 Found 272 hits with Last Name = 'gedeck' and Initial = 'p'
Found 272 hits with Last Name = 'gedeck' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
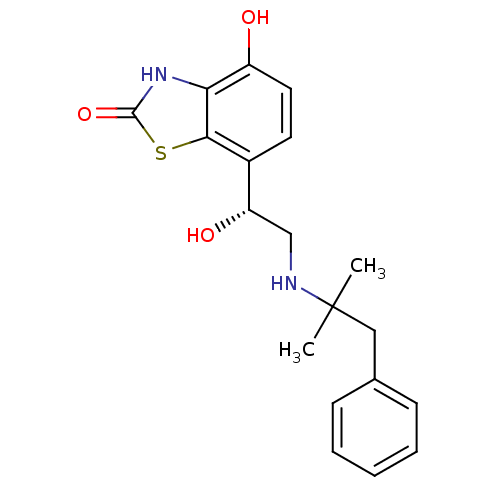
(Homo sapiens (Human)) | BDBM50324835

((R)-4-hydroxy-7-(1-hydroxy-2-(3-(methyl(phenyl)ami...)Show SMILES CN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)c1ccccc1 |r| Show InChI InChI=1S/C19H23N3O3S/c1-22(13-6-3-2-4-7-13)11-5-10-20-12-16(24)14-8-9-15(23)17-18(14)26-19(25)21-17/h2-4,6-9,16,20,23-24H,5,10-12H2,1H3,(H,21,25)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324847
((R)-4-hydroxy-7-(1-hydroxy-2-(2-methyl-1-phenylpro...)Show SMILES CC(C)(Cc1ccccc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H22N2O3S/c1-19(2,10-12-6-4-3-5-7-12)20-11-15(23)13-8-9-14(22)16-17(13)25-18(24)21-16/h3-9,15,20,22-23H,10-11H2,1-2H3,(H,21,24)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324854
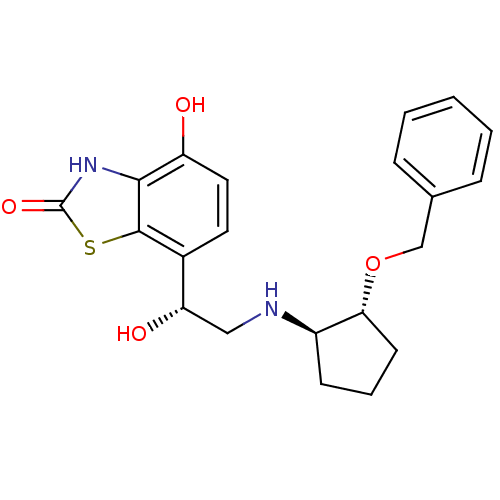
(7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1...)Show SMILES O[C@@H](CN[C@@H]1CCC[C@H]1OCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C21H24N2O4S/c24-16-10-9-14(20-19(16)23-21(26)28-20)17(25)11-22-15-7-4-8-18(15)27-12-13-5-2-1-3-6-13/h1-3,5-6,9-10,15,17-18,22,24-25H,4,7-8,11-12H2,(H,23,26)/t15-,17+,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324856
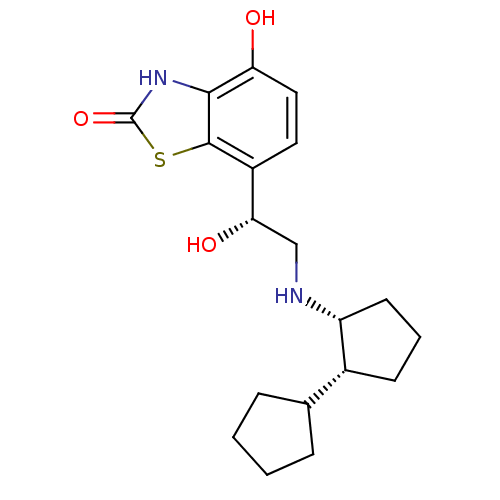
(7-((R)-2-((cis)-bi(cyclopentan)-2-ylamino)-1-hydro...)Show SMILES O[C@@H](CN[C@@H]1CCC[C@@H]1C1CCCC1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H26N2O3S/c22-15-9-8-13(18-17(15)21-19(24)25-18)16(23)10-20-14-7-3-6-12(14)11-4-1-2-5-11/h8-9,11-12,14,16,20,22-23H,1-7,10H2,(H,21,24)/t12-,14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324857
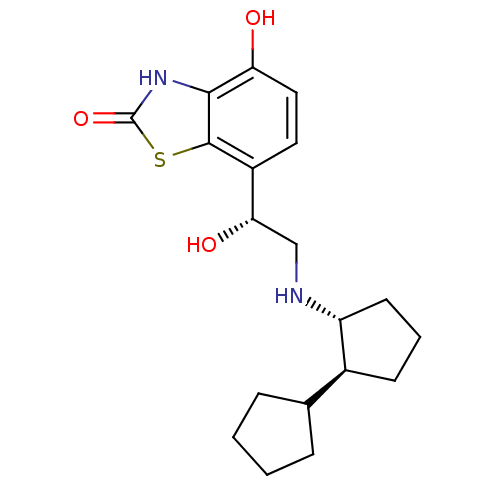
(7-((R)-2-((trans)-bi(cyclopentan)-2-ylamino)-1-hyd...)Show SMILES O[C@@H](CN[C@@H]1CCC[C@H]1C1CCCC1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H26N2O3S/c22-15-9-8-13(18-17(15)21-19(24)25-18)16(23)10-20-14-7-3-6-12(14)11-4-1-2-5-11/h8-9,11-12,14,16,20,22-23H,1-7,10H2,(H,21,24)/t12-,14+,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324854

