

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cathepsin E (Homo sapiens (Human)) | BDBM50271627 (CHEMBL507651 | N'-[(1S,2S)-2-[(4S)-1-benzyl-5-oxoi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of cathepsin E | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin E (Homo sapiens (Human)) | BDBM50271626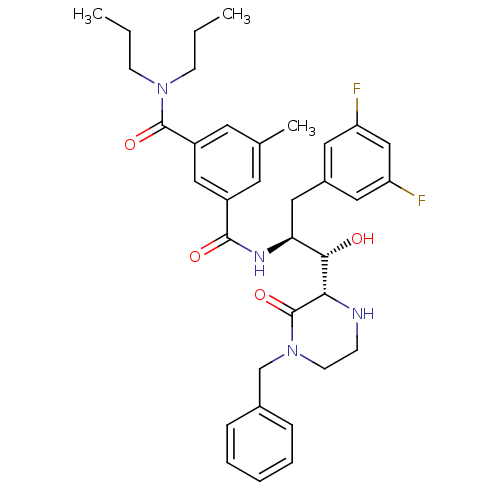 (CHEMBL509210 | N'-[(1S,2S)-2-[(2S)-4-benzyl-3-oxop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of cathepsin E | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50271627 (CHEMBL507651 | N'-[(1S,2S)-2-[(4S)-1-benzyl-5-oxoi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin D (Homo sapiens (Human)) | BDBM50271626 (CHEMBL509210 | N'-[(1S,2S)-2-[(2S)-4-benzyl-3-oxop...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of cathepsin D | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24828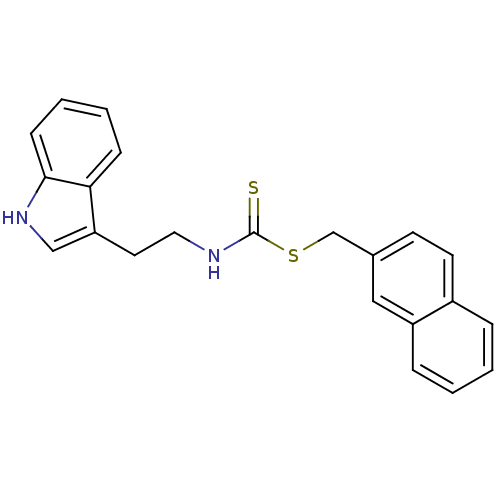 (Brassinin derivative, 16 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.16E+4 | -29.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24825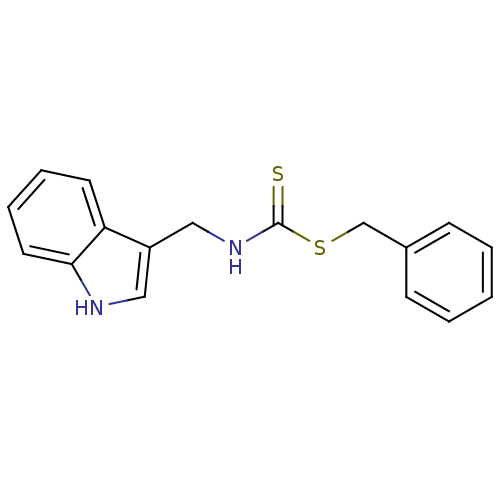 ((benzylsulfanyl)-N-(1H-indol-3-ylmethyl)carbothioa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.32E+4 | -29.