

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305851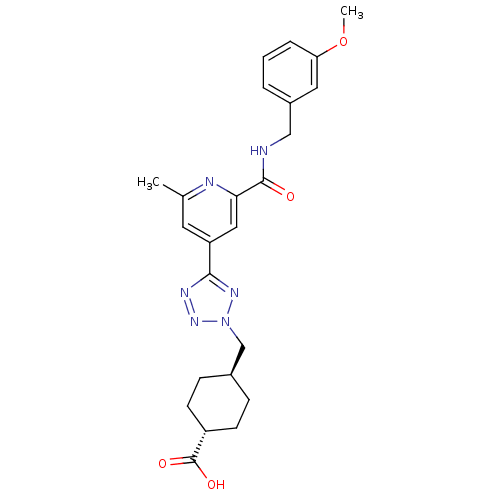 (CHEMBL596273 | trans-4-((5-(2-(3-methoxybenzylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305844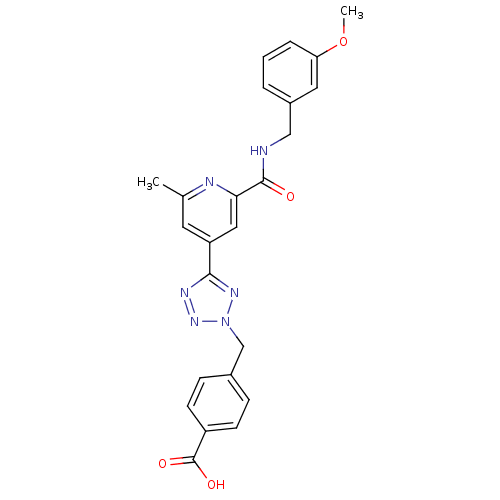 (4-((5-(2-(3-methoxybenzylcarbamoyl)-6-methylpyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305858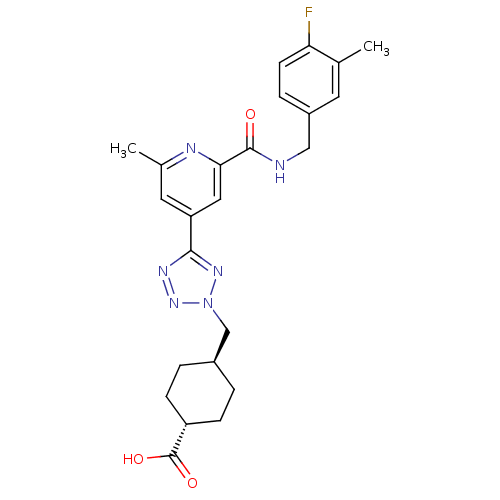 (CHEMBL605928 | trans-4-((5-(2-(4-fluoro-3-methylbe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305856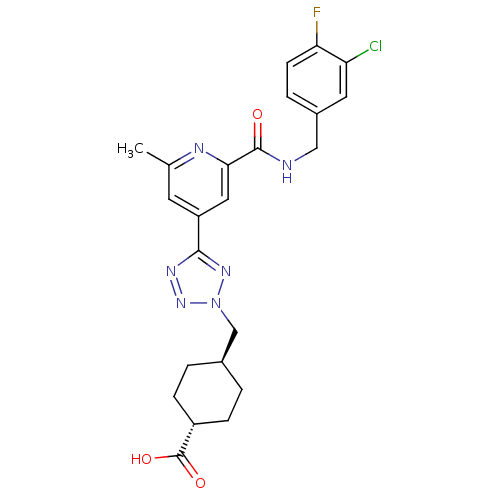 (CHEMBL603206 | trans-4-((5-(2-(3-chloro-4-fluorobe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305855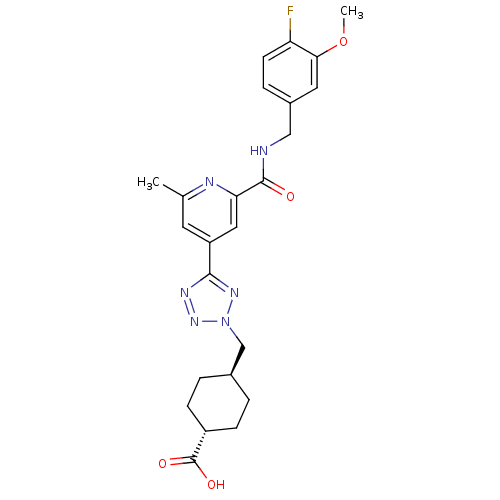 (CHEMBL595156 | trans-4-((5-(2-(4-fluoro-3-methoxyb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553757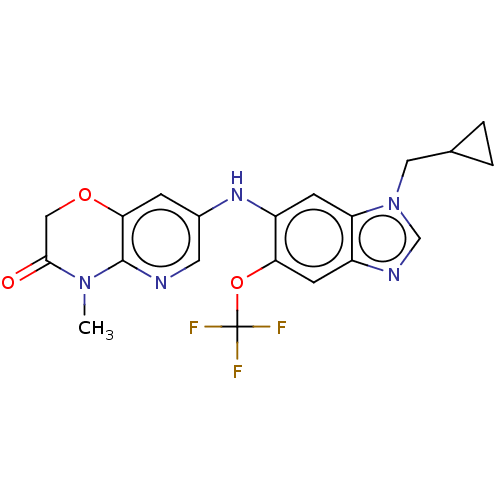 (CHEMBL4746471) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305857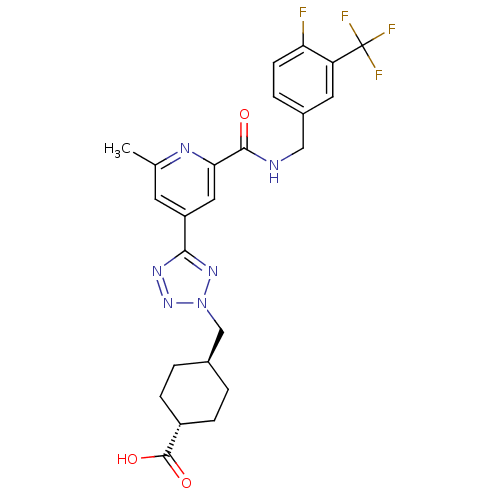 (CHEMBL594932 | trans-4-((5-(2-(4-fluoro-3-(trifluo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM419907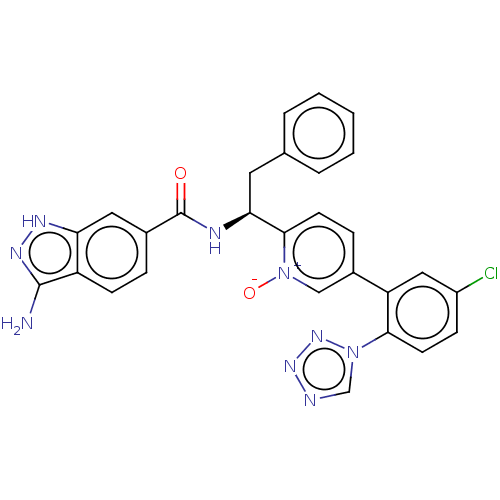 ((S)-2-(1-(3-AMMONIO-1H-INDAZOLE-6-CARBOXAMIDO)-2-P...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US10472344 (2019) BindingDB Entry DOI: 10.7270/Q2Q52S04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553762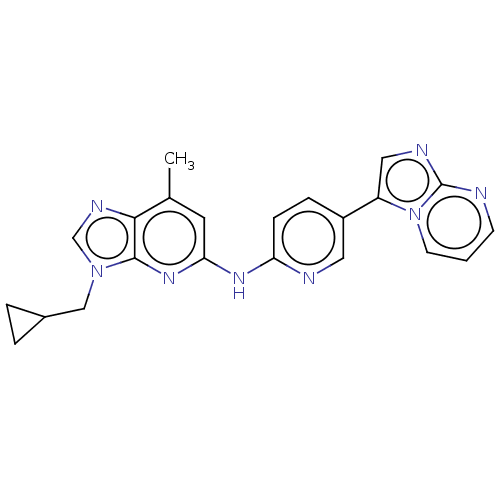 (CHEMBL4758608) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM419910 (US10472344, Example 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US10472344 (2019) BindingDB Entry DOI: 10.7270/Q2Q52S04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305854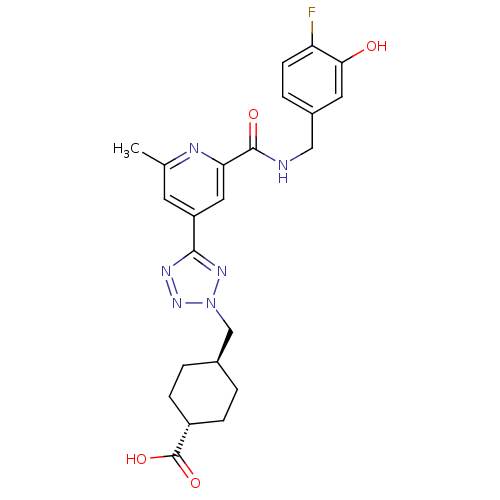 (CHEMBL604139 | trans-4-((5-(2-(4-fluoro-3-hydroxyb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305852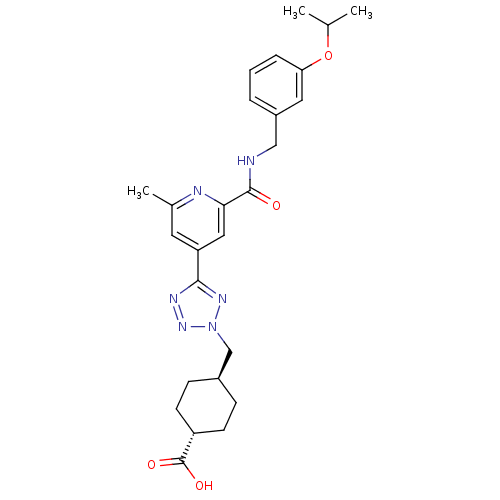 (CHEMBL603857 | trans-4-((5-(2-(3-isopropoxybenzylc...