

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053967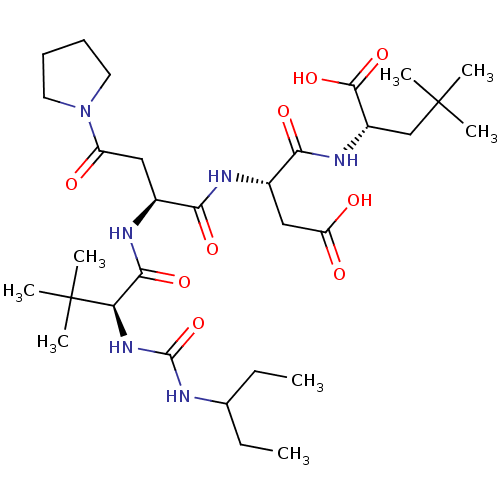 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369164 (CHEMBL1169533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50369164 (CHEMBL1169533) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476896 (CHEMBL232576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492156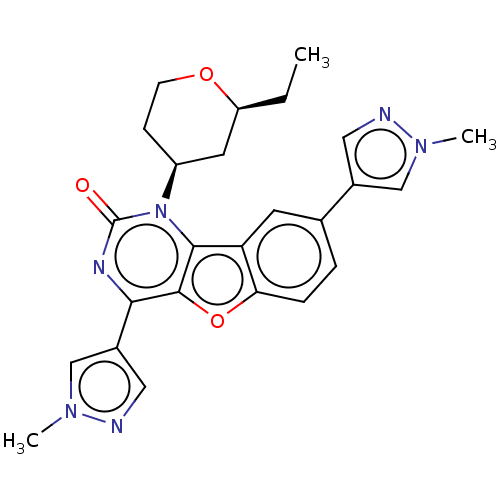 (CHEMBL2397560) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492164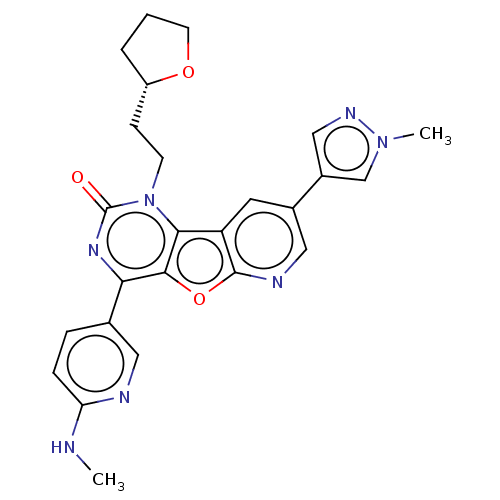 (CHEMBL2381561) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476895 (CHEMBL232379) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492155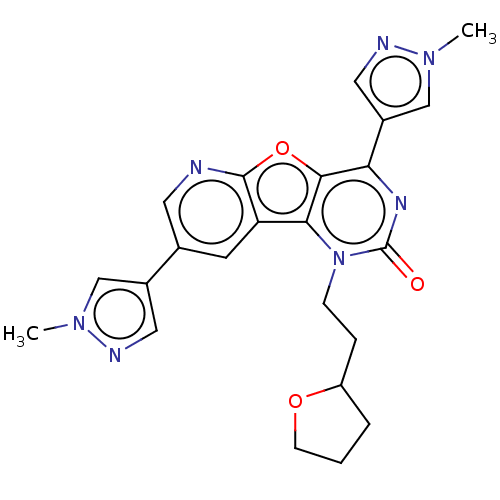 (CHEMBL2397554) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050831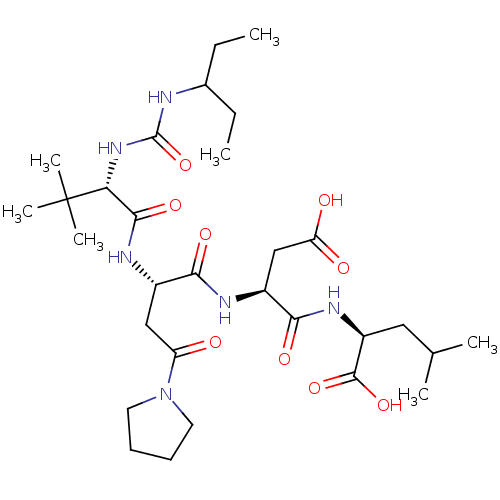 