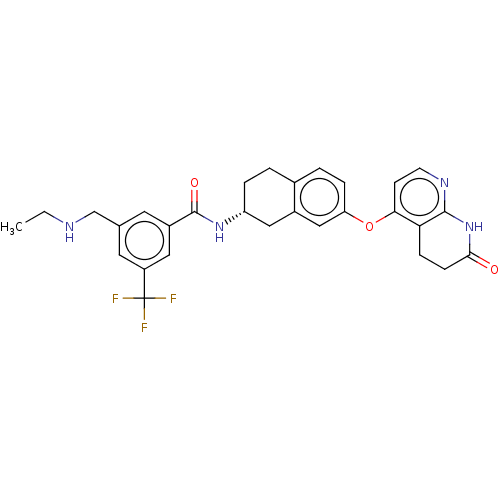
(CHEMBL3765509)Show SMILES Cl.Cl.CCNCc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3.2ClH/c1-2-33-16-17-11-20(13-21(12-17)29(30,31)32)28(38)35-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-34-27-24(25)7-8-26(37)36-27;;/h4,6,9-13,15,22,33H,2-3,5,7-8,14,16H2,1H3,(H,35,38)(H,34,36,37);2*1H/t22-;;/m1../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139153
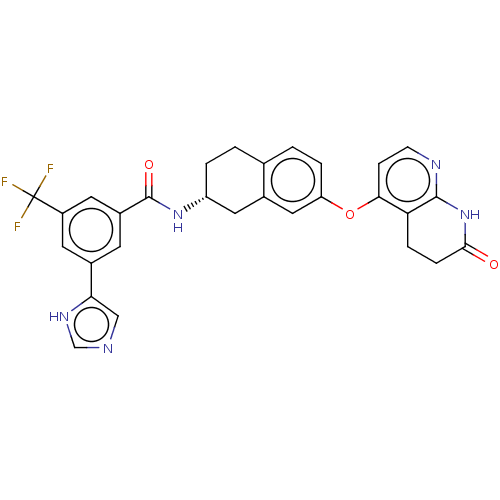
(CHEMBL3765355)Show SMILES FC(F)(F)c1cc(cc(c1)-c1cnc[nH]1)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H24F3N5O3/c30-29(31,32)20-10-18(24-14-33-15-35-24)9-19(11-20)28(39)36-21-3-1-16-2-4-22(13-17(16)12-21)40-25-7-8-34-27-23(25)5-6-26(38)37-27/h2,4,7-11,13-15,21H,1,3,5-6,12H2,(H,33,35)(H,36,39)(H,34,37,38)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139158
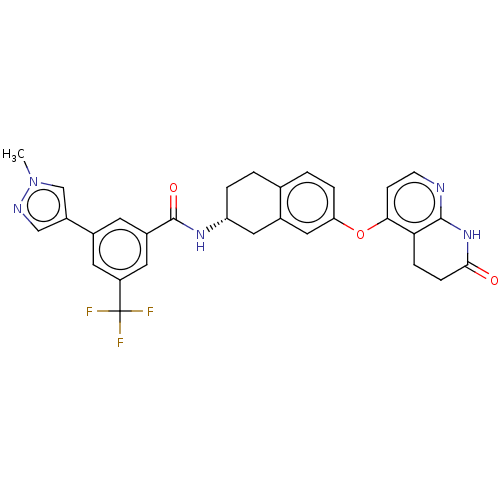
(CHEMBL3763646)Show SMILES Cl.Cn1cc(cn1)-c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C30H26F3N5O3.ClH/c1-38-16-21(15-35-38)18-10-20(12-22(11-18)30(31,32)33)29(40)36-23-4-2-17-3-5-24(14-19(17)13-23)41-26-8-9-34-28-25(26)6-7-27(39)37-28;/h3,5,8-12,14-16,23H,2,4,6-7,13H2,1H3,(H,36,40)(H,34,37,39);1H/t23-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139156
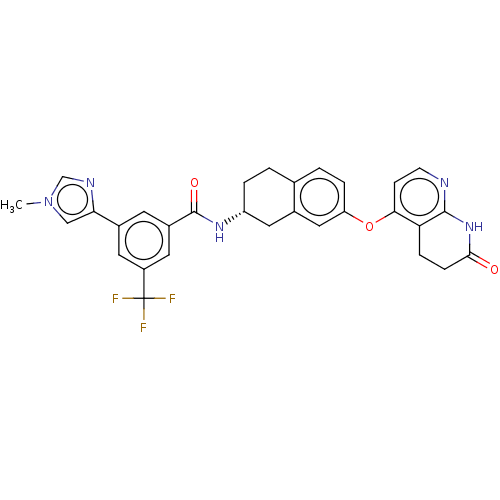
(CHEMBL3763694)Show SMILES Cl.Cl.Cn1cnc(c1)-c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C30H26F3N5O3.2ClH/c1-38-15-25(35-16-38)19-10-20(12-21(11-19)30(31,32)33)29(40)36-22-4-2-17-3-5-23(14-18(17)13-22)41-26-8-9-34-28-24(26)6-7-27(39)37-28;;/h3,5,8-12,14-16,22H,2,4,6-7,13H2,1H3,(H,36,40)(H,34,37,39);2*1H/t22-;;/m1../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139151