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24827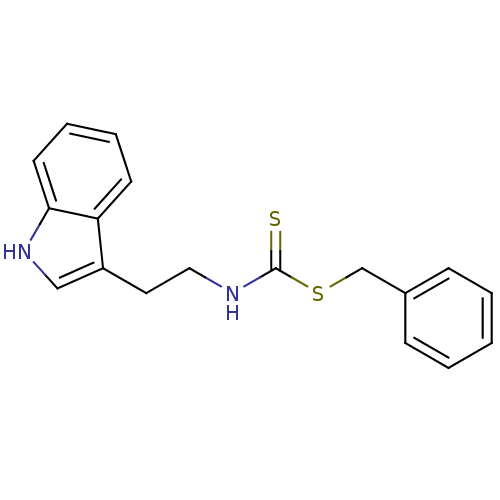 ((benzylsulfanyl)-N-[2-(1H-indol-3-yl)ethyl]carboth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 1.72E+4 | -28.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24830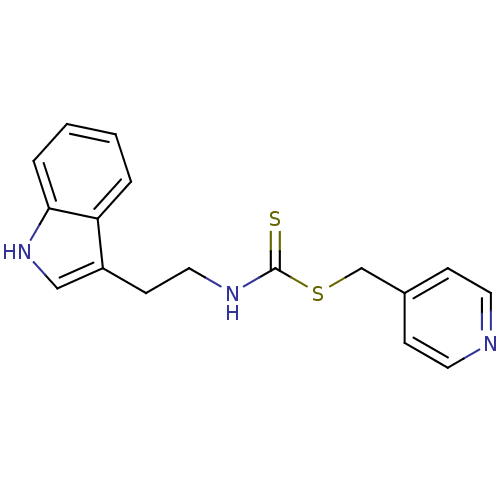 (Brassinin derivative, 18 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.05E+4 | -27.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24829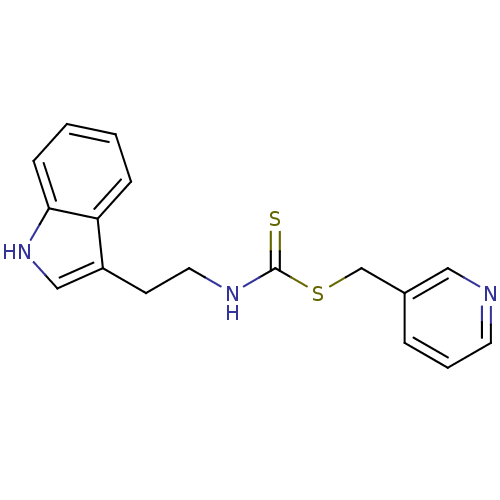 (Brassinin derivative, 17 | N-[2-(1H-indol-3-yl)eth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.84E+4 | -27.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24816 (Brassinin derivative, 4 | N-[3-(1H-indol-3-yl)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40E+4 | -26.5 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24824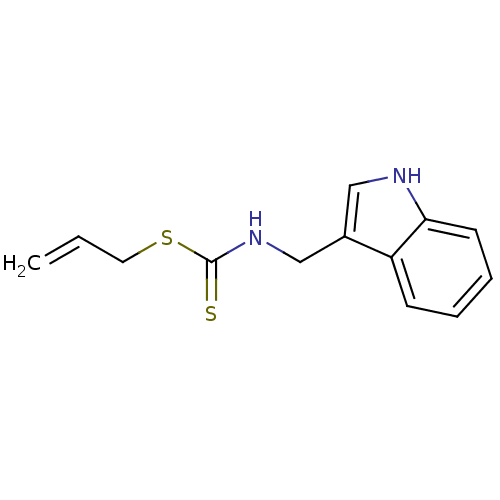 (Brassinin derivative, 12 | N-(1H-indol-3-ylmethyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70E+4 | -26.3 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24815 (Brassinin derivative, 3 | N-[2-(1-benzothiophen-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.10E+4 | -26.1 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24817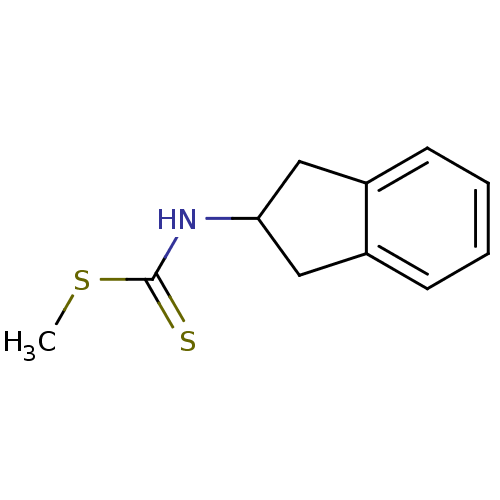 (Brassinin derivative, 5 | N-(2,3-dihydro-1H-inden-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | 4.21E+4 | -26.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24819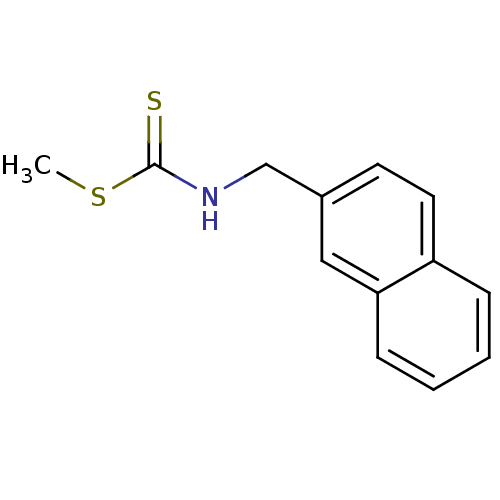 ((methylsulfanyl)-N-(naphthalen-2-ylmethyl)carbothi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.76E+4 | -25.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24821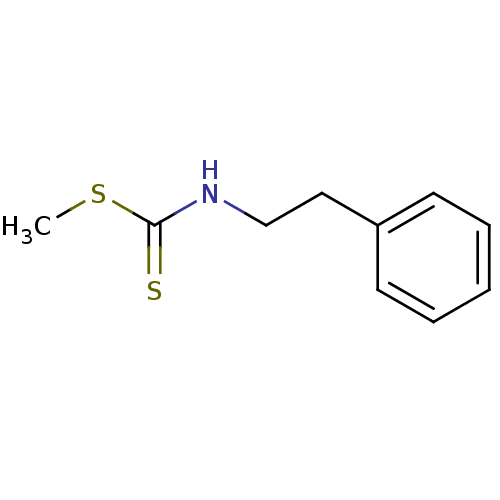 ((methylsulfanyl)-N-(2-phenylethyl)carbothioamide |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 6.24E+4 | -25.0 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24820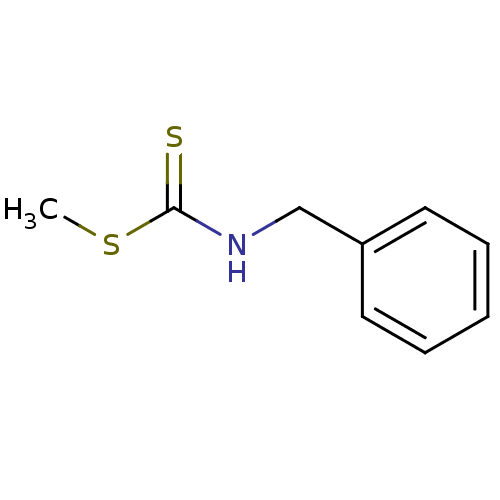 (Brassinin derivative, 8 | N-benzyl(methylsulfanyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 7.24E+4 | -24.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24814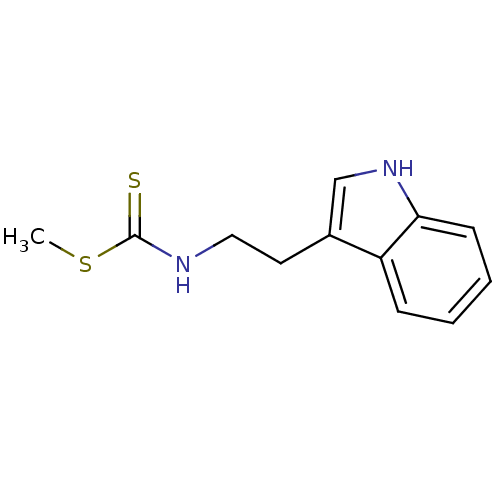 (Brassinin derivative, 2 | N-[2-(1H-indol-3-yl)ethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.25E+4 | -24.