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305850 (CHEMBL593258 | trans-4-((5-(2-(3-hydroxybenzylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553764 (CHEMBL4749423) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553761 (CHEMBL4756857) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553754 (CHEMBL4746482) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553763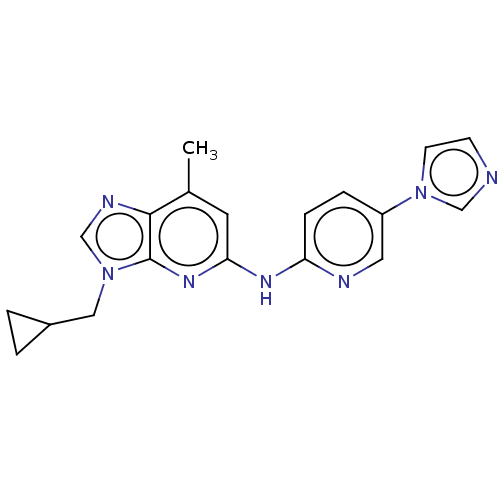 (CHEMBL4785208) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305833 (4-((5-(2-(3-methoxybenzylcarbamoyl)pyridin-4-yl)-2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM330035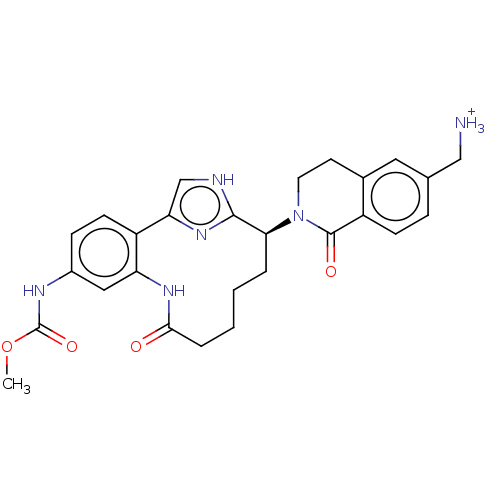 (Methyl [(7S)-7-[6-(aminomethyl)-1-oxo-3,4-dihydroi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9663527 (2017) BindingDB Entry DOI: 10.7270/Q2862JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305845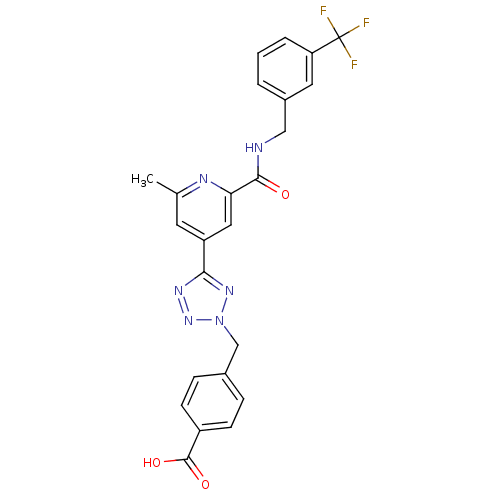 (4-((5-(2-methyl-6-(3-(trifluoromethyl)benzylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM285852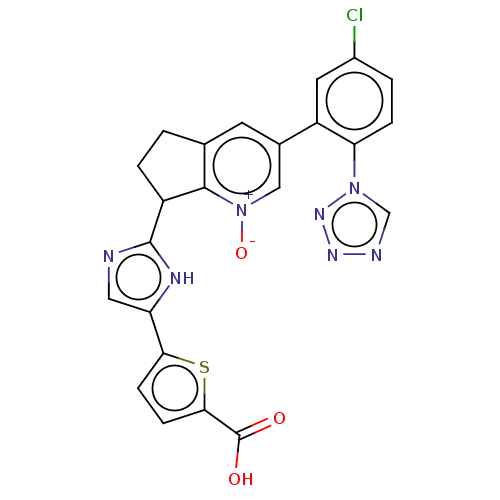 (5-(2-{3-[5-chloro-2- (1H-tetrazol-1- yl)phenyl]-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.08 