

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Competitive inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50536826 (CHEMBL4590355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 7: 735-40 (2016) Article DOI: 10.1021/acsmedchemlett.6b00167 BindingDB Entry DOI: 10.7270/Q2V69P3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50536819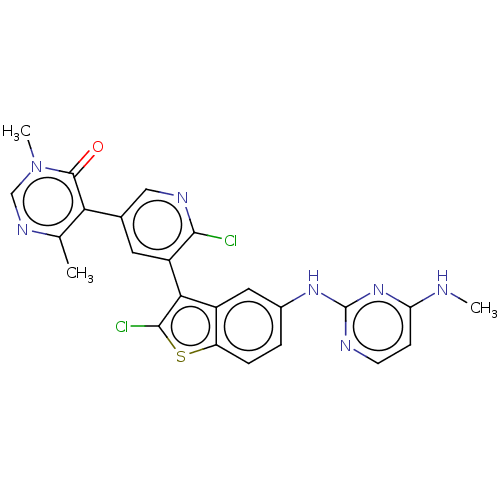 (CHEMBL4534250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 7: 735-40 (2016) Article DOI: 10.1021/acsmedchemlett.6b00167 BindingDB Entry DOI: 10.7270/Q2V69P3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin L | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 239 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin S expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Procathepsin L (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 406 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin L | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin S (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 735 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant human cathepsin S expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 909 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50517805 (CHEMBL4454648) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of human liver cathepsin B | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235302 (CHEMBL4099771) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50075098 (CHEMBL3414626 | US10143704, Compound A2 | US944606...