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053968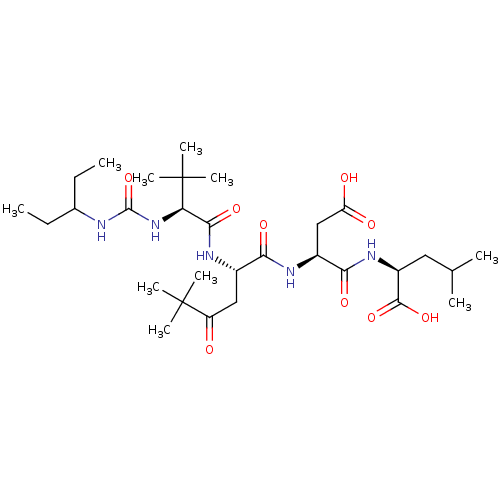 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-2-[3-(1-ethyl-p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492176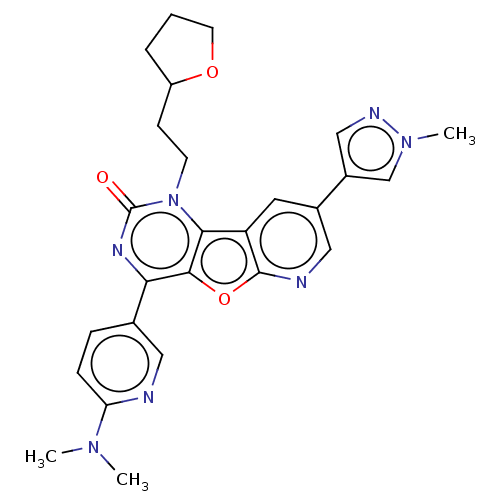 (CHEMBL2397567) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492175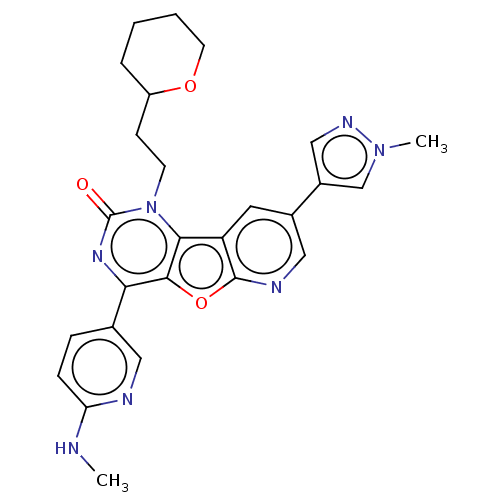 (CHEMBL2397583) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476903 (CHEMBL230188) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492163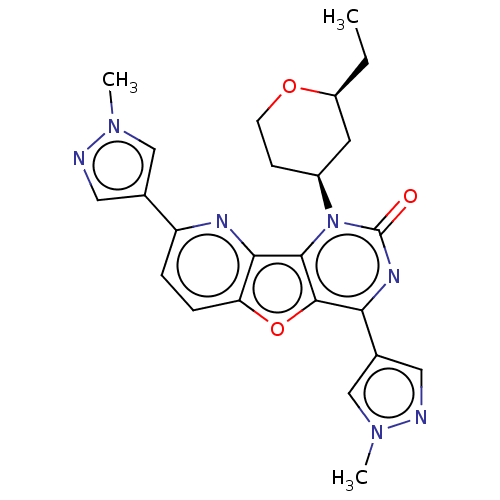 (CHEMBL2397557) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492154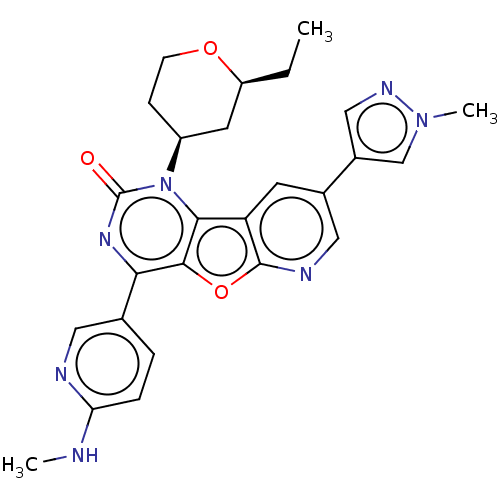 (CHEMBL2397572) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492168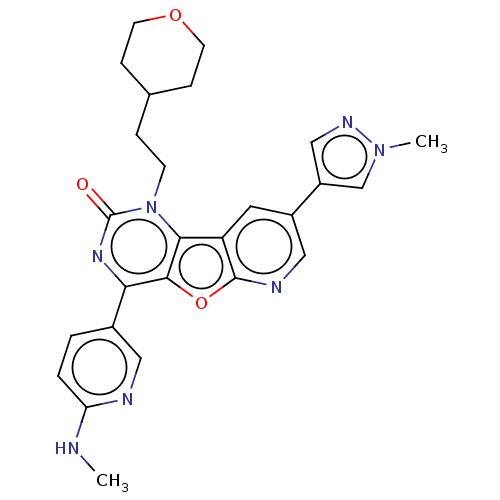 (CHEMBL2397584) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492153 (CHEMBL2397563) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492174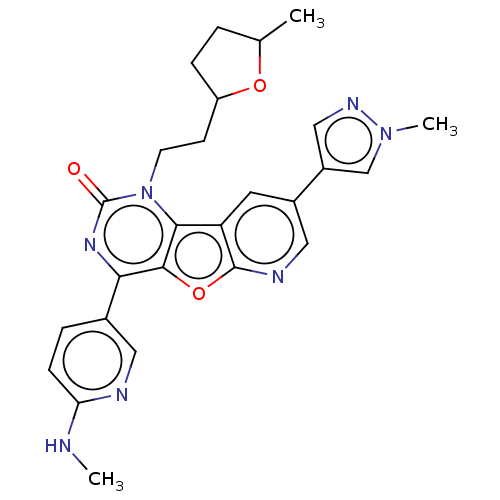 (CHEMBL2397586) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492179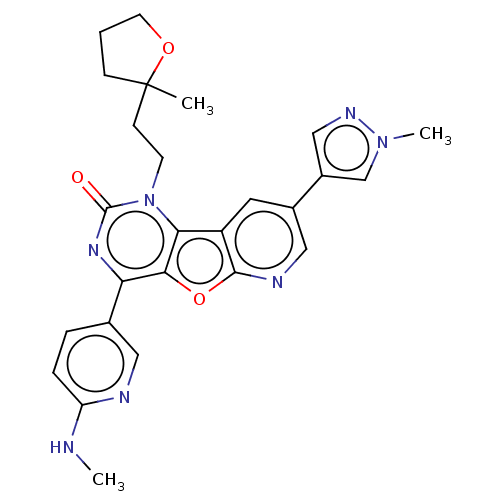 (CHEMBL2397587) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476897 (CHEMBL232178) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053981 ((S)-2-((S)-3-Carboxy-2-{(2R,5S)-2-(3,3-dimethyl-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492169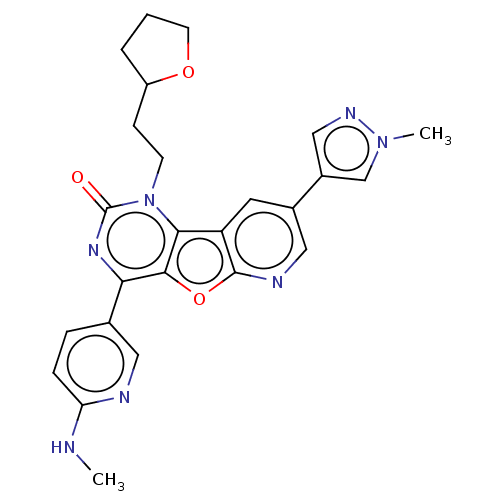 (CHEMBL2397582) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476898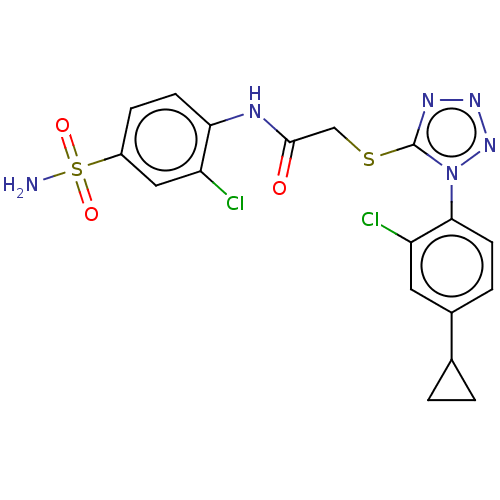 (CHEMBL232377) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492152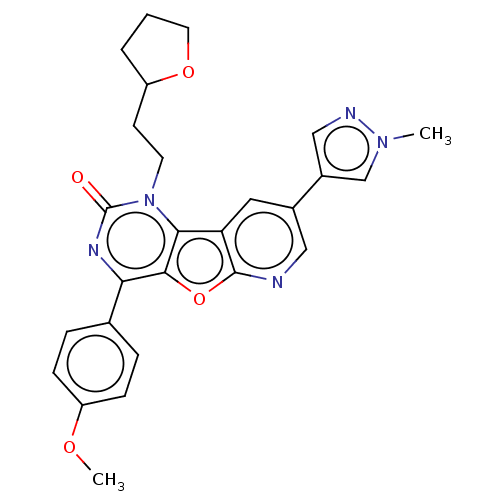 (CHEMBL2397565) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476904 (CHEMBL232575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492151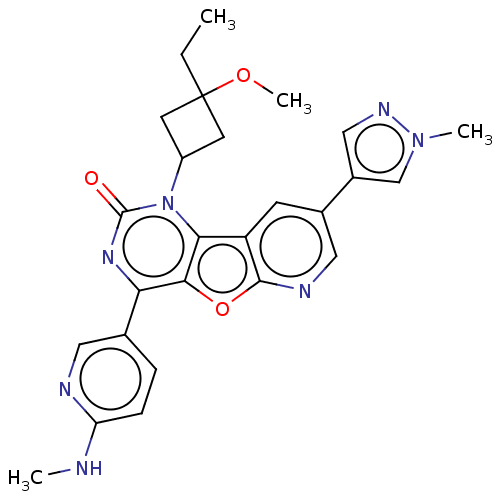 (CHEMBL2397588) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476885 (CHEMBL231030) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492162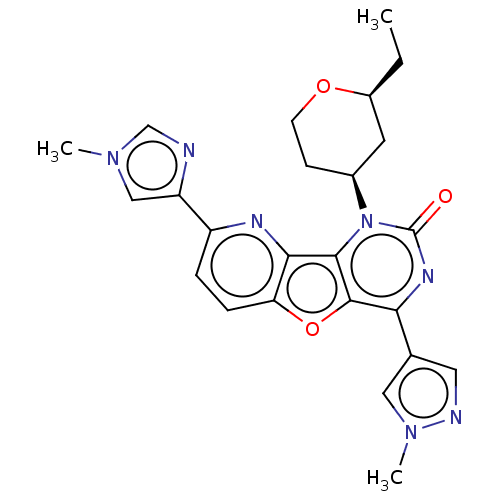 (CHEMBL2397549) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492173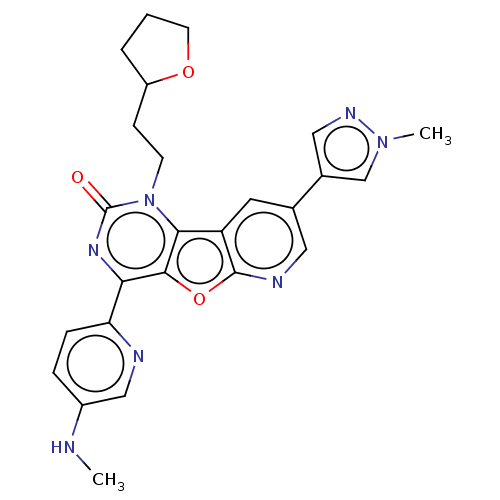 (CHEMBL2397573) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492158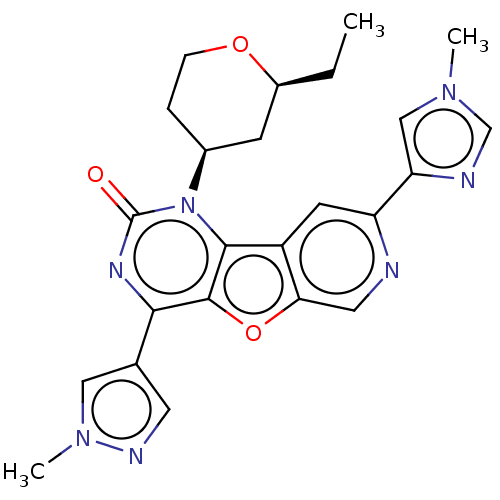 (CHEMBL2397548) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053973 ((S)-3-((S)-2-{(S)-2-[3-(1-Ethyl-propyl)-ureido]-3,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492150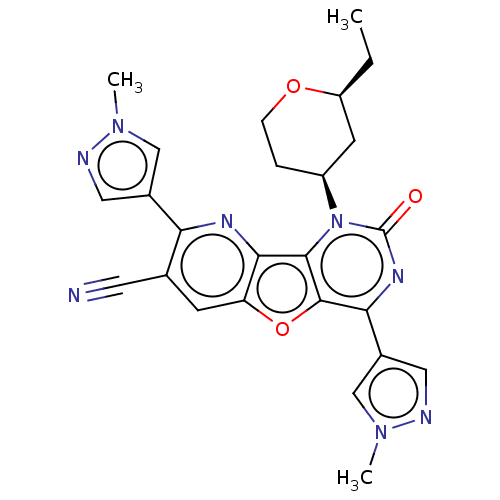 (CHEMBL2397559) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492149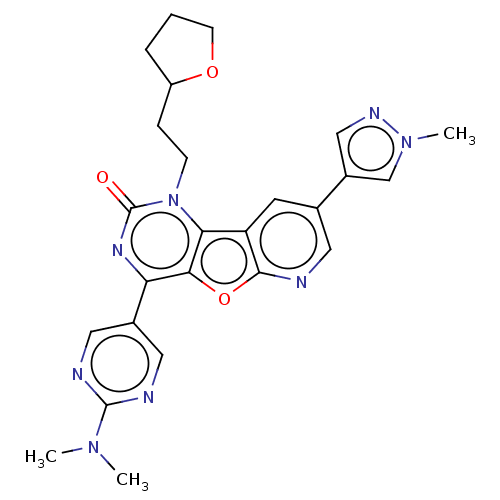 (CHEMBL2397569) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492172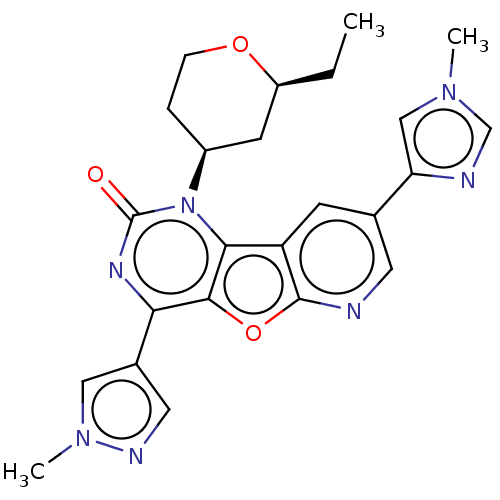 (CHEMBL2397547) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50050828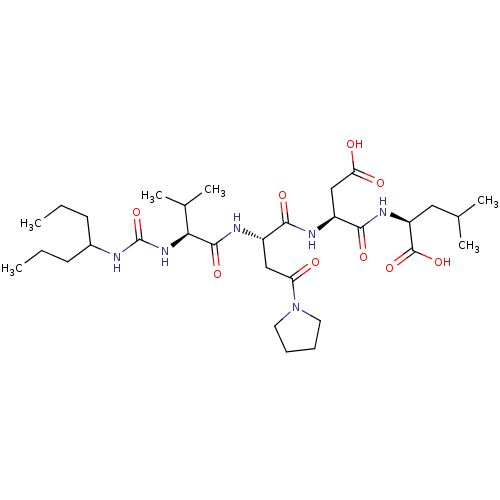 ((S)-2-[(S)-3-Carboxy-2-((S)-2-{(S)-3-methyl-2-[3-(...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ribonucleoside-diphosphate reductase large subunit/subunit M2 (Homo sapiens (Human)) | BDBM50053984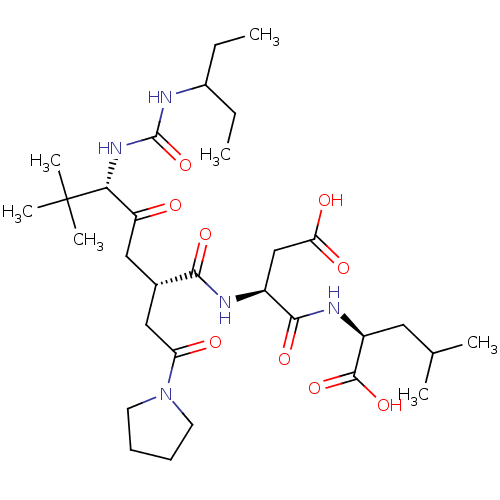 ((S)-2-{(S)-3-Carboxy-2-[(2S,5S)-5-[3-(1-ethyl-prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Bio-M£ga/Boehringer Ingelheim Research Inc. Curated by ChEMBL | Assay Description Inhibitory effect was evaluated on Herpes simplex virus (HSV) ribonucleotide reductase (RR) enzyme. | J Med Chem 39: 4173-80 (1996) Article DOI: 10.1021/jm960324r BindingDB Entry DOI: 