(CHEMBL3764870)Show SMILES Cl.Cl.OC1(CNC1)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H27F3N4O4.2ClH/c30-29(31,32)20-10-18(9-19(13-20)28(39)14-33-15-28)27(38)35-21-3-1-16-2-4-22(12-17(16)11-21)40-24-7-8-34-26-23(24)5-6-25(37)36-26;;/h2,4,7-10,12-13,21,33,39H,1,3,5-6,11,14-15H2,(H,35,38)(H,34,36,37);2*1H/t21-;;/m1../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339621
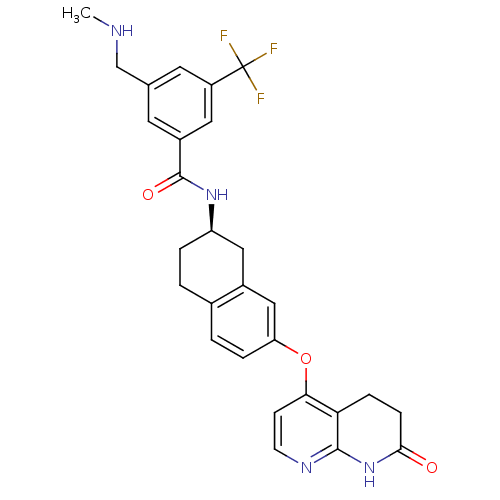
(3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...)Show SMILES CNCc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C28H27F3N4O3/c1-32-15-16-10-19(12-20(11-16)28(29,30)31)27(37)34-21-4-2-17-3-5-22(14-18(17)13-21)38-24-8-9-33-26-23(24)6-7-25(36)35-26/h3,5,8-12,14,21,32H,2,4,6-7,13,15H2,1H3,(H,34,37)(H,33,35,36)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139155
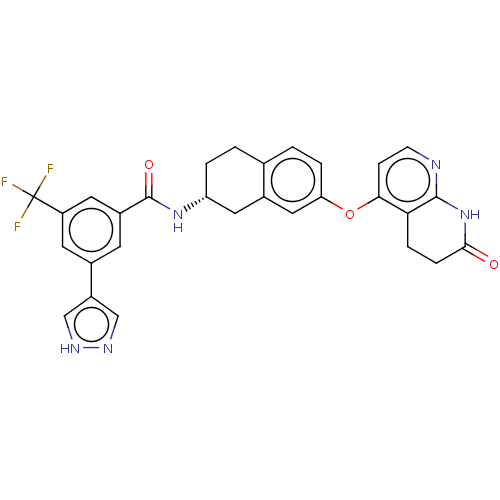
(CHEMBL3765708)Show SMILES Cl.FC(F)(F)c1cc(cc(c1)-c1cn[nH]c1)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H24F3N5O3.ClH/c30-29(31,32)21-10-17(20-14-34-35-15-20)9-19(11-21)28(39)36-22-3-1-16-2-4-23(13-18(16)12-22)40-25-7-8-33-27-24(25)5-6-26(38)37-27;/h2,4,7-11,13-15,22H,1,3,5-6,12H2,(H,34,35)(H,36,39)(H,33,37,38);1H/t22-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139148
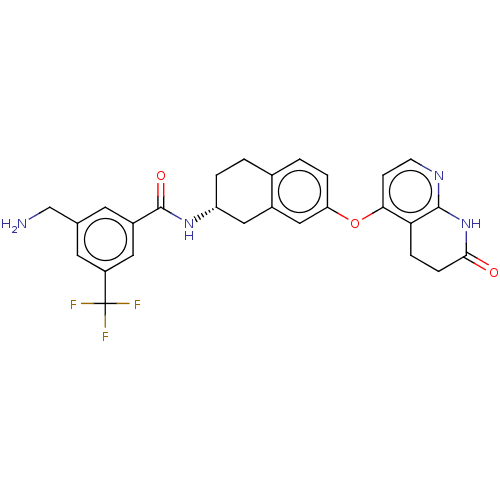
(CHEMBL3764101)Show SMILES Cl.Cl.NCc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C27H25F3N4O3.2ClH/c28-27(29,30)19-10-15(14-31)9-18(11-19)26(36)33-20-3-1-16-2-4-21(13-17(16)12-20)37-23-7-8-32-25-22(23)5-6-24(35)34-25;;/h2,4,7-11,13,20H,1,3,5-6,12,14,31H2,(H,33,36)(H,32,34,35);2*1H/t20-;;/m1../s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139159
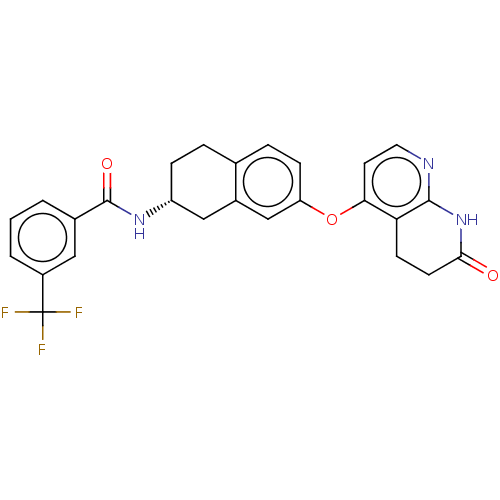
(CHEMBL3765596)Show SMILES FC(F)(F)c1cccc(c1)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C26H22F3N3O3/c27-26(28,29)18-3-1-2-16(12-18)25(34)31-19-6-4-15-5-7-20(14-17(15)13-19)35-22-10-11-30-24-21(22)8-9-23(33)32-24/h1-3,5,7,10-12,14,19H,4,6,8-9,13H2,(H,31,34)(H,30,32,33)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339615