(7-((R)-2-((1R,2R)-2-(benzyloxy)cyclopentylamino)-1...)Show SMILES O[C@@H](CN[C@@H]1CCC[C@H]1OCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C21H24N2O4S/c24-16-10-9-14(20-19(16)23-21(26)28-20)17(25)11-22-15-7-4-8-18(15)27-12-13-5-2-1-3-6-13/h1-3,5-6,9-10,15,17-18,22,24-25H,4,7-8,11-12H2,(H,23,26)/t15-,17+,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324840
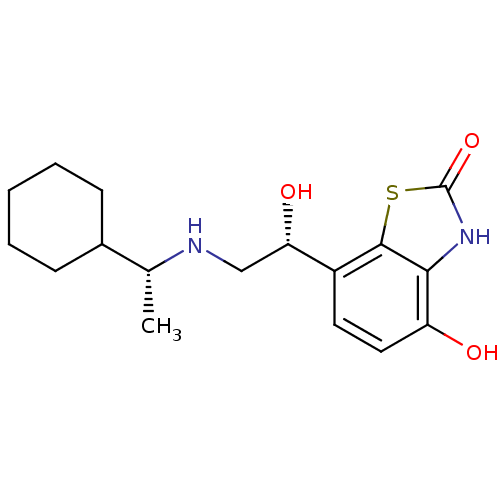
(7-((R)-2-((R)-1-cyclohexylethylamino)-1-hydroxyeth...)Show SMILES C[C@@H](NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)C1CCCCC1 |r| Show InChI InChI=1S/C17H24N2O3S/c1-10(11-5-3-2-4-6-11)18-9-14(21)12-7-8-13(20)15-16(12)23-17(22)19-15/h7-8,10-11,14,18,20-21H,2-6,9H2,1H3,(H,19,22)/t10-,14+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771
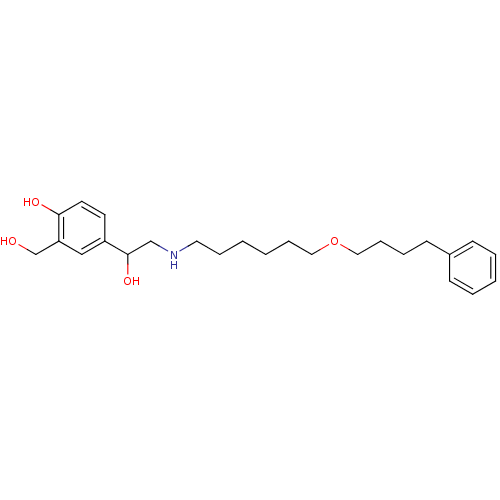
(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324846
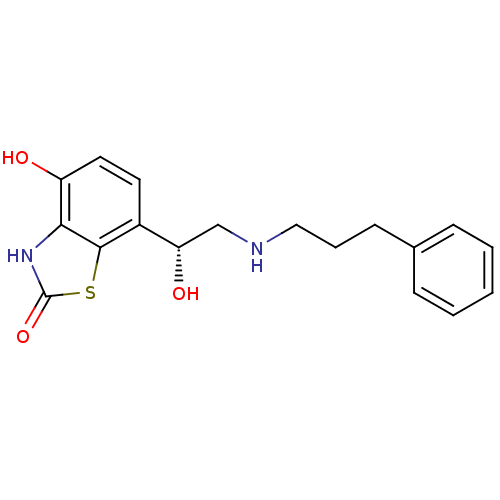
((R)-4-hydroxy-7-(1-hydroxy-2-(3-phenylpropylamino)...)Show SMILES O[C@@H](CNCCCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C18H20N2O3S/c21-14-9-8-13(17-16(14)20-18(23)24-17)15(22)11-19-10-4-7-12-5-2-1-3-6-12/h1-3,5-6,8-9,15,19,21-22H,4,7,10-11H2,(H,20,23)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324851
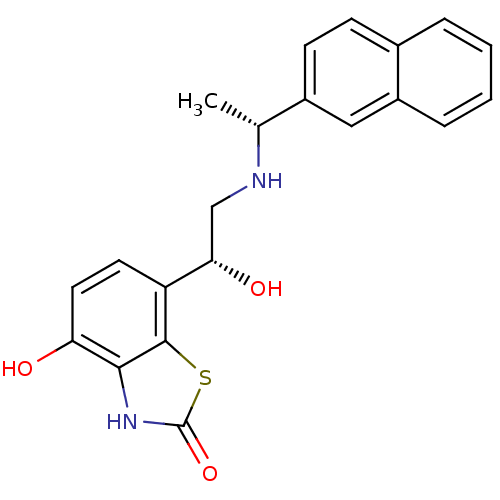
(4-hydroxy-7-((R)-1-hydroxy-2-((R)-1-(naphthalen-2-...)Show SMILES C[C@@H](NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H20N2O3S/c1-12(14-7-6-13-4-2-3-5-15(13)10-14)22-11-18(25)16-8-9-17(24)19-20(16)27-21(26)23-19/h2-10,12,18,22,24-25H,11H2,1H3,(H,23,26)/t12-,18+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324840

(7-((R)-2-((R)-1-cyclohexylethylamino)-1-hydroxyeth...)Show SMILES C[C@@H](NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)C1CCCCC1 |r| Show InChI InChI=1S/C17H24N2O3S/c1-10(11-5-3-2-4-6-11)18-9-14(21)12-7-8-13(20)15-16(12)23-17(22)19-15/h7-8,10-11,14,18,20-21H,2-6,9H2,1H3,(H,19,22)/t10-,14+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324837
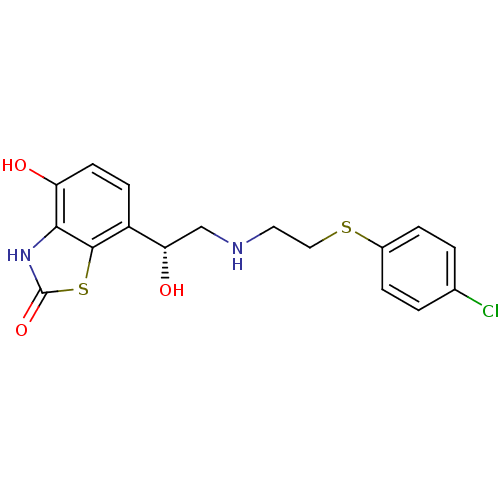