10.7270/Q28W3DZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492167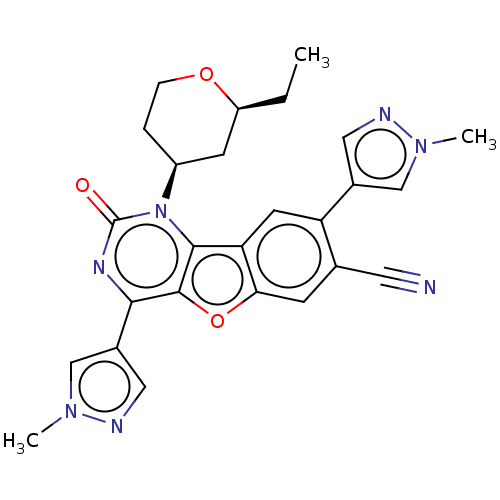 (CHEMBL2397558) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492148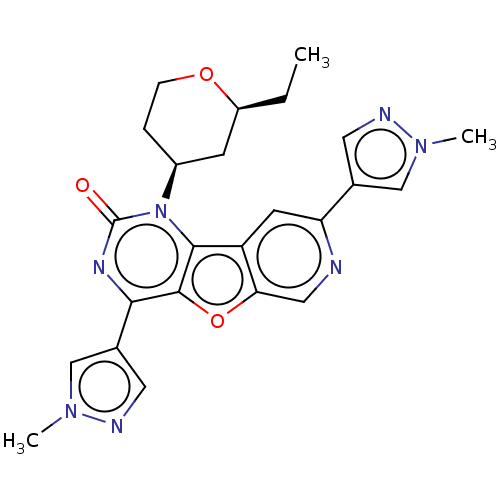 (CHEMBL2397556) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476894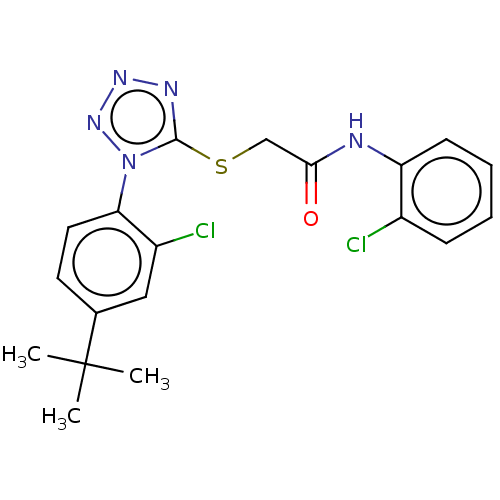 (CHEMBL230187) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476899 (CHEMBL232963) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492161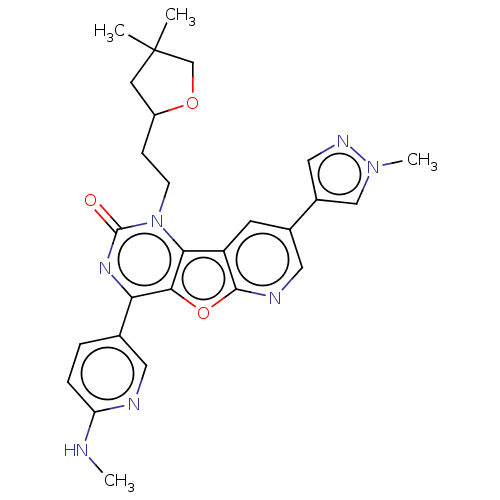 (CHEMBL2396665) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492147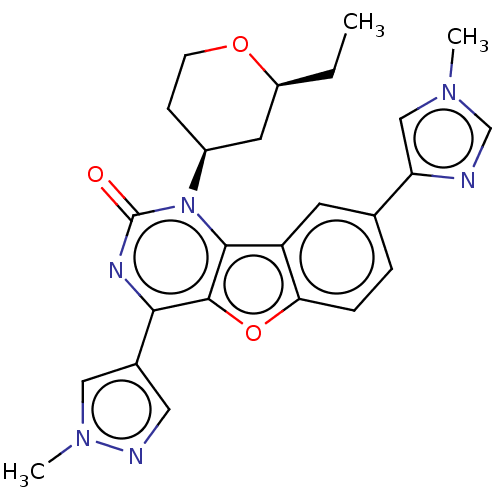 (CHEMBL2397562) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492146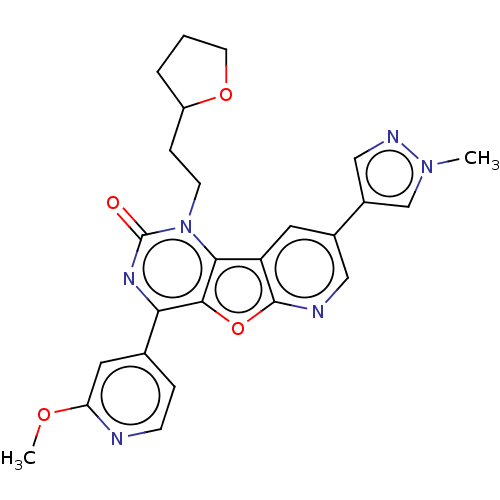 (CHEMBL2397576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50476896 (CHEMBL232576) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase K103N/Y181C mutant by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Presenilin-1 (Homo sapiens (Human)) | BDBM50272797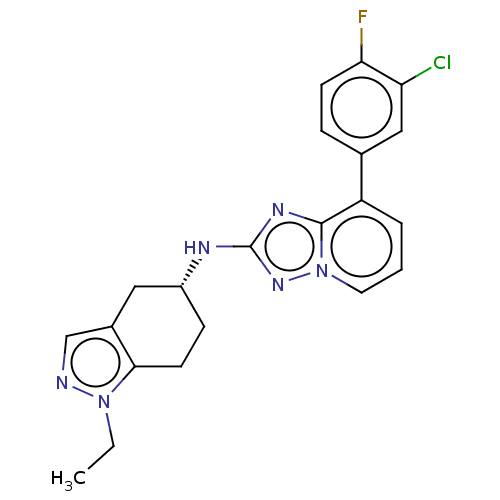 (CHEMBL4126886) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharma GmbH & Co. KG Curated by ChEMBL | Assay Description Modulation of gamma secretase in human H4 cells expressing human wild type APP assessed as inhibition of amyloid beta 42 production after 22 hrs by e... | Bioorg Med Chem 26: 3227-3241 (2018) Article DOI: 10.1016/j.bmc.2018.04.053 BindingDB Entry DOI: 10.7270/Q24X5B97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492180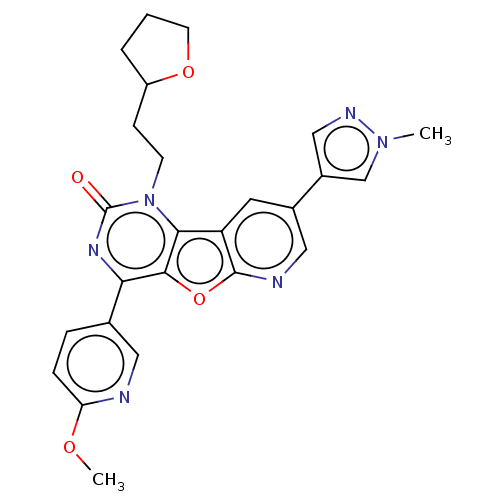 (CHEMBL2397566) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492145 (CHEMBL2397570) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM27606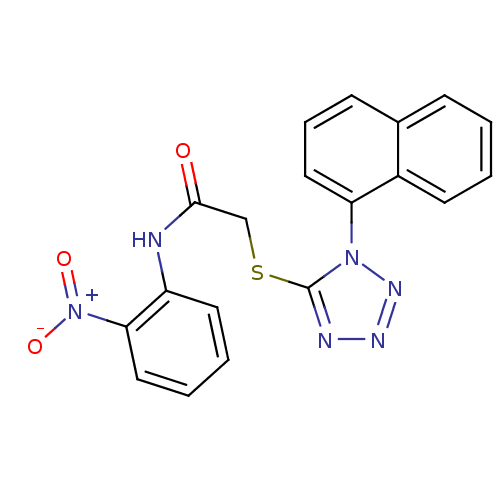 (2-{[1-(naphthalen-1-yl)-1H-1,2,3,4-tetrazol-5-yl]s...