((2S)-7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-y...)Show SMILES CN(C)Cc1cc(NC(=O)[C@H]2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C30H31F3N4O3/c1-37(2)17-18-11-23(30(31,32)33)15-24(12-18)35-29(39)21-6-3-19-7-8-25(14-22(19)13-21)40-26-9-10-34-27(16-26)36-28(38)20-4-5-20/h7-12,14-16,20-21H,3-6,13,17H2,1-2H3,(H,35,39)(H,34,36,38)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139152

(CHEMBL3764275)Show SMILES OCC1COCCN1Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C32H33F3N4O5/c33-32(34,35)23-12-19(16-39-9-10-43-18-25(39)17-40)11-22(13-23)31(42)37-24-3-1-20-2-4-26(15-21(20)14-24)44-28-7-8-36-30-27(28)5-6-29(41)38-30/h2,4,7-8,11-13,15,24-25,40H,1,3,5-6,9-10,14,16-18H2,(H,37,42)(H,36,38,41)/t24-,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Mus musculus (mouse)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse recombinant Aurora A kinase expressed in insect Sf9 cells by radioactive flashplate assay |
Proc Natl Acad Sci USA 104: 4106-11 (2007)
Article DOI: 10.1073/pnas.0608798104
BindingDB Entry DOI: 10.7270/Q25B03CS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type B-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339613

((2S)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...)Show SMILES NCc1cc(NC(=O)[C@H]2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H27F3N4O3/c29-28(30,31)21-9-16(15-32)10-22(13-21)34-27(37)19-4-1-17-5-6-23(12-20(17)11-19)38-24-7-8-33-25(14-24)35-26(36)18-2-3-18/h5-10,12-14,18-19H,1-4,11,15,32H2,(H,34,37)(H,33,35,36)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339616