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US10081617 (2018) BindingDB Entry DOI: 10.7270/Q2CR5WDN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305840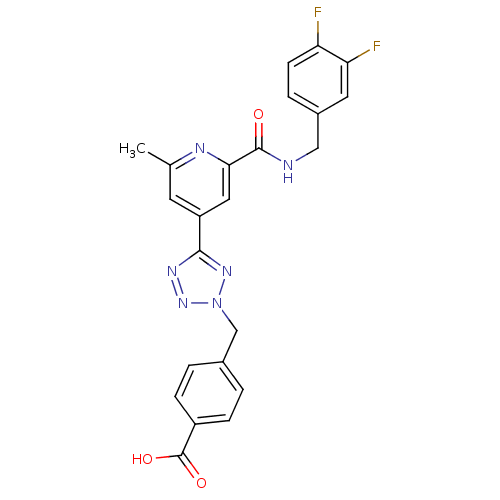 (4-((5-(2-(3,4-difluorobenzylcarbamoyl)-6-methylpyr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM419908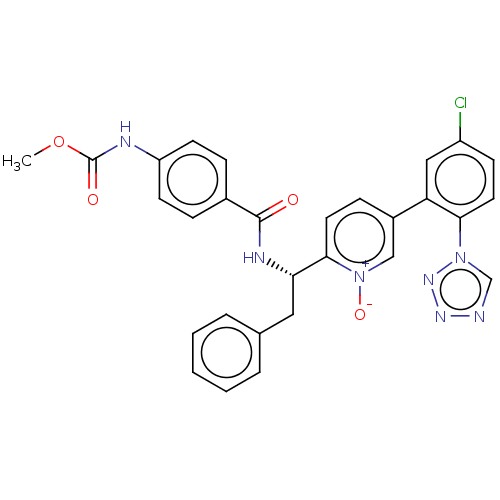 (US10472344, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US10472344 (2019) BindingDB Entry DOI: 10.7270/Q2Q52S04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM330036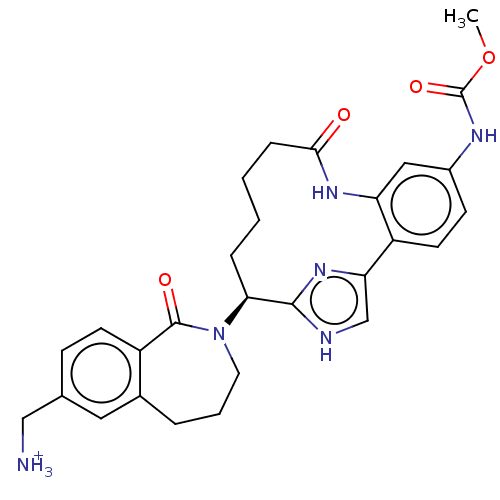 (US9663527, Example 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9663527 (2017) BindingDB Entry DOI: 10.7270/Q2862JJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553760 (CHEMBL4757188) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553753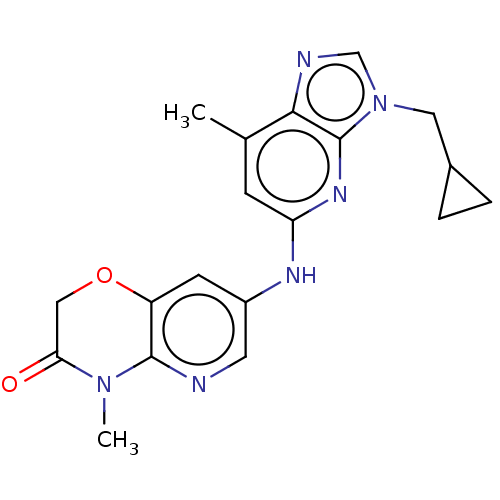 (CHEMBL4799496) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305847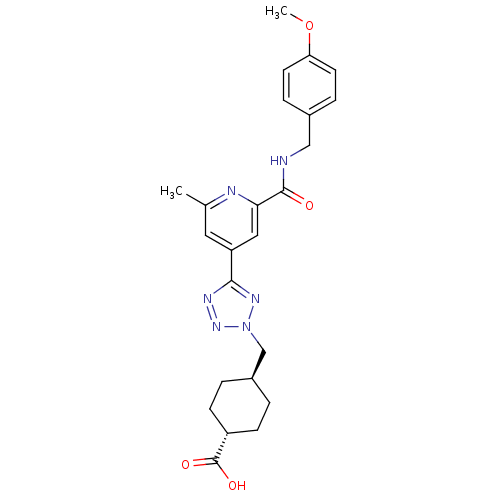 (CHEMBL596304 | trans-4-((5-(2-(4-methoxybenzylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50567601 (CHEMBL4857595) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA9 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305843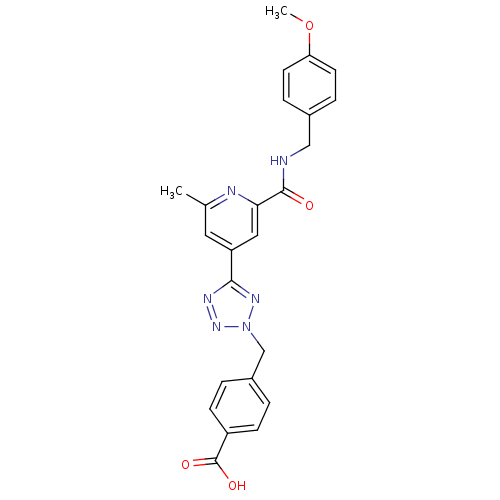 (4-((5-(2-(4-methoxybenzylcarbamoyl)-6-methylpyridi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305842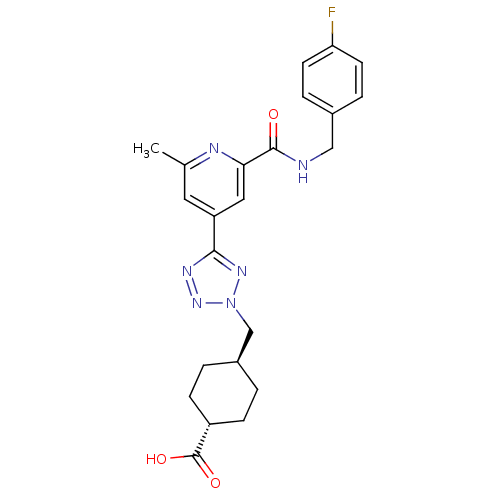 (CHEMBL603656 | trans-4-((5-(2-(4-fluorobenzylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50334354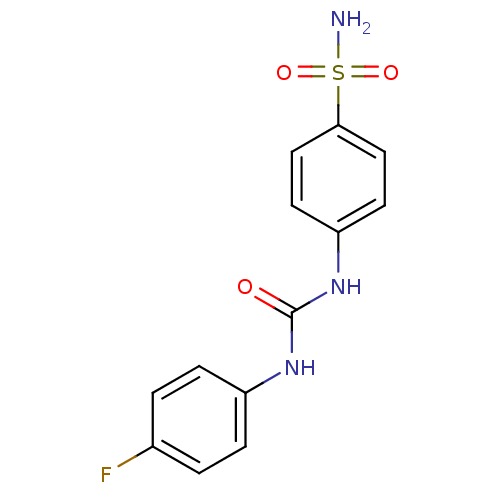 (4-(3-(4-fluorophenyl)ureido)benzenesulfonamide | 4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA12 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352466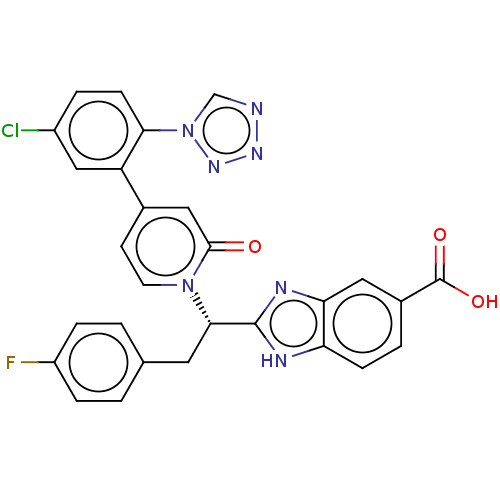 (US9809545, 3Bd) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352458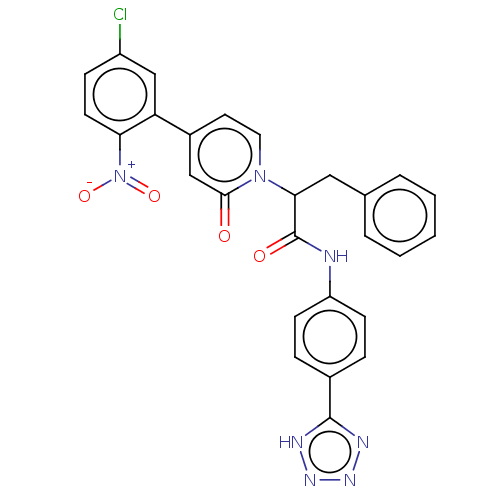 (US9809545, 1Bc) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352460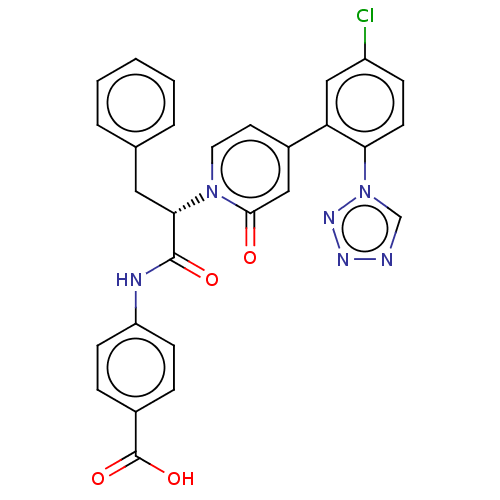 (US9809545, 1Ef) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352476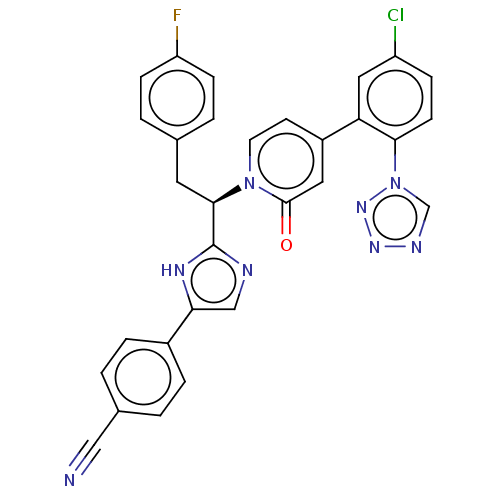 (US9809545, 4Cl) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352469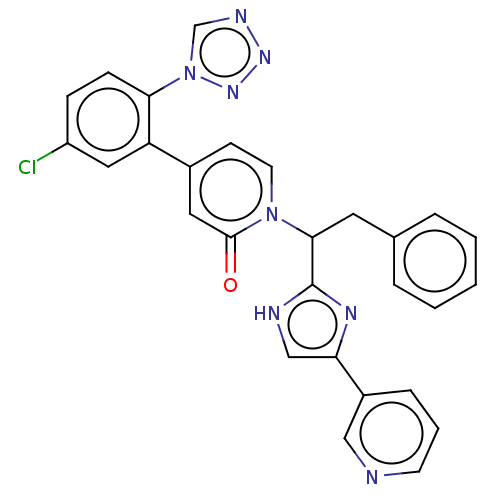 (US9809545, 4Aa) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352478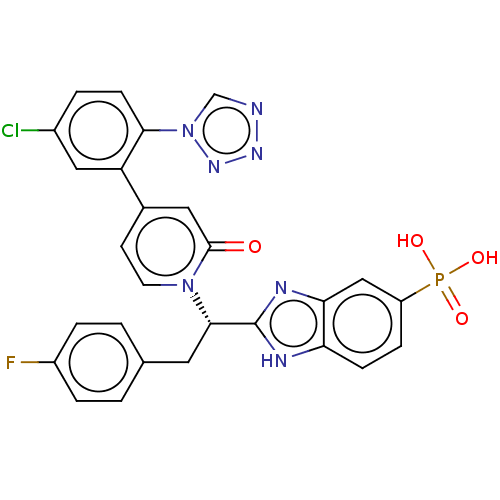 (US9809545, 5b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352464 (US9809545, 3g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50567598 (CHEMBL4853209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA12 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305838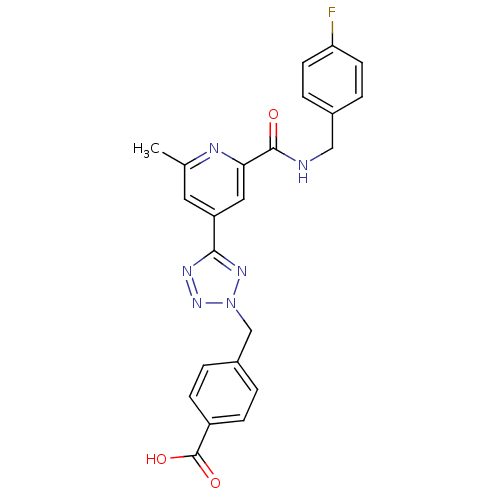 (4-((5-(2-(4-fluorobenzylcarbamoyl)-6-methylpyridin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA12 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50567599 (CHEMBL4847847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352459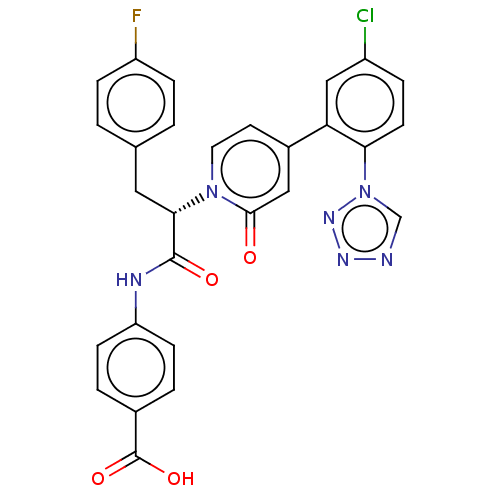 (US9809545, 