

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (GUINEA PIG) | BDBM50180190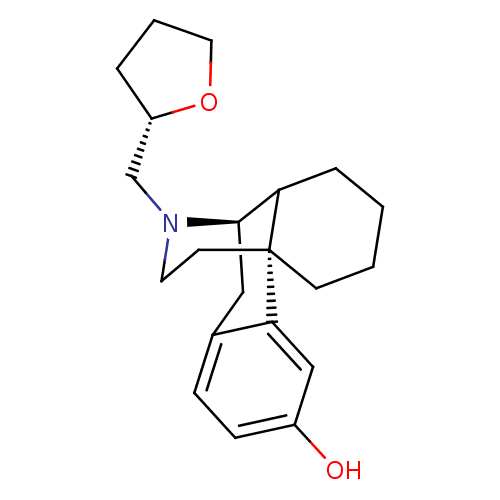 ((1R,9R)-17-[(2S)-oxolan-2-ylmethyl]-17-azatetracyc...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.0920 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50180190 ((1R,9R)-17-[(2S)-oxolan-2-ylmethyl]-17-azatetracyc...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50369519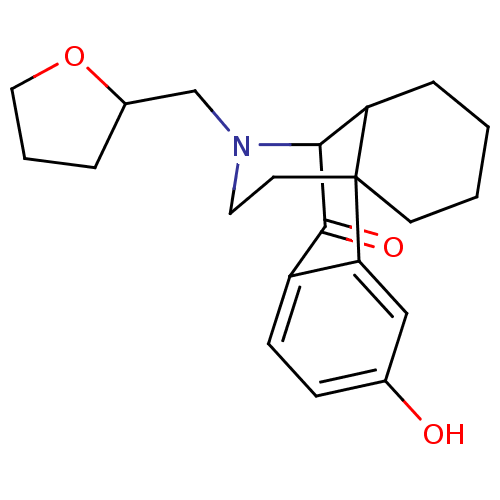 (CHEMBL1202427) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50369518 (LEVORPHANOL HYDROCHLORIDE) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50135800 ((-)-3-Hydroxy-N-cycloproypylmethylmorphinan Mandel...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50180190 ((1R,9R)-17-[(2S)-oxolan-2-ylmethyl]-17-azatetracyc...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50369519 (CHEMBL1202427) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50001023 ((2R,6R,11R)-3-Cyclopropylmethyl-6,11-dimethyl-1,2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50000091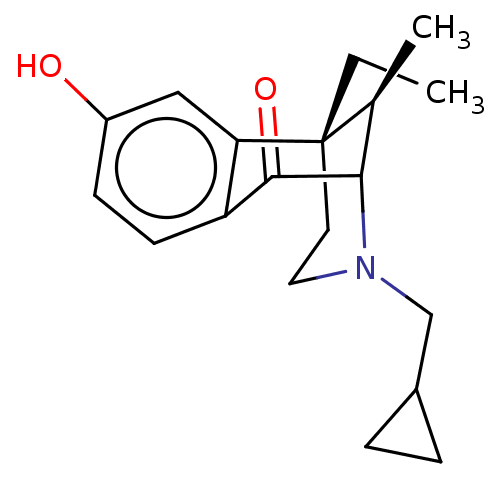 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000091 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369519 (CHEMBL1202427) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50135808 ((-)-3-Hydroxy-N-cyclobutylmethylmorphinan S(+)-Man...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50369518 (LEVORPHANOL HYDROCHLORIDE) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000091 ((6S,11R)-3-Cyclopropylmethyl-6-ethyl-8-hydroxy-11-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50365357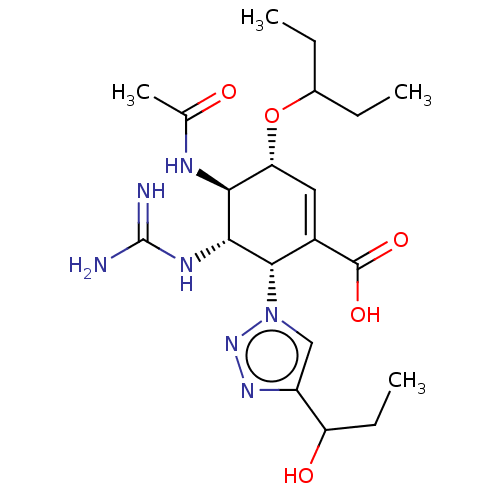 (CHEMBL4168935) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (H3N2) neuraminidase activity | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369518 (LEVORPHANOL HYDROCHLORIDE) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid UniChem Similars | DrugBank PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50365364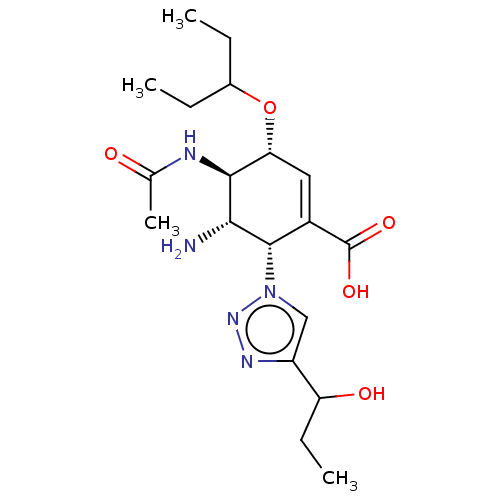 (CHEMBL4161008) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (H3N2) neuraminidase activity | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50369520 (CHEMBL1202425) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50365357 (CHEMBL4168935) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (H1N1) neuraminidase