((R)-7-(2-(2-(4-chlorophenylthio)ethylamino)-1-hydr...)Show SMILES O[C@@H](CNCCSc1ccc(Cl)cc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H17ClN2O3S2/c18-10-1-3-11(4-2-10)24-8-7-19-9-14(22)12-5-6-13(21)15-16(12)25-17(23)20-15/h1-6,14,19,21-22H,7-9H2,(H,20,23)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324850
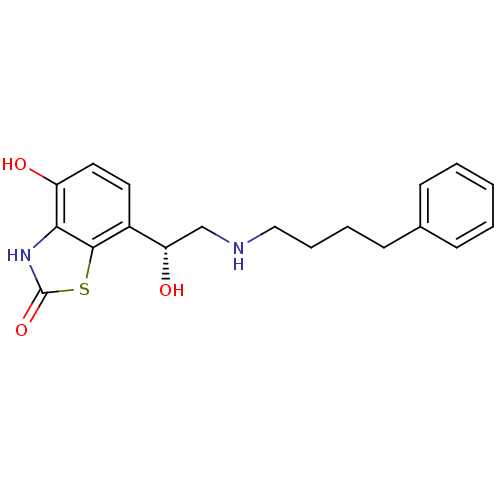
((R)-4-hydroxy-7-(1-hydroxy-2-(4-phenylbutylamino)e...)Show SMILES O[C@@H](CNCCCCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H22N2O3S/c22-15-10-9-14(18-17(15)21-19(24)25-18)16(23)12-20-11-5-4-8-13-6-2-1-3-7-13/h1-3,6-7,9-10,16,20,22-23H,4-5,8,11-12H2,(H,21,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324844
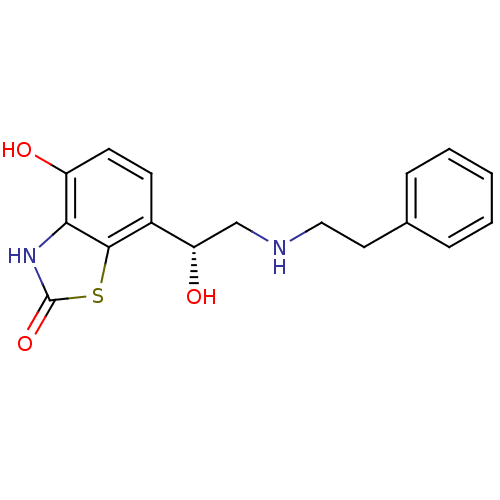
((R)-4-hydroxy-7-(1-hydroxy-2-(phenethylamino)ethyl...)Show SMILES O[C@@H](CNCCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H18N2O3S/c20-13-7-6-12(16-15(13)19-17(22)23-16)14(21)10-18-9-8-11-4-2-1-3-5-11/h1-7,14,18,20-21H,8-10H2,(H,19,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324847

((R)-4-hydroxy-7-(1-hydroxy-2-(2-methyl-1-phenylpro...)Show SMILES CC(C)(Cc1ccccc1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H22N2O3S/c1-19(2,10-12-6-4-3-5-7-12)20-11-15(23)13-8-9-14(22)16-17(13)25-18(24)21-16/h3-9,15,20,22-23H,10-11H2,1-2H3,(H,21,24)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324842
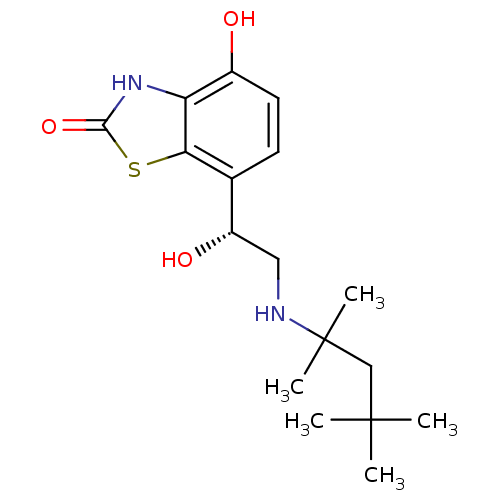
((R)-4-hydroxy-7-(1-hydroxy-2-(2,4,4-trimethylpenta...)Show SMILES CC(C)(C)CC(C)(C)NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H26N2O3S/c1-16(2,3)9-17(4,5)18-8-12(21)10-6-7-11(20)13-14(10)23-15(22)19-13/h6-7,12,18,20-21H,8-9H2,1-5H3,(H,19,22)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324846

((R)-4-hydroxy-7-(1-hydroxy-2-(3-phenylpropylamino)...)Show SMILES O[C@@H](CNCCCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C18H20N2O3S/c21-14-9-8-13(17-16(14)20-18(23)24-17)15(22)11-19-10-4-7-12-5-2-1-3-6-12/h1-3,5-6,8-9,15,19,21-22H,4,7,10-11H2,(H,20,23)/t15-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50150766
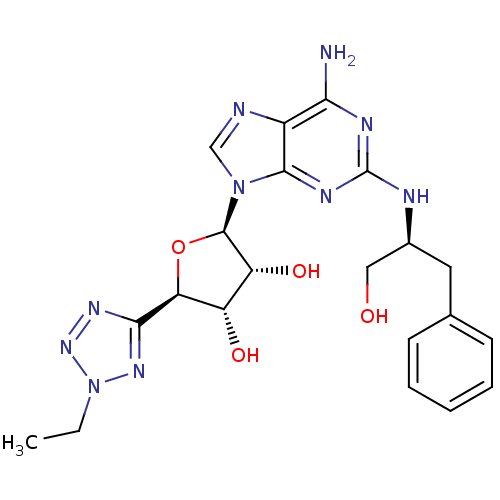