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase by scintillation proximity assay | Bioorg Med Chem Lett 17: 4437-41 (2007) Article DOI: 10.1016/j.bmcl.2007.06.012 BindingDB Entry DOI: 10.7270/Q2BR8VZ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492178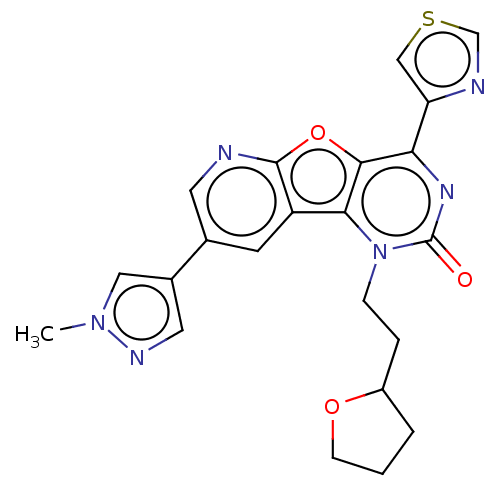 (CHEMBL2397551) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50492171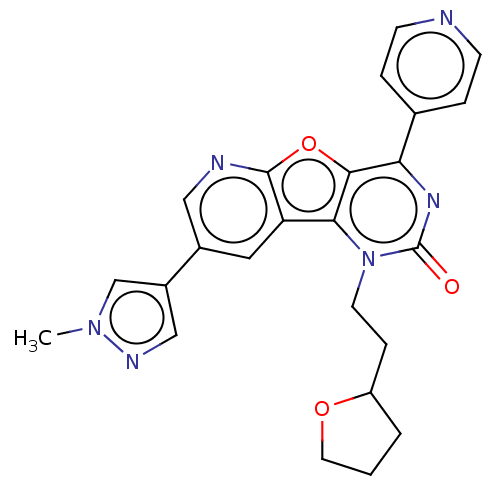 (CHEMBL2397575) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase using poly(rA)/oligo(dT)15 as substrate after 60 mins by fluorescence assay | Bioorg Med Chem Lett 23: 3967-75 (2013) Article DOI: 10.1016/j.bmcl.2013.04.043 BindingDB Entry DOI: 10.7270/Q26976HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 409 total ) | Next | Last >> |