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50529550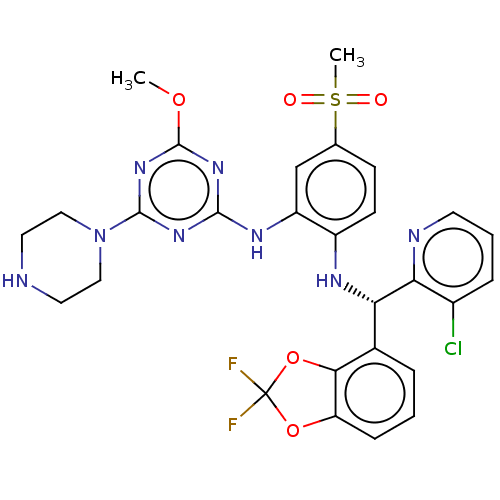 (CHEMBL4446126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... | ACS Med Chem Lett 10: 1655-1660 (2019) Article DOI: 10.1021/acsmedchemlett.9b00452 BindingDB Entry DOI: 10.7270/Q2RV0S5T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50235301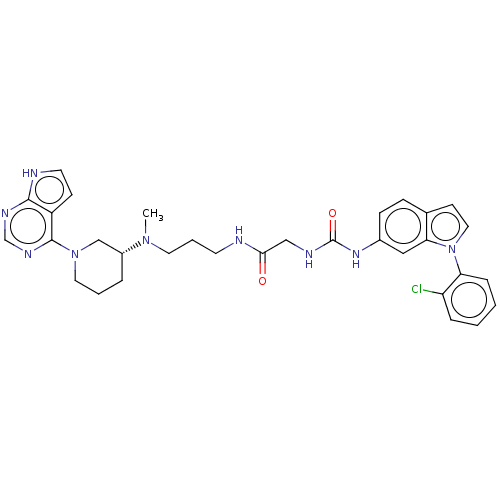 (CHEMBL4081752) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 8: 338-343 (2017) Article DOI: 10.1021/acsmedchemlett.6b00519 BindingDB Entry DOI: 10.7270/Q2WW7KZW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50529551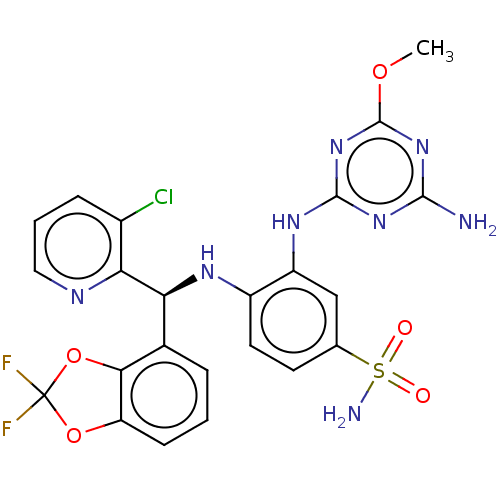 (CHEMBL4435508) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... | ACS Med Chem Lett 10: 1655-1660 (2019) Article DOI: 10.1021/acsmedchemlett.9b00452 BindingDB Entry DOI: 10.7270/Q2RV0S5T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50529554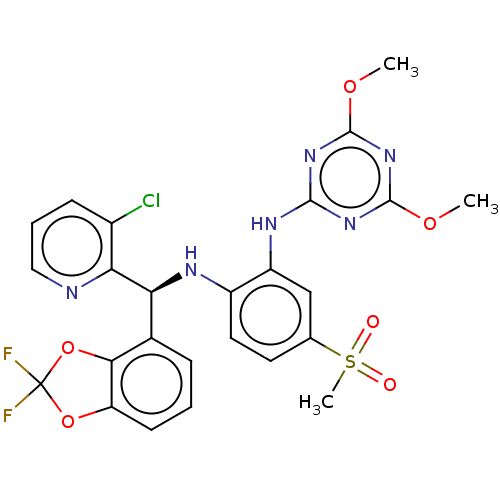 (CHEMBL4567485) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... | ACS Med Chem Lett 10: 1655-1660 (2019) Article DOI: 10.1021/acsmedchemlett.9b00452 BindingDB Entry DOI: 10.7270/Q2RV0S5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50536826 (CHEMBL4590355) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 7: 735-40 (2016) Article DOI: 10.1021/acsmedchemlett.6b00167 BindingDB Entry DOI: 10.7270/Q2V69P3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324297 (4-(1-Piperazin-1-yl[2,6]naphthyridin-3-yl)pyridin-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50529544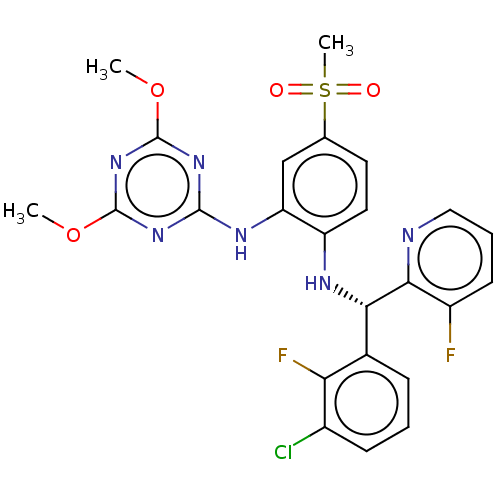 (CHEMBL4557484) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... | ACS Med Chem Lett 10: 1655-1660 (2019) Article DOI: 10.1021/acsmedchemlett.9b00452 BindingDB Entry DOI: 10.7270/Q2RV0S5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324315 (CHEMBL1214998 | Cyclohexyl-[4-(1-piperazin-1-yl[2,...