1Ea) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM352461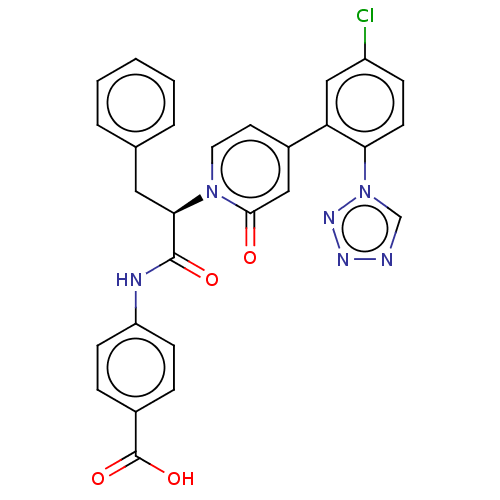 (US9809545, 1Ff) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US9809545 (2017) BindingDB Entry DOI: 10.7270/Q24Q7X4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50567598 (CHEMBL4853209) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA9 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50305849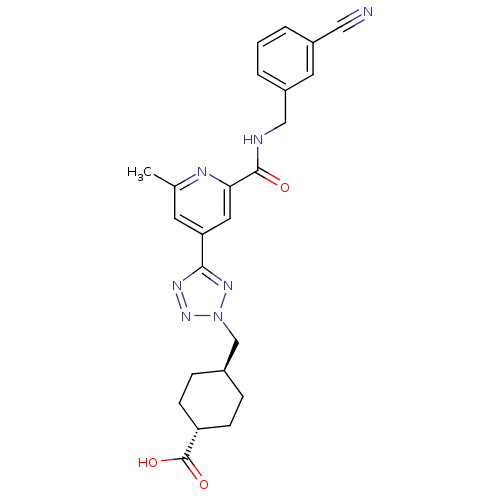 (CHEMBL593257 | trans-4-((5-(2-(3-cyanobenzylcarbam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant full length MMP13 assessed as type 3 collagen cleavage activity after 18 hrs | Bioorg Med Chem Lett 20: 576-80 (2010) Article DOI: 10.1016/j.bmcl.2009.11.081 BindingDB Entry DOI: 10.7270/Q2JS9QHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM419912 (US10472344, Example 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | UniChem | US Patent | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp. US Patent | Assay Description Factor XIa determinations were made in 50 mM HEPES