((2R,3R,4S,5R)-2-(6-amino-2-((S)-1-hydroxy-3-phenyl...)Show SMILES CCn1nnc(n1)[C@H]1O[C@H]([C@H](O)[C@@H]1O)n1cnc2c(N)nc(N[C@H](CO)Cc3ccccc3)nc12 Show InChI InChI=1S/C21H26N10O4/c1-2-31-28-18(27-29-31)16-14(33)15(34)20(35-16)30-10-23-13-17(22)25-21(26-19(13)30)24-12(9-32)8-11-6-4-3-5-7-11/h3-7,10,12,14-16,20,32-34H,2,8-9H2,1H3,(H3,22,24,25,26)/t12-,14-,15+,16-,20+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM86453

(CAS_73573-87-2 | Formoterol | NSC_3083544)Show InChI InChI=1S/C19H24N2O4/c1-13(9-14-3-6-16(25-2)7-4-14)20-11-19(24)15-5-8-18(23)17(10-15)21-12-22/h3-8,10,12-13,19-20,23-24H,9,11H2,1-2H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324851

(4-hydroxy-7-((R)-1-hydroxy-2-((R)-1-(naphthalen-2-...)Show SMILES C[C@@H](NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)c1ccc2ccccc2c1 |r| Show InChI InChI=1S/C21H20N2O3S/c1-12(14-7-6-13-4-2-3-5-15(13)10-14)22-11-18(25)16-8-9-17(24)19-20(16)27-21(26)23-19/h2-10,12,18,22,24-25H,11H2,1H3,(H,23,26)/t12-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324837

((R)-7-(2-(2-(4-chlorophenylthio)ethylamino)-1-hydr...)Show SMILES O[C@@H](CNCCSc1ccc(Cl)cc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H17ClN2O3S2/c18-10-1-3-11(4-2-10)24-8-7-19-9-14(22)12-5-6-13(21)15-16(12)25-17(23)20-15/h1-6,14,19,21-22H,7-9H2,(H,20,23)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390424
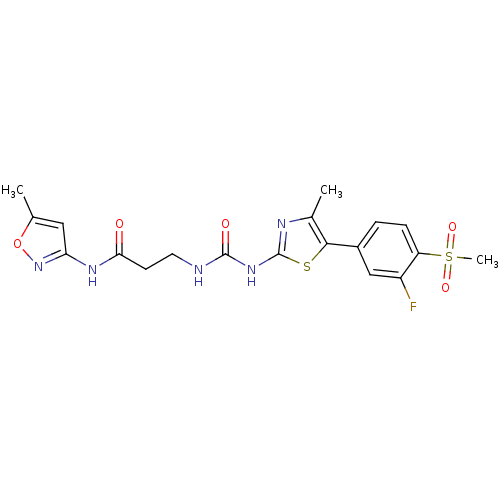
(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM25771

(1-hydroxy-2-naphthoic acid;4-[1-hydroxy-2-[6-(4-ph...)Show InChI InChI=1S/C25H37NO4/c27-20-23-18-22(13-14-24(23)28)25(29)19-26-15-7-1-2-8-16-30-17-9-6-12-21-10-4-3-5-11-21/h3-5,10-11,13-14,18,25-29H,1-2,6-9,12,15-17,19-20H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151724
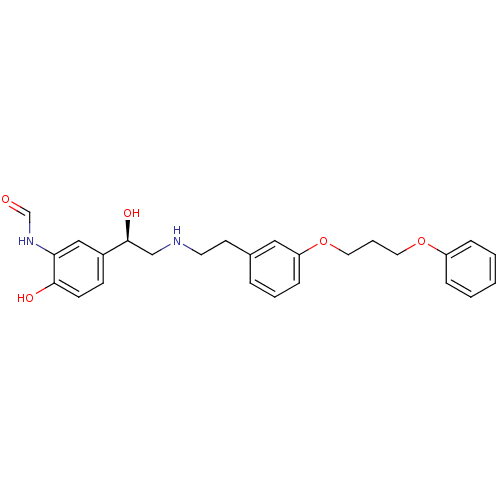
(CHEMBL183948 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[3-(...)Show SMILES O[C@@H](CNCCc1cccc(OCCCOc2ccccc2)c1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H30N2O5/c29-19-28-24-17-21(10-11-25(24)30)26(31)18-27-13-12-20-6-4-9-23(16-20)33-15-5-14-32-22-7-2-1-3-8-22/h1-4,6-11,16-17,19,26-27,30-31H,5,12-15,18H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324844