(7-({2-[(Cyclopropylcarbonyl)amino]pyridin-4-yl}oxy...)Show SMILES CC(C)NCc1cc(NC(=O)C2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F Show InChI InChI=1S/C31H33F3N4O3/c1-18(2)36-17-19-11-24(31(32,33)34)15-25(12-19)37-30(40)22-6-3-20-7-8-26(14-23(20)13-22)41-27-9-10-35-28(16-27)38-29(39)21-4-5-21/h7-12,14-16,18,21-22,36H,3-6,13,17H2,1-2H3,(H,37,40)(H,35,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of wild-type B-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339614

((2R)-N-[3-(aminomethyl)-5-(trifluoromethyl)phenyl]...)Show SMILES NCc1cc(NC(=O)[C@@H]2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F |r| Show InChI InChI=1S/C28H27F3N4O3/c29-28(30,31)21-9-16(15-32)10-22(13-21)34-27(37)19-4-1-17-5-6-23(12-20(17)11-19)38-24-7-8-33-25(14-24)35-26(36)18-2-3-18/h5-10,12-14,18-19H,1-4,11,15,32H2,(H,34,37)(H,33,35,36)/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339611
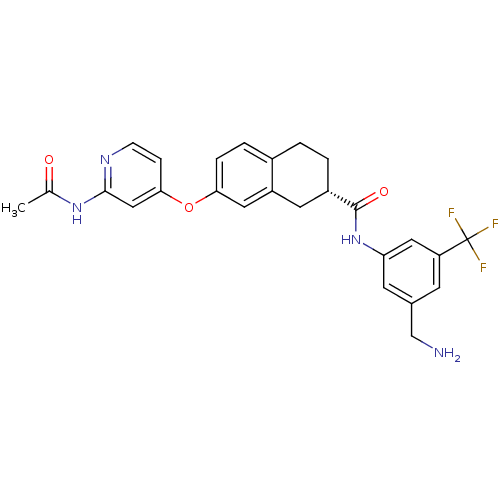
((2S)-7-{[2-(acetylamino)pyridin-4-yl]oxy}-N-[3-(am...)Show SMILES CC(=O)Nc1cc(Oc2ccc3CC[C@@H](Cc3c2)C(=O)Nc2cc(CN)cc(c2)C(F)(F)F)ccn1 |r| Show InChI InChI=1S/C26H25F3N4O3/c1-15(34)32-24-13-23(6-7-31-24)36-22-5-4-17-2-3-18(10-19(17)11-22)25(35)33-21-9-16(14-30)8-20(12-21)26(27,28)29/h4-9,11-13,18H,2-3,10,14,30H2,1H3,(H,33,35)(H,31,32,34)/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM16673

(4-[4-({[4-chloro-3-(trifluoromethyl)phenyl]carbamo...)Show SMILES CNC(=O)c1cc(Oc2ccc(NC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C21H16ClF3N4O3/c1-26-19(30)18-11-15(8-9-27-18)32-14-5-2-12(3-6-14)28-20(31)29-13-4-7-17(22)16(10-13)21(23,24)25/h2-11H,1H3,(H,26,30)(H2,28,29,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of KDR |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339612

(CHEMBL1688868 | N-[3-(Aminomethyl)-5-(trifluoromet...)Show SMILES NCc1cc(NC(=O)C2CCc3ccc(Oc4ccnc(NC(=O)C5CC5)c4)cc3C2)cc(c1)C(F)(F)F Show InChI InChI=1S/C28H27F3N4O3/c29-28(30,31)21-9-16(15-32)10-22(13-21)34-27(37)19-4-1-17-5-6-23(12-20(17)11-19)38-24-7-8-33-25(14-24)35-26(36)18-2-3-18/h5-10,12-14,18-19H,1-4,11,15,32H2,(H,34,37)(H,33,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Discoidin domain-containing receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of DDR2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339623

(4-[3-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...)Show SMILES CNC(=O)c1cc(Oc2cccc(CCC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)c2)ccn1 Show InChI InChI=1S/C23H19ClF3N3O3/c1-28-22(32)20-13-17(9-10-29-20)33-16-4-2-3-14(11-16)5-8-21(31)30-15-6-7-19(24)18(12-15)23(25,26)27/h2-4,6-7,9-13H,5,8H2,1H3,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139154