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24813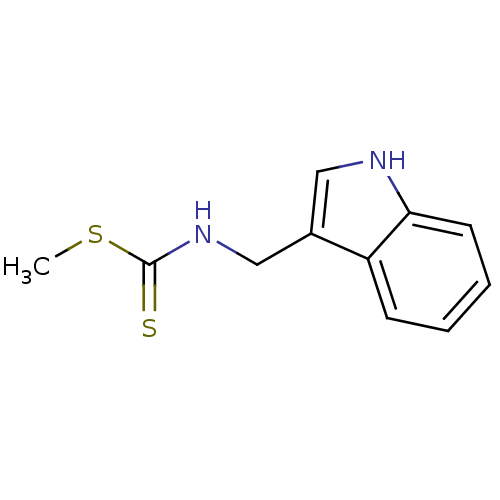 (Brassinin, 1 | N-(1H-indol-3-ylmethyl)(methylsulfa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 9.77E+4 | -23.8 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24822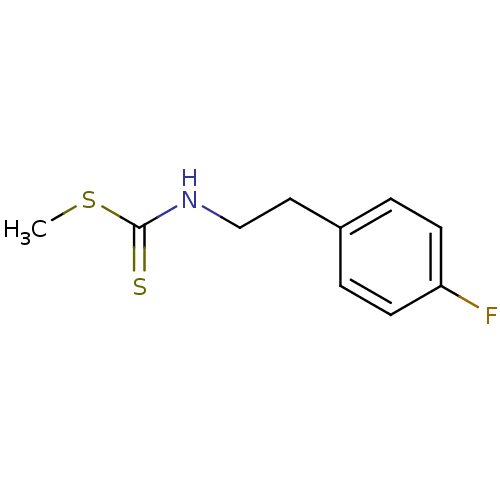 (Brassinin derivative, 10 | N-[2-(4-fluorophenyl)et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.49E+5 | -22.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24818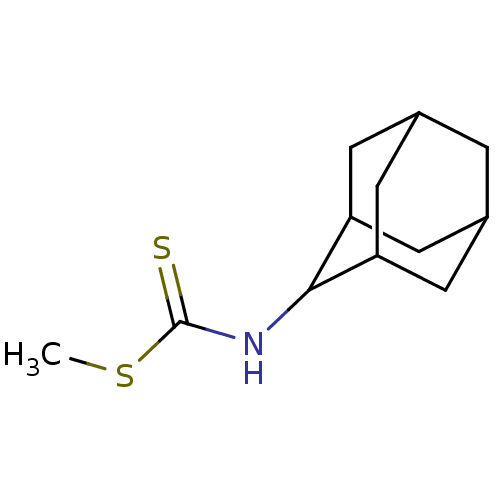 (Brassinin derivative, 6 | N-(adamantan-2-yl)(methy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+5 | -22.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24834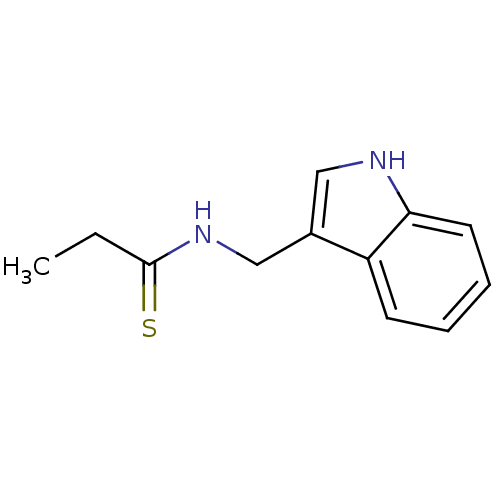 (N-(1H-indol-3-ylmethyl)propanethioamide | thioamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.02E+5 | -21.9 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24836 (4-(1H-indol-3-ylmethyl)-2-methyl-1,3-thiazole | th...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.29E+5 | -20.7 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24832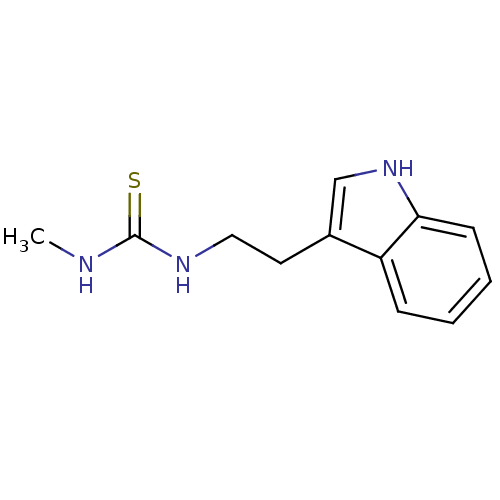 (1-[2-(1H-indol-3-yl)ethyl]-3-methylthiourea | thio...