((R)-4-hydroxy-7-(1-hydroxy-2-(phenethylamino)ethyl...)Show SMILES O[C@@H](CNCCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H18N2O3S/c20-13-7-6-12(16-15(13)19-17(22)23-16)14(21)10-18-9-8-11-4-2-1-3-5-11/h1-7,14,18,20-21H,8-10H2,(H,19,22)/t14-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324830
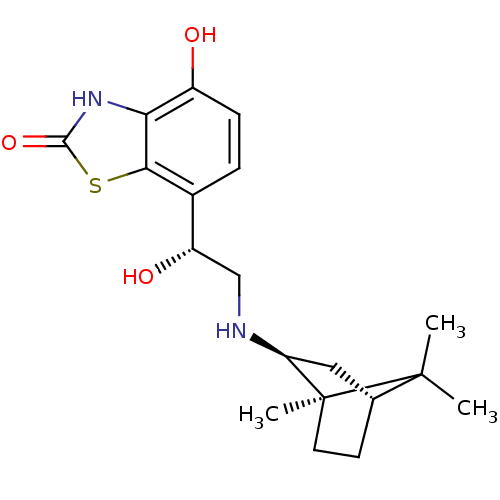
(4-hydroxy-7-((R)-1-hydroxy-2-((1R,2S,4R)-1,7,7-tri...)Show SMILES CC1(C)[C@@H]2CC[C@@]1(C)[C@H](C2)NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12 |r,THB:10:8:4.5:1| Show InChI InChI=1S/C19H26N2O3S/c1-18(2)10-6-7-19(18,3)14(8-10)20-9-13(23)11-4-5-12(22)15-16(11)25-17(24)21-15/h4-5,10,13-14,20,22-23H,6-9H2,1-3H3,(H,21,24)/t10-,13+,14+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324831
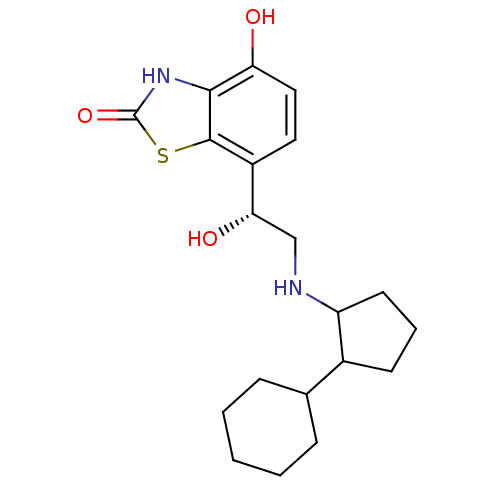
((R)-7-(2-(2-cyclohexylcyclopentylamino)-1-hydroxye...)Show SMILES O[C@@H](CNC1CCCC1C1CCCCC1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C20H28N2O3S/c23-16-10-9-14(19-18(16)22-20(25)26-19)17(24)11-21-15-8-4-7-13(15)12-5-2-1-3-6-12/h9-10,12-13,15,17,21,23-24H,1-8,11H2,(H,22,25)/t13?,15?,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390409
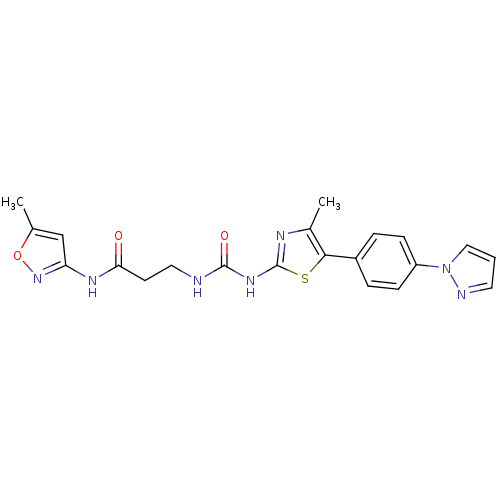
(CHEMBL1986603)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(cc2)-n2cccn2)no1 Show InChI InChI=1S/C21H21N7O3S/c1-13-12-17(27-31-13)25-18(29)8-10-22-20(30)26-21-24-14(2)19(32-21)15-4-6-16(7-5-15)28-11-3-9-23-28/h3-7,9,11-12H,8,10H2,1-2H3,(H,25,27,29)(H2,22,24,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324834
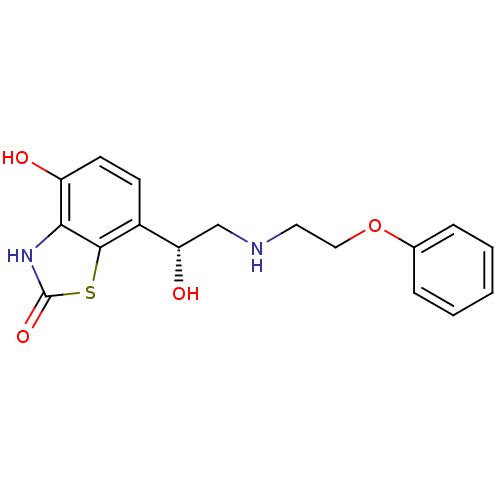
((R)-4-hydroxy-7-(1-hydroxy-2-(2-phenoxyethylamino)...)Show SMILES O[C@@H](CNCCOc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H18N2O4S/c20-13-7-6-12(16-15(13)19-17(22)24-16)14(21)10-18-8-9-23-11-4-2-1-3-5-11/h1-7,14,18,20-21H,8-10H2,(H,19,22)/t14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309479
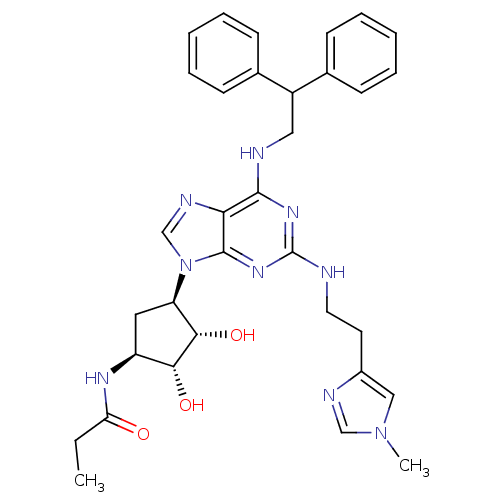