buffer at pH 7.4 containing 150 mM NaCl, 5 mM CaCl2, and 0.1% PEG 8000 (polyethylene glycol; JT Ba... | US Patent US10472344 (2019) BindingDB Entry DOI: 10.7270/Q2Q52S04 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM400240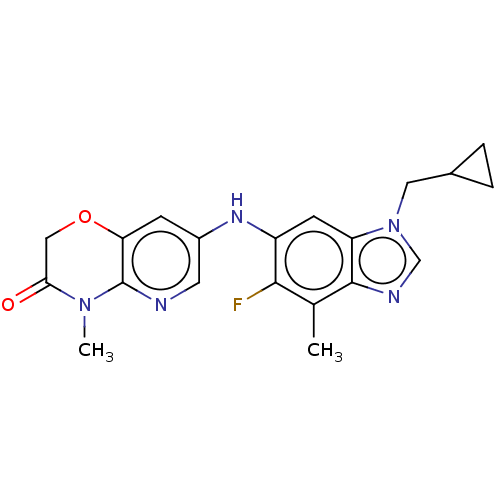 (7-((1-(Cyclopropylmethyl)-5-fluoro-4-methyl-1H-ben...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50553759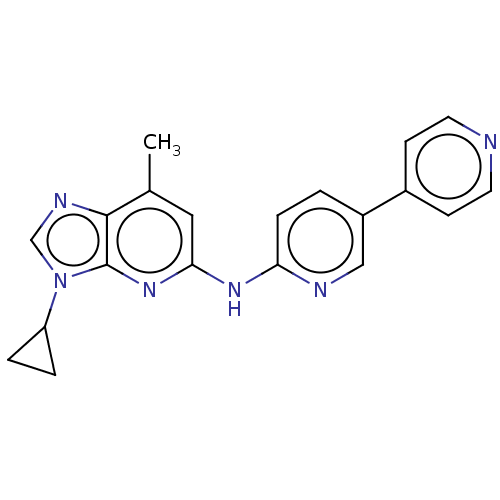 (CHEMBL4760677) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]-Ro15-1788 from human GABAA alpha5beta3gamma2 by scintillation proximity assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127536 BindingDB Entry DOI: 10.7270/Q2MW2MSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50567598 (CHEMBL4853209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 pre-incubated for 15 mins measured by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113360 BindingDB Entry DOI: 10.7270/Q2M61Q1S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3317 total ) | Next | Last >> |