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 3.42E+5 | -20.6 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24826 ((hexylsulfanyl)-N-(1H-indol-3-ylmethyl)carbothioam...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.64E+5 | -20.4 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24823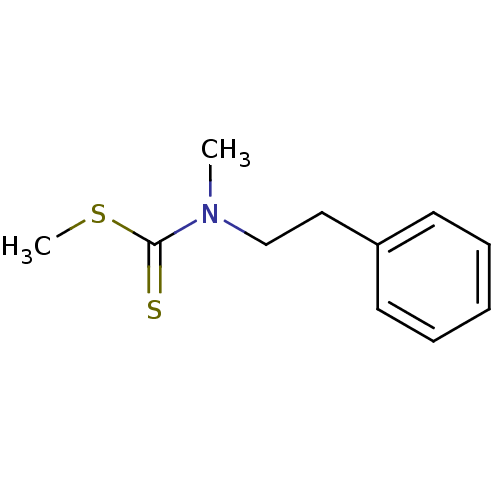 (Brassinin derivative, 11 | N-methyl(methylsulfanyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.27E+6 | -17.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Indoleamine 2,3-dioxygenase 1 (Homo sapiens (Human)) | BDBM24835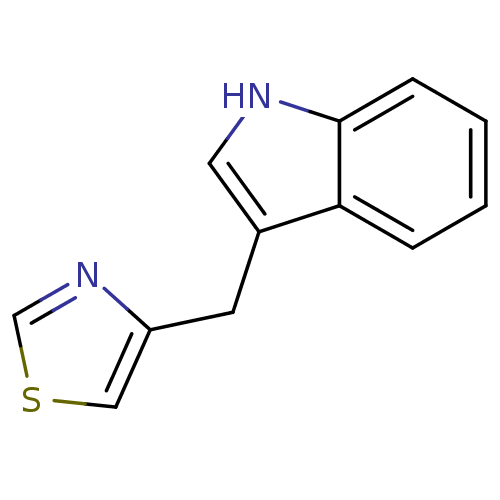 (4-(1H-indol-3-ylmethyl)-1,3-thiazole | thiazole, 2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.29E+6 | -17.2 | n/a | n/a | n/a | n/a | n/a | 6.5 | 37 |
Bryn Mawr College | Assay Description The IC50 inhibition assays were performed in a 96-well microtiter plate format using purified recombinant IDO, which was added to the substrate, L-tr... | J Med Chem 49: 684-92 (2006) Article DOI: 10.1021/jm0508888 BindingDB Entry DOI: 