(CHEMBL3765074)Show SMILES FC(F)(F)c1cc(cc(c1)-c1cc[nH]n1)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H24F3N5O3/c30-29(31,32)20-12-18(24-7-10-34-37-24)11-19(13-20)28(39)35-21-3-1-16-2-4-22(15-17(16)14-21)40-25-8-9-33-27-23(25)5-6-26(38)36-27/h2,4,7-13,15,21H,1,3,5-6,14H2,(H,34,37)(H,35,39)(H,33,36,38)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339622
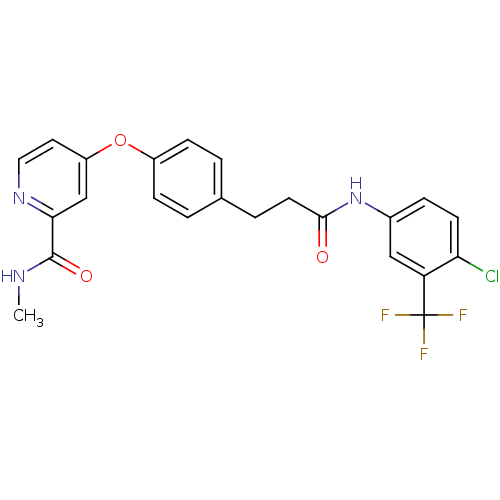
(4-[4-(3-{[4-Chloro-3-(trifluoromethyl)phenyl]amino...)Show SMILES CNC(=O)c1cc(Oc2ccc(CCC(=O)Nc3ccc(Cl)c(c3)C(F)(F)F)cc2)ccn1 Show InChI InChI=1S/C23H19ClF3N3O3/c1-28-22(32)20-13-17(10-11-29-20)33-16-6-2-14(3-7-16)4-9-21(31)30-15-5-8-19(24)18(12-15)23(25,26)27/h2-3,5-8,10-13H,4,9H2,1H3,(H,28,32)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339610
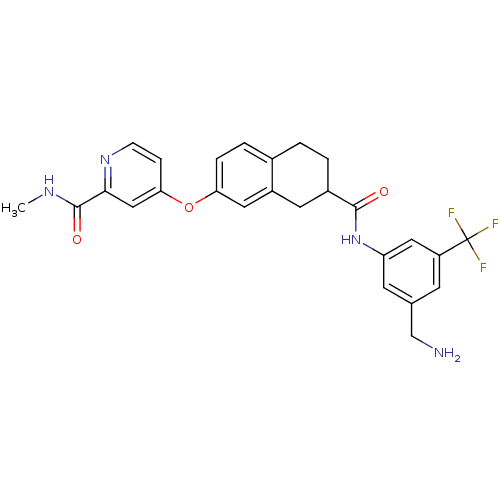
(4-{[7-({[3-(Aminomethyl)-5-(trifluoromethyl)phenyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3CCC(Cc3c2)C(=O)Nc2cc(CN)cc(c2)C(F)(F)F)ccn1 Show InChI InChI=1S/C26H25F3N4O3/c1-31-25(35)23-13-22(6-7-32-23)36-21-5-4-16-2-3-17(10-18(16)11-21)24(34)33-20-9-15(14-30)8-19(12-20)26(27,28)29/h4-9,11-13,17H,2-3,10,14,30H2,1H3,(H,31,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339625