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324306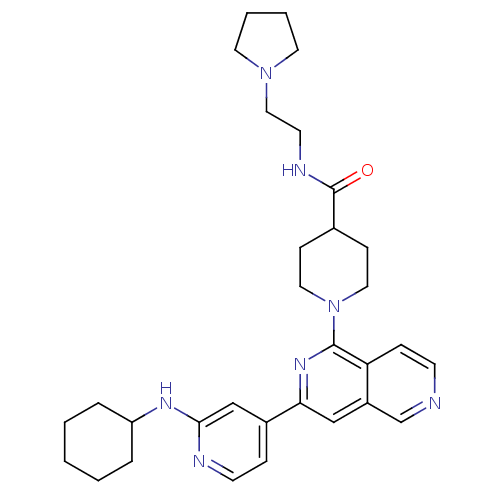 (1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324298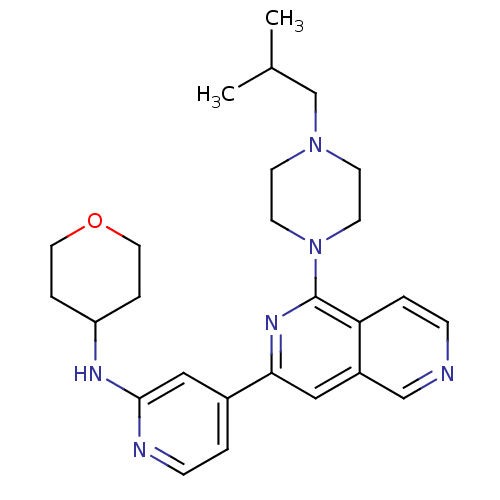 (CHEMBL1215712 | {4-[1-(4-Isobutylpiperazin-1-yl)[2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324296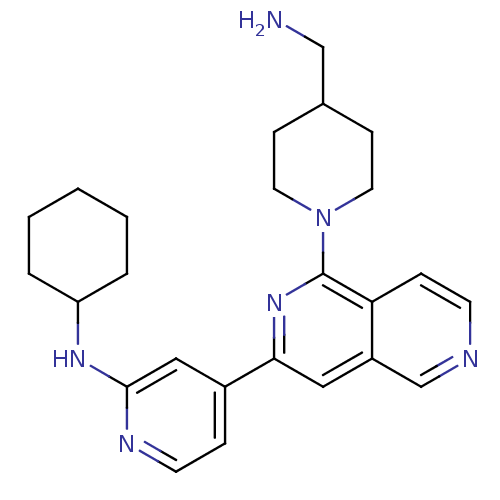 (1-[3-(2-Cyclohexylaminopyridin-4-yl)[2,6]naphthyri...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324305 (CHEMBL1214711 | N-(2-(pyrrolidin-1-yl)ethyl)-1-(3-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324314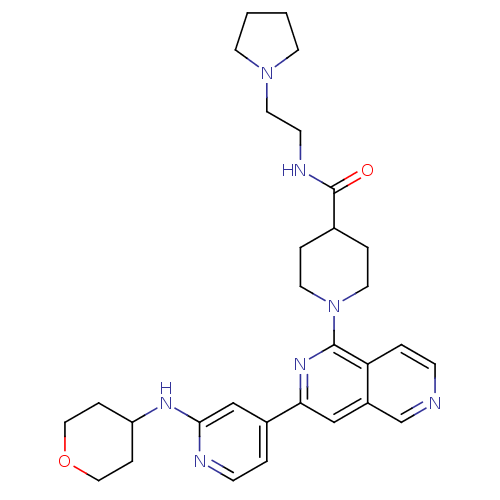 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158429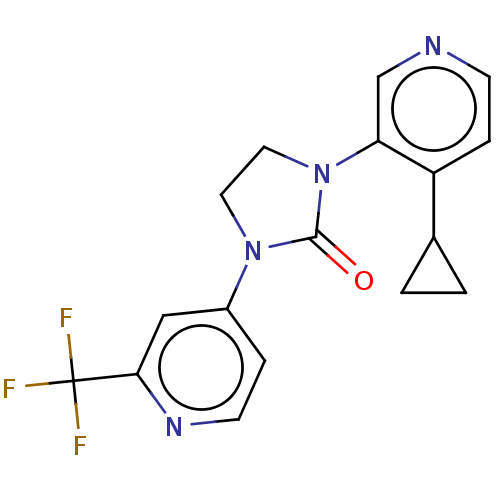 (US9029399, 2A | US9339501, 2A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158429 (US9029399, 2A | US9339501, 2A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50536819 (CHEMBL4534250) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosomes as substrate preincubated for 30 mins followed by substrate a... | ACS Med Chem Lett 7: 735-40 (2016) Article DOI: 10.1021/acsmedchemlett.6b00167 BindingDB Entry DOI: 10.7270/Q2V69P3G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158474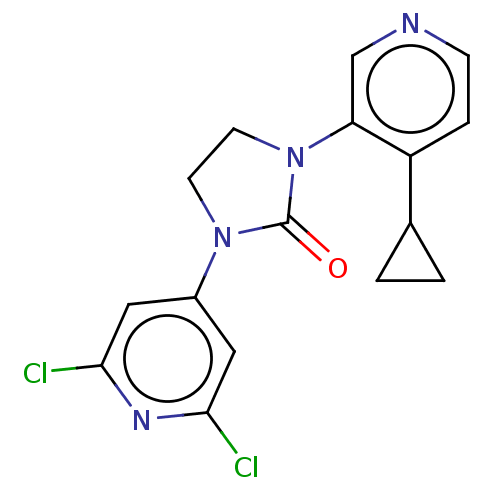 (US9029399, 46A | US9339501, 46A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158474 (US9029399, 46A | US9339501, 46A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM132104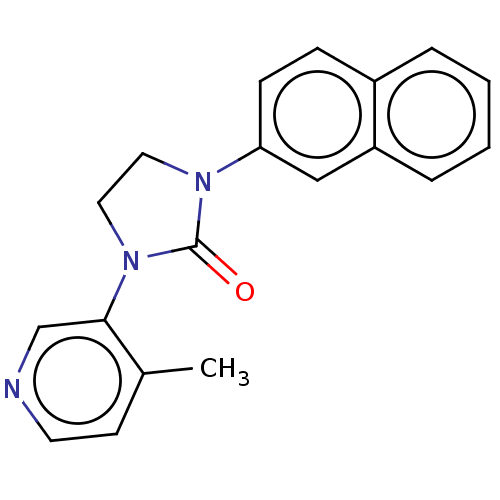 (USRE45173, 14A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324312 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324313 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324294 (CHEMBL1215643 | Cyclohexyl-{4-[1-(4-cyclopropylmet...