(CHEMBL591423 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...)Show SMILES CCC(=O)N[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3cn(C)cn3)nc12 |r| Show InChI InChI=1S/C33H39N9O3/c1-3-27(43)38-25-16-26(30(45)29(25)44)42-20-37-28-31(39-33(40-32(28)42)34-15-14-23-18-41(2)19-36-23)35-17-24(21-10-6-4-7-11-21)22-12-8-5-9-13-22/h4-13,18-20,24-26,29-30,44-45H,3,14-17H2,1-2H3,(H,38,43)(H2,34,35,39,40)/t25-,26+,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50077647
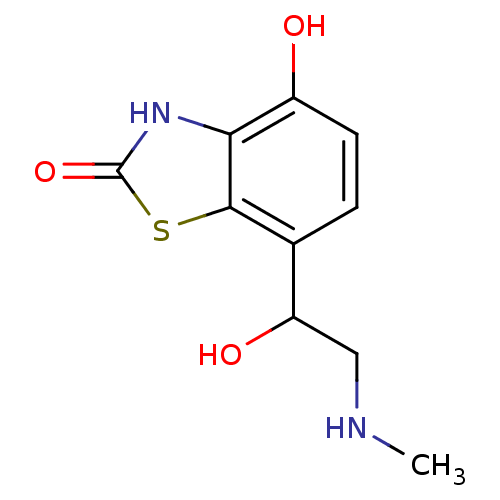
(4-Hydroxy-7-(1-hydroxy-2-methylamino-ethyl)-3H-ben...)Show InChI InChI=1S/C10H12N2O3S/c1-11-4-7(14)5-2-3-6(13)8-9(5)16-10(15)12-8/h2-3,7,11,13-14H,4H2,1H3,(H,12,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
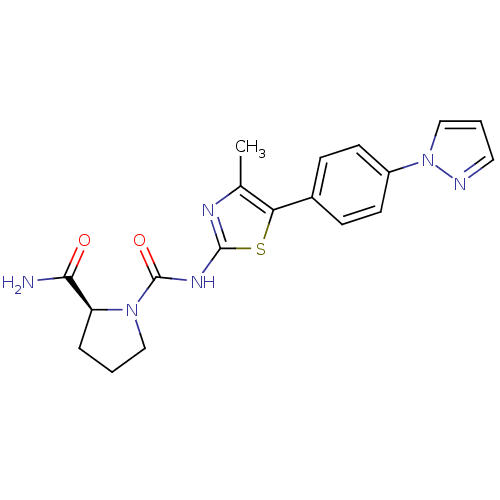
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309480
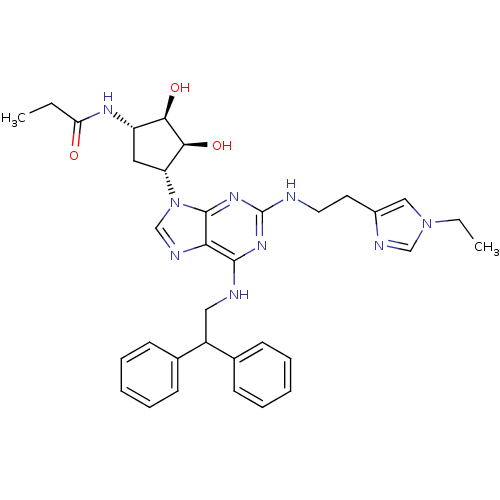
(CHEMBL591356 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...)Show SMILES CCC(=O)N[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3cn(CC)cn3)nc12 |r| Show InChI InChI=1S/C34H41N9O3/c1-3-28(44)39-26-17-27(31(46)30(26)45)43-21-38-29-32(36-18-25(22-11-7-5-8-12-22)23-13-9-6-10-14-23)40-34(41-33(29)43)35-16-15-24-19-42(4-2)20-37-24/h5-14,19-21,25-27,30-31,45-46H,3-4,15-18H2,1-2H3,(H,39,44)(H2,35,36,40,41)/t26-,27+,30+,31-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309478
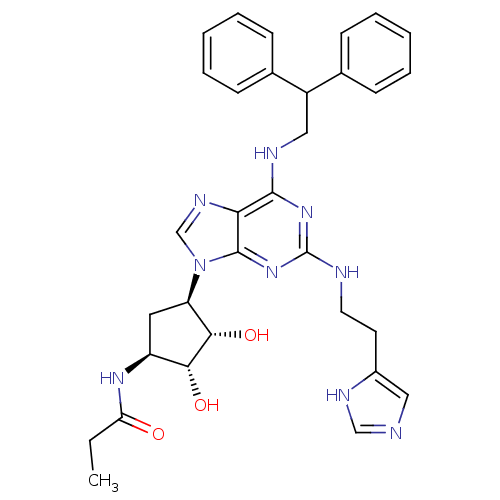
(CHEMBL601514 | N-((2S,3S,4R,5R)-5-(2-(2-(1H-imidaz...)Show SMILES CCC(=O)N[C@H]1C[C@H]([C@H](O)[C@@H]1O)n1cnc2c(NCC(c3ccccc3)c3ccccc3)nc(NCCc3cnc[nH]3)nc12 |r| Show InChI InChI=1S/C32H37N9O3/c1-2-26(42)38-24-15-25(29(44)28(24)43)41-19-37-27-30(39-32(40-31(27)41)34-14-13-22-16-33-18-36-22)35-17-23(20-9-5-3-6-10-20)21-11-7-4-8-12-21/h3-12,16,18-19,23-25,28-29,43-44H,2,13-15,17H2,1H3,(H,33,36)(H,38,42)(H2,34,35,39,40)/t24-,25+,28+,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309481
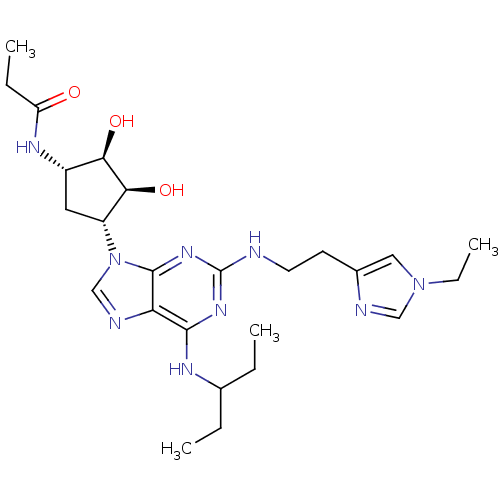
(CHEMBL592541 | N-((2S,3S,4R,5R)-5-(2-(2-(1-ethyl-1...)Show SMILES CCC(CC)Nc1nc(NCCc2cn(CC)cn2)nc2n(cnc12)[C@@H]1C[C@H](NC(=O)CC)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C25H39N9O3/c1-5-15(6-2)29-23-20-24(32-25(31-23)26-10-9-16-12-33(8-4)13-27-16)34(14-28-20)18-11-17(21(36)22(18)37)30-19(35)7-3/h12-15,17-18,21-22,36-37H,5-11H2,1-4H3,(H,30,35)(H2,26,29,31,32)/t17-,18+,21+,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309494