activity | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50365364 (CHEMBL4161008) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (H1N1) neuraminidase activity | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50369520 (CHEMBL1202425) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50369520 (CHEMBL1202425) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50083924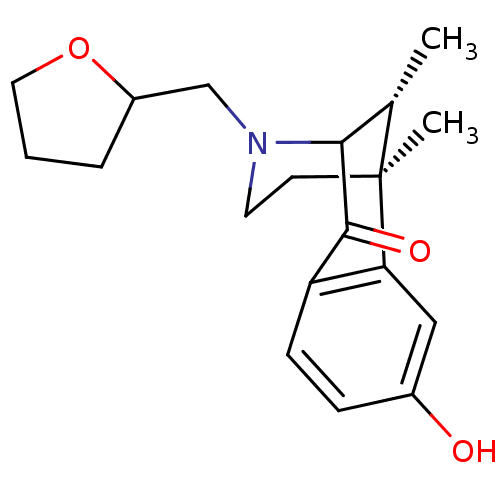 (8-Hydroxy-6,11-dimethyl-3-(tetrahydro-furan-2-ylme...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor kappa 1 by displacing the radioligand [3H]U-69593 from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50347134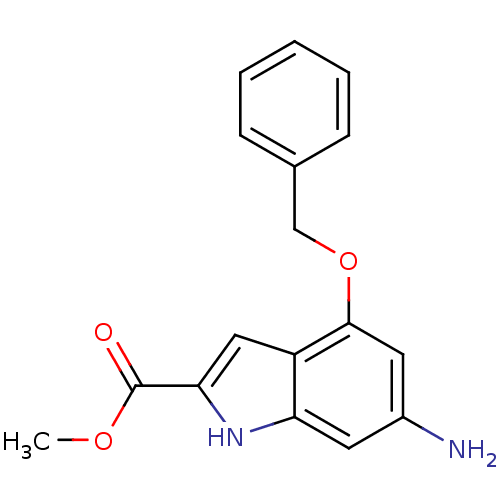 (CHEMBL1797249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis pantothenate synthetase using ATP by Dixon plot analysis | Bioorg Med Chem Lett 21: 3943-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.021 BindingDB Entry DOI: 10.7270/Q2X63N93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50365366 (CHEMBL1232591) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Paris/2590/2009(H1N1)) neuraminidase activity by fluorometric assay | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminase kidney isoform, mitochondrial (Homo sapiens (Human)) | BDBM108460 (CHEMBL2178393 | US11191732, Example 1 | US8604016,...) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johns Hopkins University Curated by ChEMBL | Assay Description Uncompetitive inhibition of human kidney glutaminase (124 to 669) assessed as reduction of glutamine hydrolysis by double-reciprocal plot analysis | J Med Chem 55: 10551-63 (2012) Article DOI: 10.1021/jm301191p BindingDB Entry DOI: 10.7270/Q2VD70M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (GUINEA PIG) | BDBM50083924 (8-Hydroxy-6,11-dimethyl-3-(tetrahydro-furan-2-ylme...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor mu 1 by displacing the radioligand [3H]DAMGO from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50365366 (CHEMBL1232591) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/HongKong/156/97 (H5N1)) neuraminidase activity by fluorometric assay | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pantothenate synthetase (Mycobacterium tuberculosis (strain ATCC 25618 / H3...) | BDBM50347134 (CHEMBL1797249) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Medical Sciences and Peking Union Medical College Curated by ChEMBL | Assay Description Competitive inhibition of Mycobacterium tuberculosis pantothenate synthetase using beta-alanine by Dixon plot analysis | Bioorg Med Chem Lett 21: 3943-6 (2011) Article DOI: 10.1016/j.bmcl.2011.05.021 BindingDB Entry DOI: 10.7270/Q2X63N93 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50083924 (8-Hydroxy-6,11-dimethyl-3-(tetrahydro-furan-2-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Binding affinity towards Opioid receptor delta 1 by displacing the radioligand [3H]naltrindole from guinea pig brain membrane | J Med Chem 43: 114-22 (2000) BindingDB Entry DOI: 10.7270/Q20V8DGH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (strain A/Memphis/1/1971 H3N2)) | BDBM50365366 (CHEMBL1232591) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 2.19E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Shandong University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Paris/908/97 (H3N2)) neuraminidase activity by fluorometric assay | J Med Chem 61: 6379-6397 (2018) Article DOI: 10.1021/acs.jmedchem.8b00929 BindingDB Entry DOI: 10.7270/Q2H41V0C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592748 (CHEMBL5193123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592743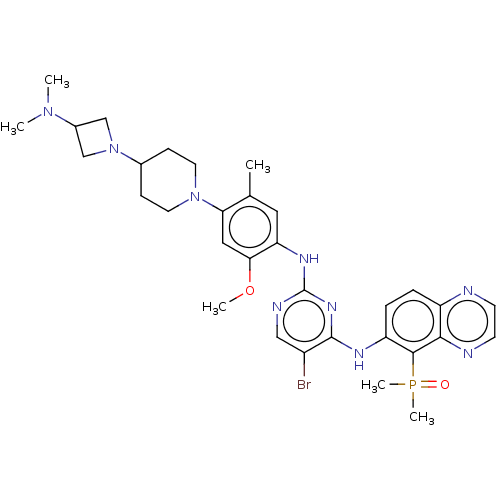 (CHEMBL5186680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592747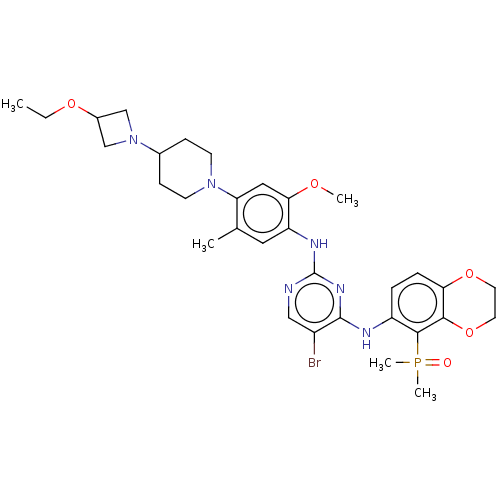 (CHEMBL5195904) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592741 (CHEMBL5180388) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592752 (CHEMBL5188940) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592752 (CHEMBL5188940) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592745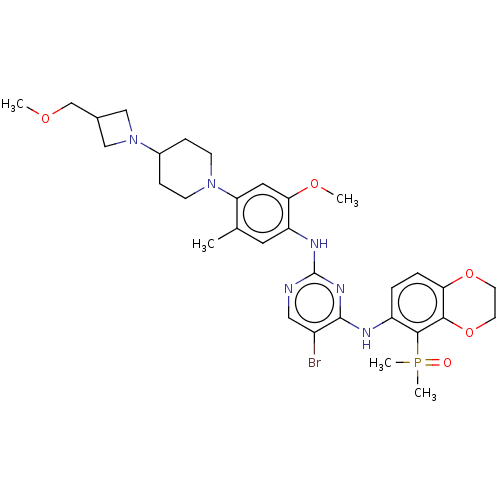 (CHEMBL5188373) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592743 (CHEMBL5186680) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592748 (CHEMBL5193123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Epidermal growth factor receptor (Homo sapiens (Human)) | BDBM50592738 (CHEMBL5199678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1016/j.bmc.2022.116907 BindingDB Entry DOI: 10.7270/Q2J96BC1 | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1042 total ) | Next | Last >> |