(4-{[7-({[4-(Aminomethyl)-3-(trifluoromethyl)phenyl...)Show SMILES CNC(=O)c1cc(Oc2ccc3CCC(Cc3c2)C(=O)Nc2ccc(CN)c(c2)C(F)(F)F)ccn1 Show InChI InChI=1S/C26H25F3N4O3/c1-31-25(35)23-13-21(8-9-32-23)36-20-7-5-15-2-3-16(10-18(15)11-20)24(34)33-19-6-4-17(14-30)22(12-19)26(27,28)29/h4-9,11-13,16H,2-3,10,14,30H2,1H3,(H,31,35)(H,33,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50343948
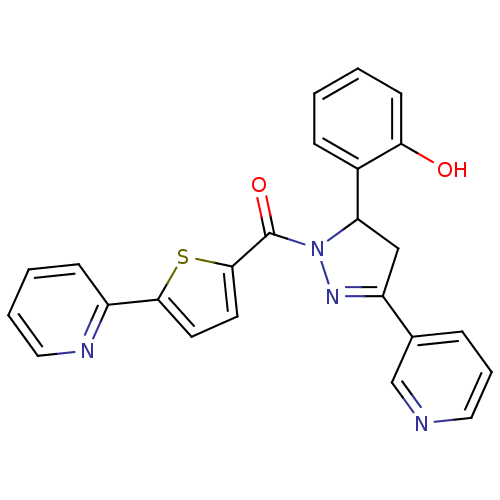
((5-(2-hydroxyphenyl)-3-(pyridin-3-yl)-4,5-dihydro-...)Show SMILES Oc1ccccc1C1CC(=NN1C(=O)c1ccc(s1)-c1ccccn1)c1cccnc1 |c:10| Show InChI InChI=1S/C24H18N4O2S/c29-21-9-2-1-7-17(21)20-14-19(16-6-5-12-25-15-16)27-28(20)24(30)23-11-10-22(31-23)18-8-3-4-13-26-18/h1-13,15,20,29H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of B-Raf |
Bioorg Med Chem Lett 20: 4800-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.113
BindingDB Entry DOI: 10.7270/Q2MP53M9 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EPHA2 |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50343948

((5-(2-hydroxyphenyl)-3-(pyridin-3-yl)-4,5-dihydro-...)Show SMILES Oc1ccccc1C1CC(=NN1C(=O)c1ccc(s1)-c1ccccn1)c1cccnc1 |c:10| Show InChI InChI=1S/C24H18N4O2S/c29-21-9-2-1-7-17(21)20-14-19(16-6-5-12-25-15-16)27-28(20)24(30)23-11-10-22(31-23)18-8-3-4-13-26-18/h1-13,15,20,29H,14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf |
Bioorg Med Chem Lett 20: 4800-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.113
BindingDB Entry DOI: 10.7270/Q2MP53M9 |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
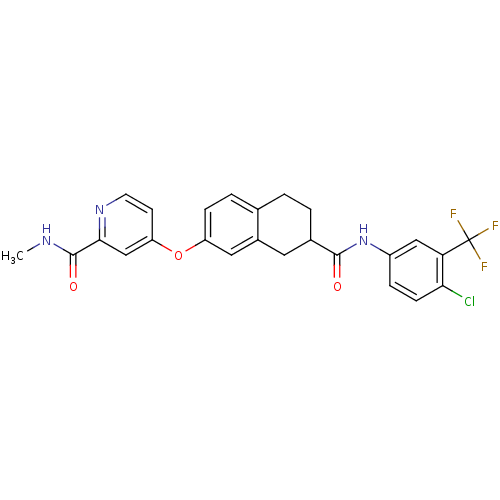
(Homo sapiens (Human)) | BDBM50339624
(CHEMBL1688864 | rac-4-{[7-({[4-Chloro-3-(trifluoro...)Show SMILES CNC(=O)c1cc(Oc2ccc3CCC(Cc3c2)C(=O)Nc2ccc(Cl)c(c2)C(F)(F)F)ccn1 Show InChI InChI=1S/C25H21ClF3N3O3/c1-30-24(34)22-13-19(8-9-31-22)35-18-6-4-14-2-3-15(10-16(14)11-18)23(33)32-17-5-7-21(26)20(12-17)25(27,28)29/h4-9,11-13,15H,2-3,10H2,1H3,(H,30,34)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-Raf assessed as reduction in [33P]ATP incorporation into biotinylated substrate peptide after 3 hrs by flash plate assay |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139158

(CHEMBL3763646)Show SMILES Cl.Cn1cc(cn1)-c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C30H26F3N5O3.ClH/c1-38-16-21(15-35-38)18-10-20(12-22(11-18)30(31,32)33)29(40)36-23-4-2-17-3-5-24(14-19(17)13-23)41-26-8-9-34-28-25(26)6-7-27(39)37-28;/h3,5,8-12,14-16,23H,2,4,6-7,13H2,1H3,(H,36,40)(H,34,37,39);1H/t23-;/m1./s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK5/p35NCK (unknown origin) using histone H1 as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM31093