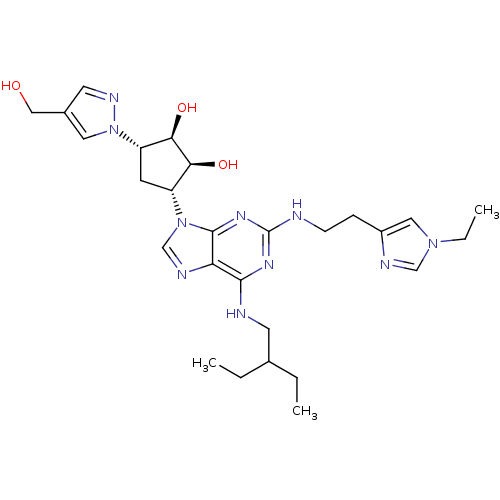
((1R,2S,3R,5S)-3-(2-(2-(1-ethyl-1H-imidazol-4-yl)et...)Show SMILES CCC(CC)CNc1nc(NCCc2cn(CC)cn2)nc2n(cnc12)[C@@H]1C[C@@H]([C@@H](O)[C@H]1O)n1cc(CO)cn1 |r| Show InChI InChI=1S/C27H40N10O3/c1-4-17(5-2)10-29-25-22-26(34-27(33-25)28-8-7-19-13-35(6-3)15-30-19)36(16-31-22)20-9-21(24(40)23(20)39)37-12-18(14-38)11-32-37/h11-13,15-17,20-21,23-24,38-40H,4-10,14H2,1-3H3,(H2,28,29,33,34)/t20-,21+,23+,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151719
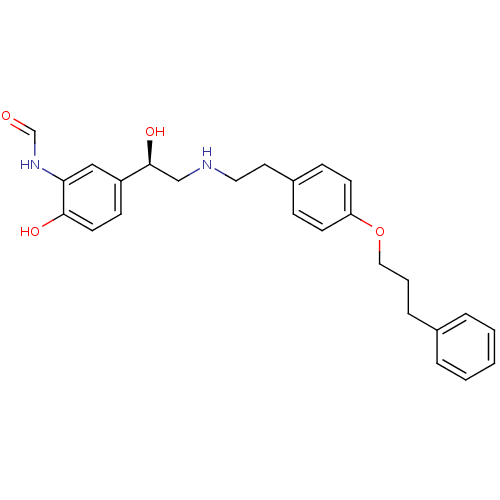
(CHEMBL363329 | N-[2-Hydroxy-5-(1-hydroxy-2-{2-[4-(...)Show SMILES O[C@@H](CNCCc1ccc(OCCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C26H30N2O4/c29-19-28-24-17-22(10-13-25(24)30)26(31)18-27-15-14-21-8-11-23(12-9-21)32-16-4-7-20-5-2-1-3-6-20/h1-3,5-6,8-13,17,19,26-27,30-31H,4,7,14-16,18H2,(H,28,29)/t26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324855
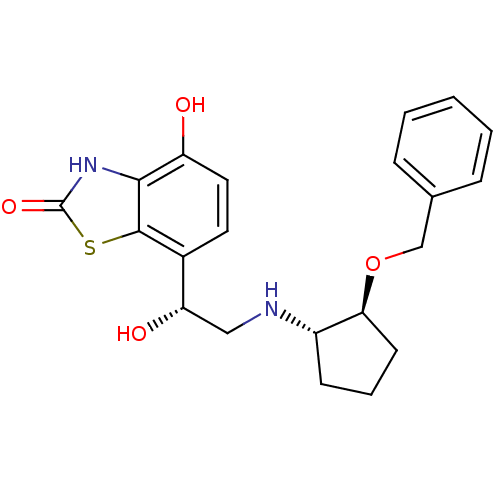
(7-((R)-2-((1S,2S)-2-(benzyloxy)cyclopentylamino)-1...)Show SMILES O[C@@H](CN[C@H]1CCC[C@@H]1OCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C21H24N2O4S/c24-16-10-9-14(20-19(16)23-21(26)28-20)17(25)11-22-15-7-4-8-18(15)27-12-13-5-2-1-3-6-13/h1-3,5-6,9-10,15,17-18,22,24-25H,4,7-8,11-12H2,(H,23,26)/t15-,17-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324833
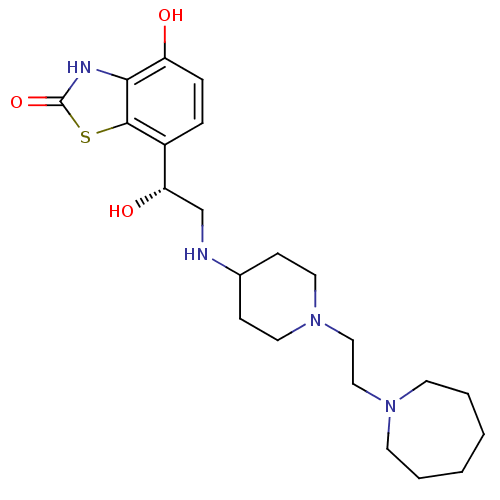
((R)-7-(2-(1-(2-(azepan-1-yl)ethyl)piperidin-4-ylam...)Show SMILES O[C@@H](CNC1CCN(CCN2CCCCCC2)CC1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C22H34N4O3S/c27-18-6-5-17(21-20(18)24-22(29)30-21)19(28)15-23-16-7-11-26(12-8-16)14-13-25-9-3-1-2-4-10-25/h5-6,16,19,23,27-28H,1-4,7-15H2,(H,24,29)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324857

(7-((R)-2-((trans)-bi(cyclopentan)-2-ylamino)-1-hyd...)Show SMILES O[C@@H](CN[C@@H]1CCC[C@H]1C1CCCC1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H26N2O3S/c22-15-9-8-13(18-17(15)21-19(24)25-18)16(23)10-20-14-7-3-6-12(14)11-4-1-2-5-11/h8-9,11-12,14,16,20,22-23H,1-7,10H2,(H,21,24)/t12-,14+,16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324827