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324292 (CHEMBL1215641 | Cyclohexyl-{4-[1-(4-methylpiperazi...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase D1 (Homo sapiens (Human)) | BDBM50324309 (1-{3-[2-(Tetrahydropyran-4-ylamino)pyridin-4-yl][2...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of PKD1 by TR-FRET assay | J Med Chem 53: 5400-21 (2010) Article DOI: 10.1021/jm100075z BindingDB Entry DOI: 10.7270/Q2T153T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM132137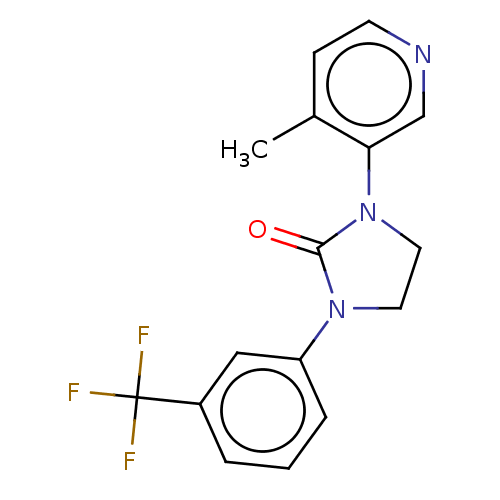 (USRE45173, 70A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C eta type (Homo sapiens (Human)) | BDBM50391897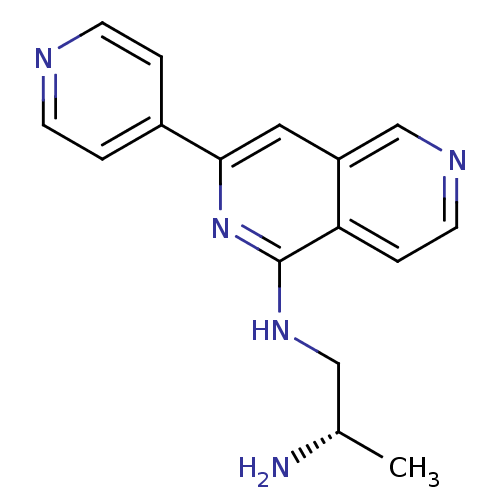 (CHEMBL2147537) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of recombinant human PKCeta assessed as [33P]-ATP incorporation into tridecapeptide substrate after 60 mins by scintillation proximity ass... | Bioorg Med Chem Lett 21: 7367-72 (2011) Article DOI: 10.1016/j.bmcl.2011.10.025 BindingDB Entry DOI: 10.7270/Q22J6CZ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50529549 (CHEMBL4448208) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... | ACS Med Chem Lett 10: 1655-1660 (2019) Article DOI: 10.1021/acsmedchemlett.9b00452 BindingDB Entry DOI: 10.7270/Q2RV0S5T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin K (Homo sapiens (Human)) | BDBM50517806 (CHEMBL4541720) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jilin University Curated by ChEMBL | Assay Description Inhibition of recombinant His-tagged human cathepsin K expressed in Escherichia coli | Bioorg Med Chem 27: 1034-1042 (2019) Article DOI: 10.1016/j.bmc.2019.02.003 BindingDB Entry DOI: 