

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carnitine O-palmitoyltransferase 1, muscle isoform (Rattus norvegicus) | BDBM50046145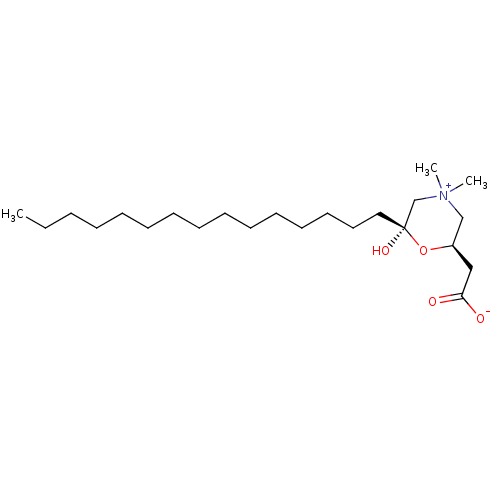 (2-[6-hydroxy-4,4-dimethyl-6-pentadecyl-(2R,6S)-1,4...) | Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit carnitine palmitoyltransferase I activity in intact mitochondria from rat heart | J Med Chem 36: 237-42 (1993) BindingDB Entry DOI: 10.7270/Q2P849ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carnitine O-palmitoyltransferase 1, liver isoform (Rattus norvegicus) | BDBM50046145 (2-[6-hydroxy-4,4-dimethyl-6-pentadecyl-(2R,6S)-1,4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Compound was evaluated for its ability to inhibit carnitine palmitoyltransferase I activity in intact mitochondria from rat liver | J Med Chem 36: 237-42 (1993) BindingDB Entry DOI: 10.7270/Q2P849ZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230709 (CHEMBL295954) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230703 (CHEMBL296591) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230702 (CHEMBL48695) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230712 (CHEMBL52232) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230714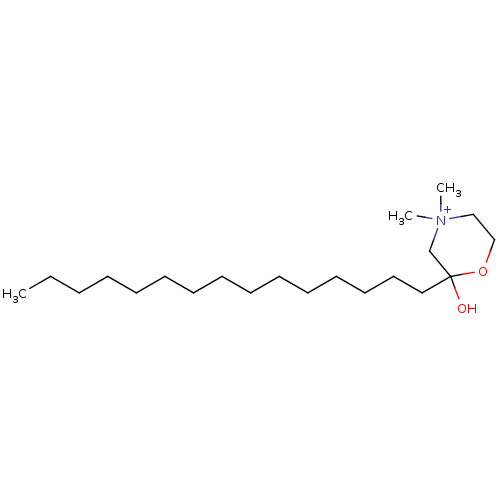 (CHEMBL48551) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230705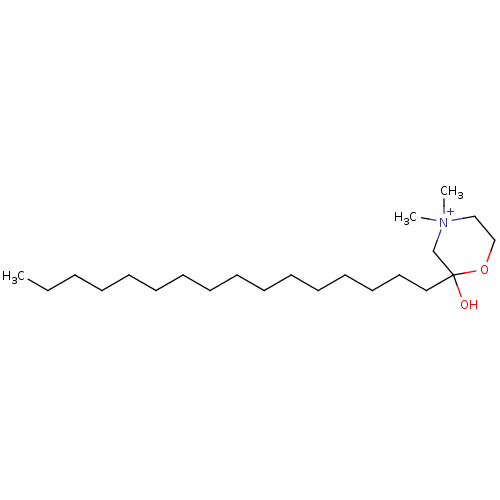 (CHEMBL49951) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230711 (CHEMBL441041) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230704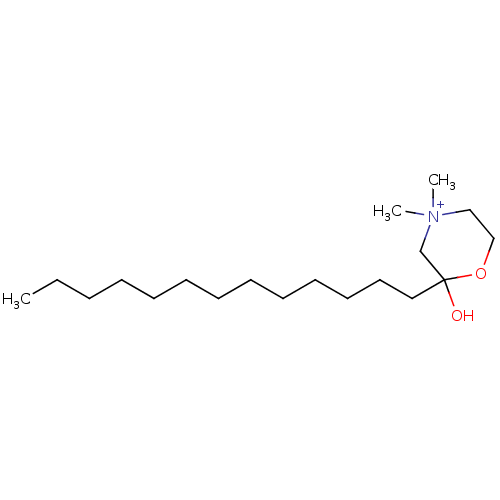 (CHEMBL416830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230713 (CHEMBL301124) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230701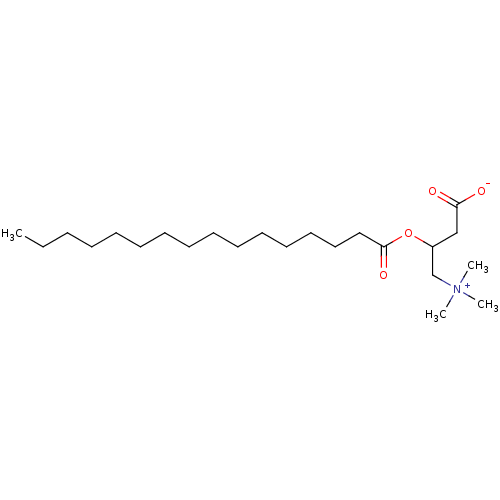 (CHEBI:73067 | Palmitoyl-carnitine) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50046145 (2-[6-hydroxy-4,4-dimethyl-6-pentadecyl-(2R,6S)-1,4...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carnitine O-palmitoyltransferase 1, liver isoform (Rattus norvegicus) | BDBM50284163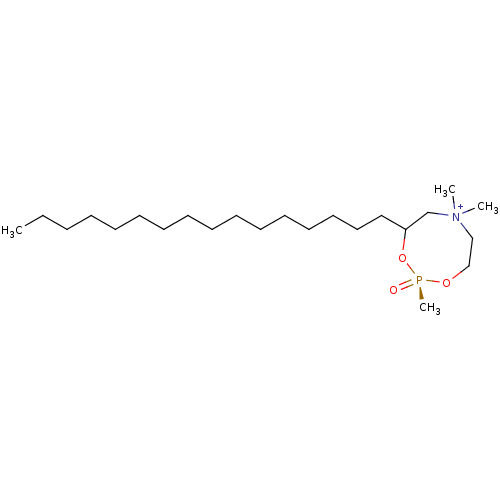 ((S)-4-Hexadecyl-2,6,6-trimethyl-2-oxo-2lambda*5*-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency of the compound against Carnitine Palmitoyltransferase (CPT-II) | Bioorg Med Chem Lett 4: 883-886 (1994) Article DOI: 10.1016/S0960-894X(01)80256-0 BindingDB Entry DOI: 10.7270/Q2RX9C15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C zeta type (Rattus norvegicus) | BDBM50230710 (CHEMBL49275) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Louisiana State University Curated by ChEMBL | Assay Description Inhibition of protein kinase C (PKC) purified from rat brain | J Med Chem 36: 177-8 (1993) BindingDB Entry DOI: 10.7270/Q2Z321VQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carnitine O-palmitoyltransferase 2, mitochondrial (Homo sapiens (Human)) | BDBM50284164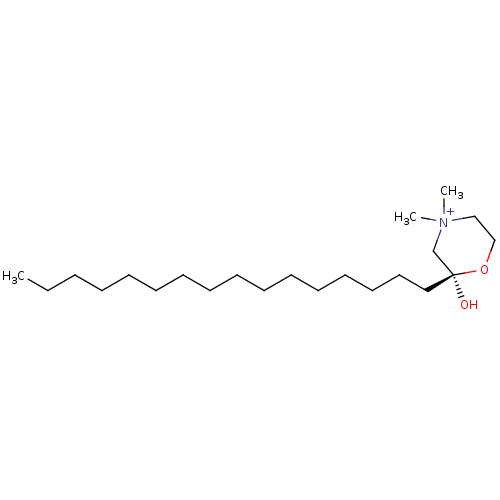 ((S)-2-Hexadecyl-2-hydroxy-4,4-dimethyl-morpholin-4...