(4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...)Show SMILES OC(=O)c1ccc(Nc2ncc3CN=C(c4cc(Cl)ccc4-c3n2)c2c(F)cccc2F)cc1 |c:13| Show InChI InChI=1S/C25H15ClF2N4O2/c26-15-6-9-17-18(10-15)23(21-19(27)2-1-3-20(21)28)29-11-14-12-30-25(32-22(14)17)31-16-7-4-13(5-8-16)24(33)34/h1-10,12H,11H2,(H,33,34)(H,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Aurora A Thr288 autophosphorylation in human HeLa cells after 1 hr |
Proc Natl Acad Sci USA 104: 4106-11 (2007)
Article DOI: 10.1073/pnas.0608798104
BindingDB Entry DOI: 10.7270/Q25B03CS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50139153

(CHEMBL3765355)Show SMILES FC(F)(F)c1cc(cc(c1)-c1cnc[nH]1)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H24F3N5O3/c30-29(31,32)20-10-18(24-14-33-15-35-24)9-19(11-20)28(39)36-21-3-1-16-2-4-22(13-17(16)12-21)40-25-7-8-34-27-23(25)5-6-26(38)37-27/h2,4,7-11,13-15,21H,1,3,5-6,12H2,(H,33,35)(H,36,39)(H,34,37,38)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK4/Cyclin D1 (unknown origin) using RPPTLSPIPHIPR peptide as substrate in presence of [gamma-33P]ATP |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50339608

(3-(1-Amino-1-methylethyl)-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CC(C)(N)c1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-28(2,33)19-11-18(12-20(15-19)29(30,31)32)27(38)35-21-5-3-16-4-6-22(14-17(16)13-21)39-24-9-10-34-26-23(24)7-8-25(37)36-26/h4,6,9-12,14-15,21H,3,5,7-8,13,33H2,1-2H3,(H,35,38)(H,34,36,37)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339621

(3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...)Show SMILES CNCc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C28H27F3N4O3/c1-32-15-16-10-19(12-20(11-16)28(29,30)31)27(37)34-21-4-2-17-3-5-22(14-18(17)13-21)38-24-8-9-33-26-23(24)6-7-25(36)35-26/h3,5,8-12,14,21,32H,2,4,6-7,13,15H2,1H3,(H,34,37)(H,33,35,36)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |
RAF proto-oncogene serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50339621

(3-[(methylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,8-...)Show SMILES CNCc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C28H27F3N4O3/c1-32-15-16-10-19(12-20(11-16)28(29,30)31)27(37)34-21-4-2-17-3-5-22(14-18(17)13-21)38-24-8-9-33-26-23(24)6-7-25(36)35-26/h3,5,8-12,14,21,32H,2,4,6-7,13,15H2,1H3,(H,34,37)(H,33,35,36)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of raf kinase in human A375 cells assessed as decrease in MEK-mediated ERK phosphorylation after 3 hrs using 3,3',5,5'-tetramethylbenzidin... |
J Med Chem 54: 1836-46 (2011)
Article DOI: 10.1021/jm101479y
BindingDB Entry DOI: 10.7270/Q2DB824F |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase B-raf
(Homo sapiens (Human)) | BDBM50339609

(3-[(Dimethylamino)methyl]-N-{(2R)-7-[(7-oxo-5,6,7,...)Show SMILES CN(C)Cc1cc(cc(c1)C(F)(F)F)C(=O)N[C@@H]1CCc2ccc(Oc3ccnc4NC(=O)CCc34)cc2C1 |r| Show InChI InChI=1S/C29H29F3N4O3/c1-36(2)16-17-11-20(13-21(12-17)29(30,31)32)28(38)34-22-5-3-18-4-6-23(15-19(18)14-22)39-25-9-10-33-27-24(25)7-8-26(37)35-27/h4,6,9-13,15,22H,3,5,7-8,14,16H2,1-2H3,(H,34,38)(H,33,35,37)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. 40 Landsdowne Street, Cambridge, MA 02139, United States.
Curated by ChEMBL
| Assay Description
Inhibition of CDK2/Cyclin E (unknown origin) expressed in baculoviral infected insect Sf9 cells using histone H1 as substrate in presence of [gamma-3... |
Bioorg Med Chem Lett 26: 1156-60 (2016)
Article DOI: 10.1016/j.bmcl.2016.01.049
BindingDB Entry DOI: 10.7270/Q29P33HD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data