10.7270/Q2M048TS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132182 (USRE45173, 121A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158428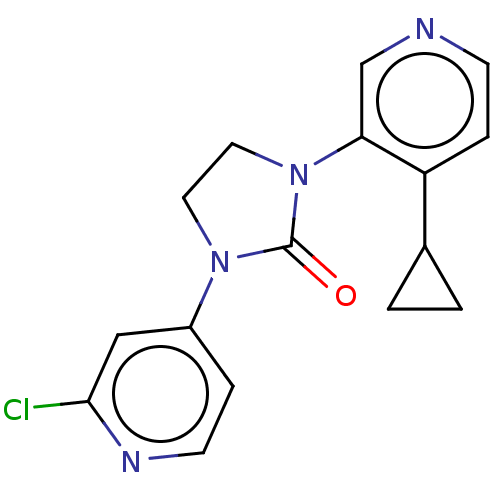 (US9029399, 1A | US9339501, 1A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132216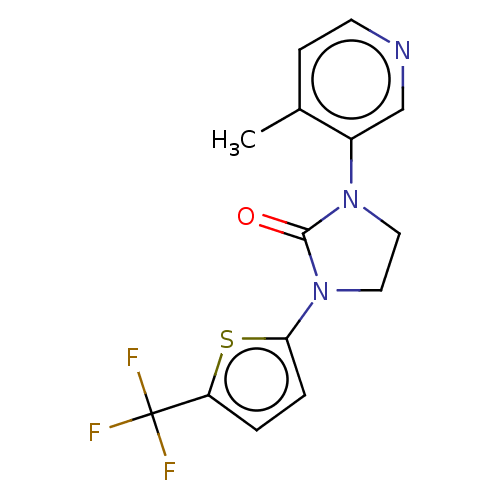 (USRE45173, 155A | USRE45173, 192A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158445 (US9029399, 19A | US9339501, 19A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9029399 (2015) BindingDB Entry DOI: 10.7270/Q28G8JFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM158428 (US9029399, 1A | US9339501, 1A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM132216 (USRE45173, 155A | USRE45173, 192A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Homo sapiens (Human)) | BDBM158445 (US9029399, 19A | US9339501, 19A) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | 7.5 | n/a |
NOVARTIS AG US Patent | Assay Description Assay method was adopted from a published protocol with some modifications to suit our requirements (Dmitry N Grigoryev et al, Analytical Biochemistr... | US Patent US9339501 (2016) BindingDB Entry DOI: 10.7270/Q26T0KG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Steroid 17-alpha-hydroxylase/17,20 lyase (Rattus norvegicus (Rat)) | BDBM132121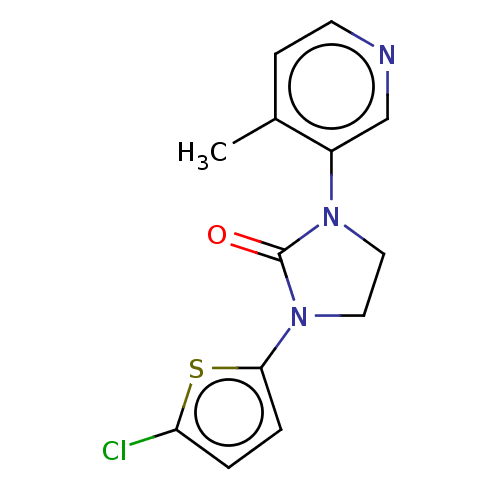 (USRE45173, 41A) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description Cytochrome P450, 17-20 lyase (CYP17-lyase) assay development using recombinant human CYP17 enzyme and 17-alpha -hydroxy pregnenolone [21-3H] as the s... | US Patent USRE45173 (2014) BindingDB Entry DOI: 10.7270/Q2MW2FT2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 908 total ) | Next | Last >> |