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 2.35E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency of the compound against Carnitine Palmitoyltransferase (CPT-II); Range = 32-235 microM | Bioorg Med Chem Lett 4: 883-886 (1994) Article DOI: 10.1016/S0960-894X(01)80256-0 BindingDB Entry DOI: 10.7270/Q2RX9C15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carnitine O-palmitoyltransferase 1, liver isoform (Rattus norvegicus) | BDBM50284163 ((S)-4-Hexadecyl-2,6,6-trimethyl-2-oxo-2lambda*5*-[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 3.05E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency of the compound against Carnitine Palmitoyltransferase (CPT-II) | Bioorg Med Chem Lett 4: 883-886 (1994) Article DOI: 10.1016/S0960-894X(01)80256-0 BindingDB Entry DOI: 10.7270/Q2RX9C15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal carnitine O-octanoyltransferase (Homo sapiens (Human)) | BDBM50284163 ((S)-4-Hexadecyl-2,6,6-trimethyl-2-oxo-2lambda*5*-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 4.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency of the compound against carnitine octanoyltransferase (COT) | Bioorg Med Chem Lett 4: 883-886 (1994) Article DOI: 10.1016/S0960-894X(01)80256-0 BindingDB Entry DOI: 10.7270/Q2RX9C15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal carnitine O-octanoyltransferase (Homo sapiens (Human)) | BDBM50284163 ((S)-4-Hexadecyl-2,6,6-trimethyl-2-oxo-2lambda*5*-[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article | n/a | n/a | 9.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency of the compound against carnitine octanoyltransferase (COT) | Bioorg Med Chem Lett 4: 883-886 (1994) Article DOI: 10.1016/S0960-894X(01)80256-0 BindingDB Entry DOI: 10.7270/Q2RX9C15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisomal carnitine O-octanoyltransferase (Homo sapiens (Human)) | BDBM50284164 ((S)-2-Hexadecyl-2-hydroxy-4,4-dimethyl-morpholin-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4.00E+6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory potency of the compound against carnitine octanoyltransferase (COT); Range = 1300-4000 microM | Bioorg Med Chem Lett 4: 883-886 (1994) Article DOI: 10.1016/S0960-894X(01)80256-0 BindingDB Entry DOI: 10.7270/Q2RX9C15 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| LanC-like protein 2 (Homo sapiens (Human)) | BDBM50558735 (CHEMBL4788758) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to LANCL2 (unknown origin) by surface plasmon resonance analysis | Citation and Details BindingDB Entry DOI: 10.7270/Q2Q243XP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||