10.7270/Q2QR4VDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271628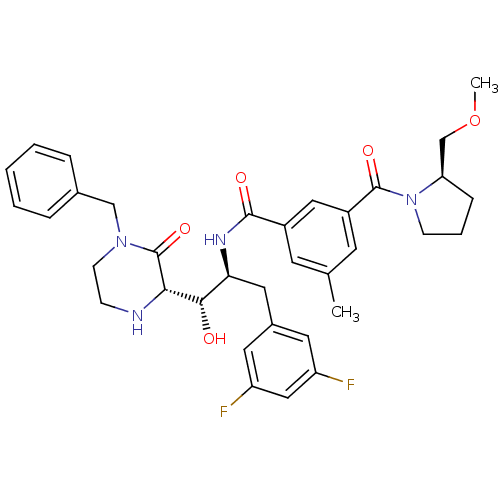 (CHEMBL510659 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271629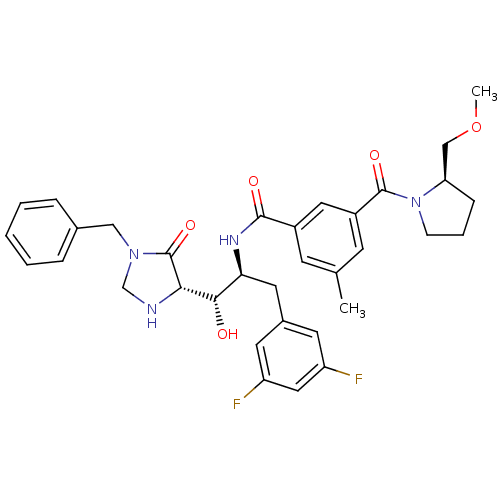 (CHEMBL451950 | N-((1S,2S)-1-((S)-1-benzyl-5-oxoimi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271631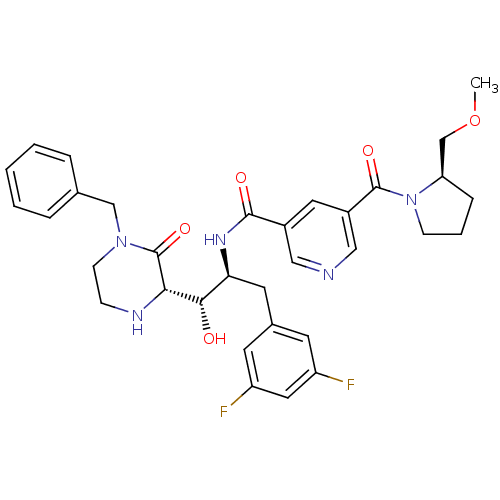 (CHEMBL511110 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271626 (CHEMBL509210 | N'-[(1S,2S)-2-[(2S)-4-benzyl-3-oxop...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271705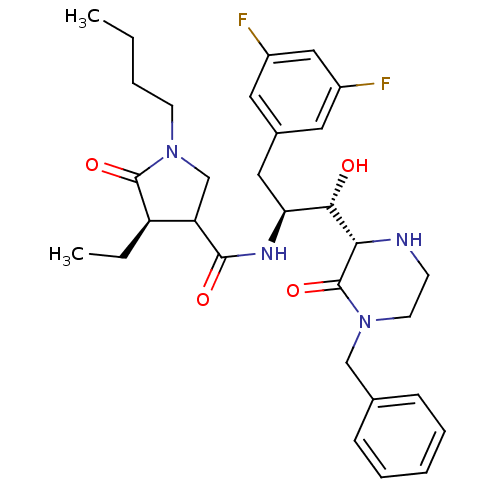 ((4R)-N-((1S,2S)-1-((S)-4-benzyl-3-oxopiperazin-2-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271630 (CHEMBL453413 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271703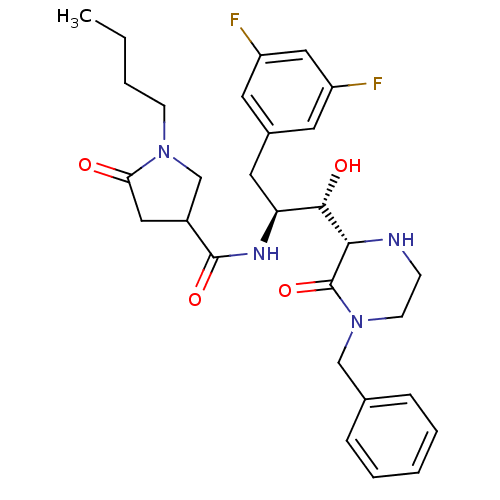 (CHEMBL491071 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271862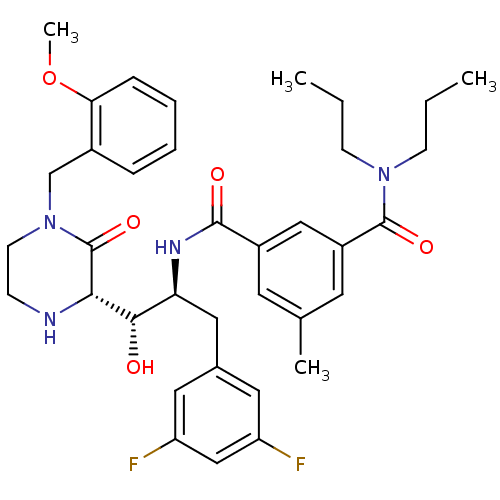 (CHEMBL445721 | N1-((1S,2S)-1-((S)-4-(2-methoxybenz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271627 (CHEMBL507651 | N'-[(1S,2S)-2-[(4S)-1-benzyl-5-oxoi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50227337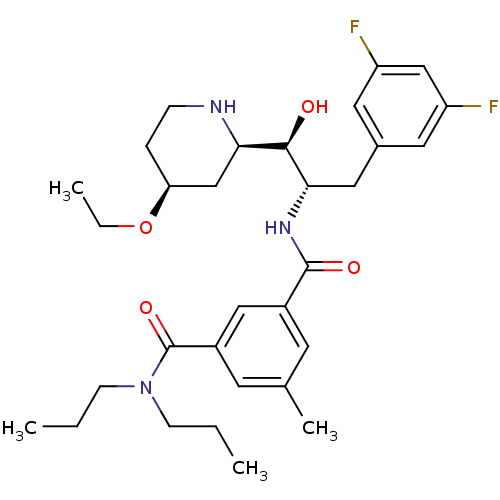 (CHEMBL257278 | N'-{(1S,2R)-1-(3,5-DIFLUOROBENZYL)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271632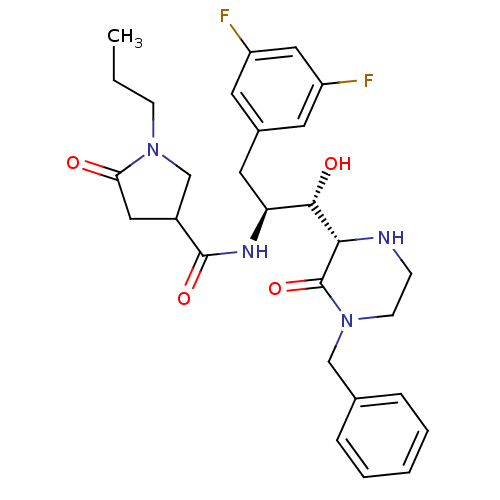 (CHEMBL490463 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271810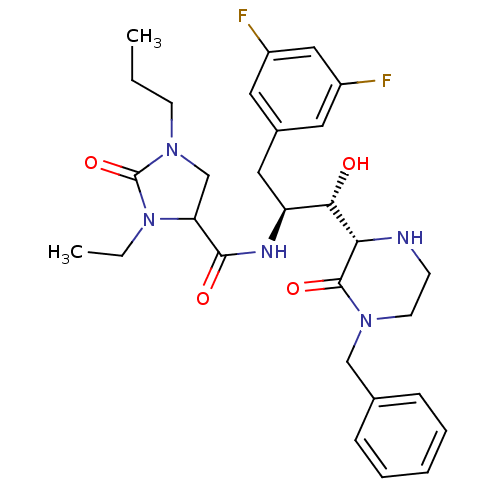 (CHEMBL491660 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271861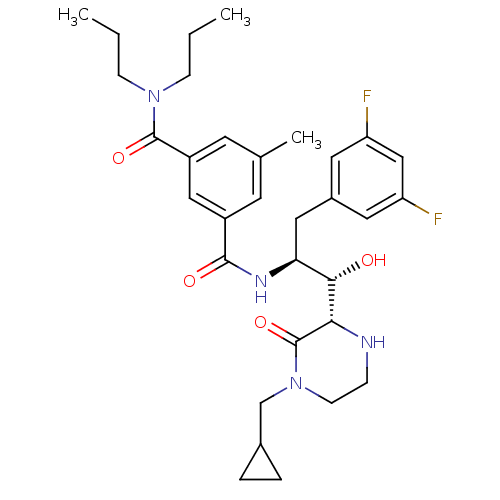 (CHEMBL507571 | N1-((1S,2S)-1-((S)-4-(cyclopropylme...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271768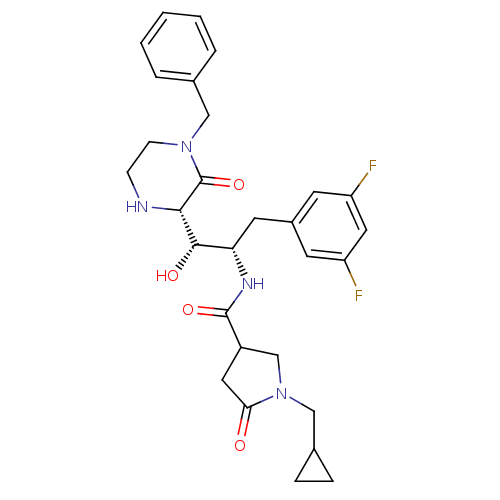 (CHEMBL491659 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271811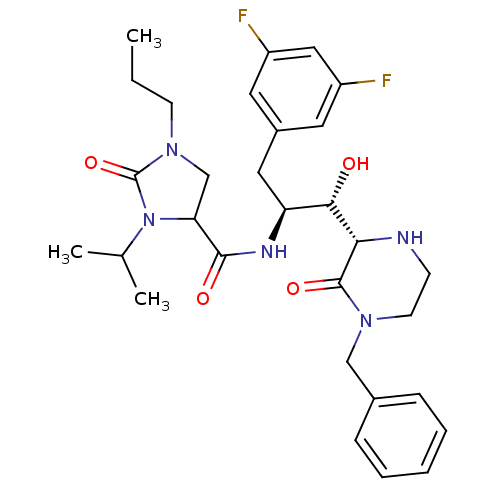 (CHEMBL508095 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271770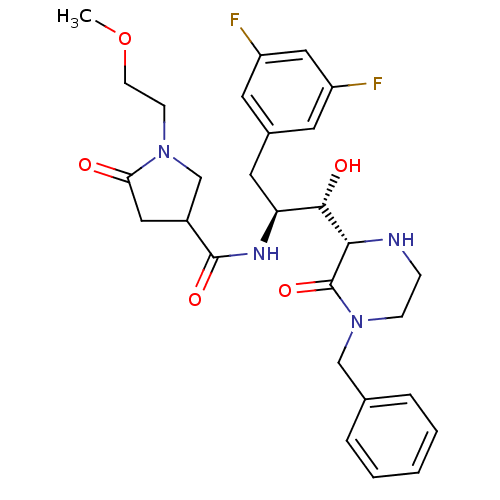 (CHEMBL521794 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271860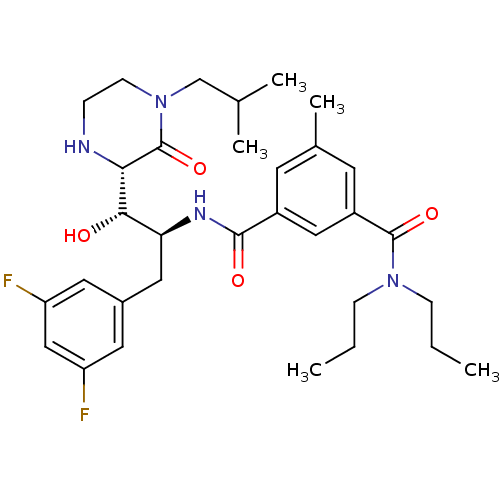 (CHEMBL507330 | N1-((1S,2S)-3-(3,5-difluorophenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50227345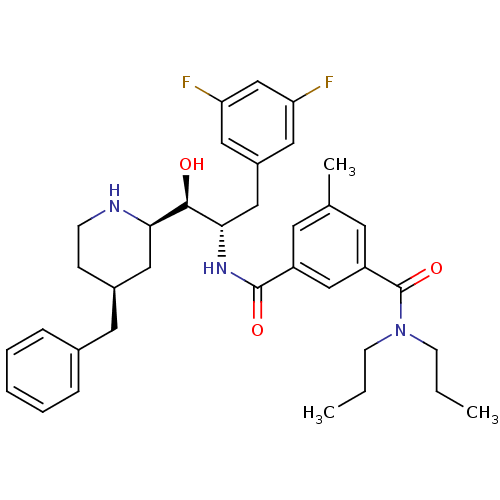 (CHEMBL255194 | N'-[(1S,2R)-2-[(2R,4S)-4-benzylpipe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271767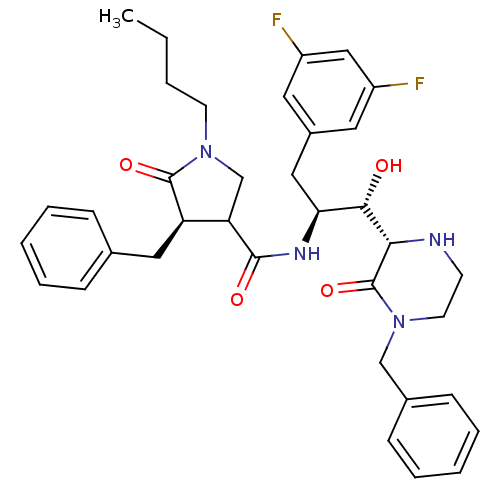 ((4R)-4-benzyl-N-((1S,2S)-1-((S)-4-benzyl-3-oxopipe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271863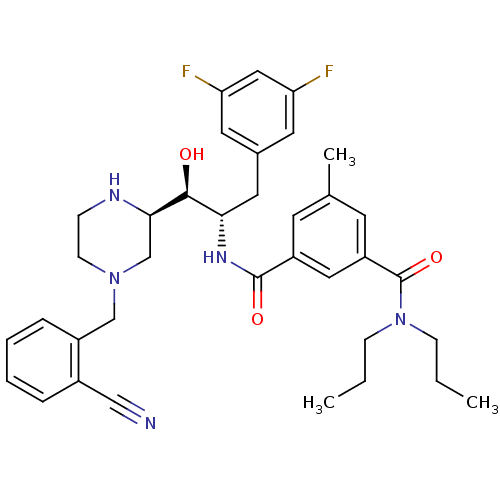 (CHEMBL455237 | N1-((1S,2S)-1-((R)-4-(2-cyanobenzyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271812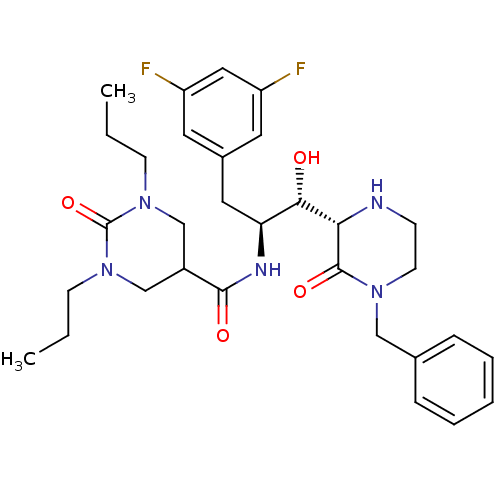 (CHEMBL502121 | N-((1S,2S)-1-((S)-4-benzyl-3-oxopip...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271769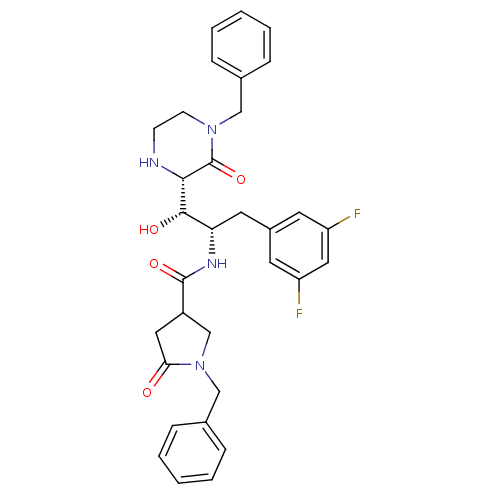 (1-benzyl-N-((1S,2S)-1-((S)-4-benzyl-3-oxopiperazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 2 (Homo sapiens (Human)) | BDBM50271626 (CHEMBL509210 | N'-[(1S,2S)-2-[(2S)-4-benzyl-3-oxop...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE2 | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50271864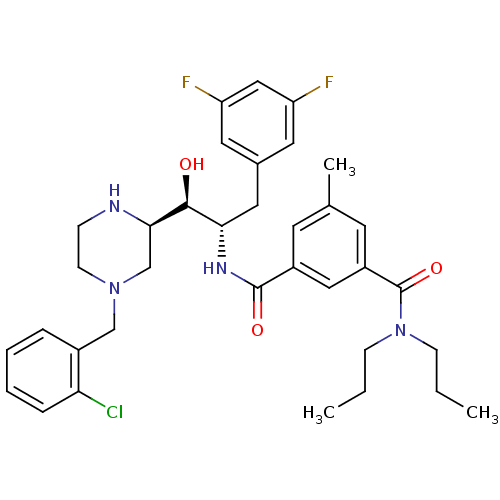 (CHEMBL476216 | N1-((1S,2S)-1-((R)-4-(2-chlorobenzy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Inhibition of BACE1 (unknown origin) | Bioorg Med Chem Lett 18: 3236-41 (2008) Article DOI: 10.1016/j.bmcl.2008.04.050 BindingDB Entry DOI: 10.7270/Q2PZ58MR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 93 total ) | Next | Last >> |