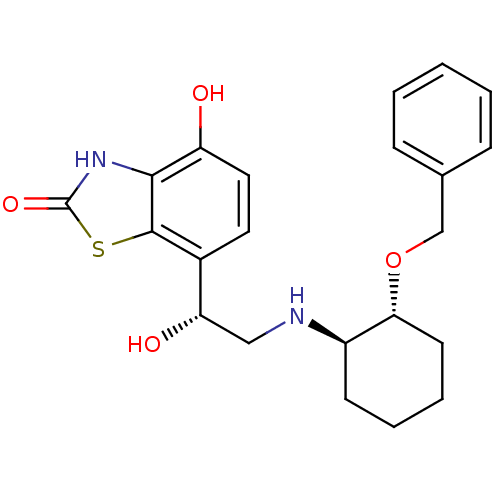
(7-((R)-2-((1R,2R)-2-(benzyloxy)cyclohexylamino)-1-...)Show SMILES O[C@@H](CN[C@@H]1CCCC[C@H]1OCc1ccccc1)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C22H26N2O4S/c25-17-11-10-15(21-20(17)24-22(27)29-21)18(26)12-23-16-8-4-5-9-19(16)28-13-14-6-2-1-3-7-14/h1-3,6-7,10-11,16,18-19,23,25-26H,4-5,8-9,12-13H2,(H,24,27)/t16-,18+,19-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151721

(CHEMBL363260 | N-(2-Hydroxy-5-{(R)-1-hydroxy-2-[2-...)Show SMILES O[C@@H](CNCCc1ccc(OCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C25H28N2O4/c28-18-27-23-16-21(8-11-24(23)29)25(30)17-26-14-12-20-6-9-22(10-7-20)31-15-13-19-4-2-1-3-5-19/h1-11,16,18,25-26,29-30H,12-15,17H2,(H,27,28)/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309487
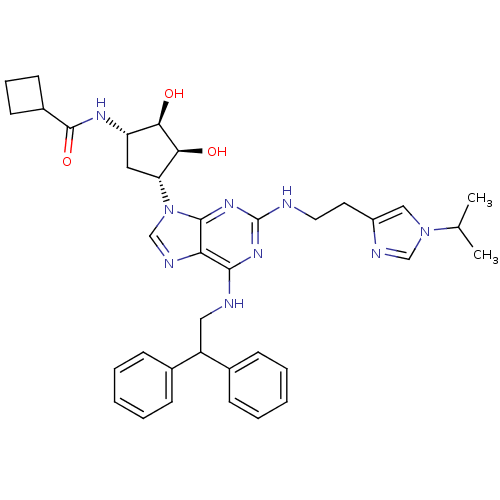
(CHEMBL601515 | N-((2S,3S,4R,5R)-5-(6-(2,2-diphenyl...)Show SMILES CC(C)n1cnc(CCNc2nc(NCC(c3ccccc3)c3ccccc3)c3ncn([C@@H]4C[C@H](NC(=O)C5CCC5)[C@@H](O)[C@H]4O)c3n2)c1 |r| Show InChI InChI=1S/C37H45N9O3/c1-23(2)45-20-27(40-21-45)16-17-38-37-43-34(39-19-28(24-10-5-3-6-11-24)25-12-7-4-8-13-25)31-35(44-37)46(22-41-31)30-18-29(32(47)33(30)48)42-36(49)26-14-9-15-26/h3-8,10-13,20-23,26,28-30,32-33,47-48H,9,14-19H2,1-2H3,(H,42,49)(H2,38,39,43,44)/t29-,30+,32+,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50309482
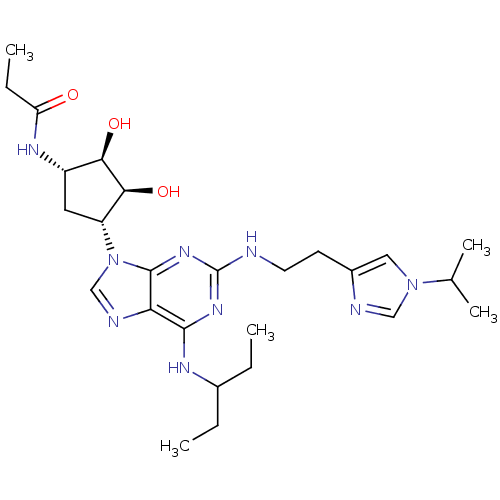
(CHEMBL592540 | N-((2S,3S,4R,5R)-3,4-dihydroxy-5-(2...)Show SMILES CCC(CC)Nc1nc(NCCc2cn(cn2)C(C)C)nc2n(cnc12)[C@@H]1C[C@H](NC(=O)CC)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C26H41N9O3/c1-6-16(7-2)30-24-21-25(33-26(32-24)27-10-9-17-12-34(13-28-17)15(4)5)35(14-29-21)19-11-18(22(37)23(19)38)31-20(36)8-3/h12-16,18-19,22-23,37-38H,6-11H2,1-5H3,(H,31,36)(H2,27,30,32,33)/t18-,19+,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]NECA from human recombinant adenosine A2A receptor expressed in Sf21 cells co-expressing GalphaS2, beta4, gamma2 |
Bioorg Med Chem Lett 20: 1219-24 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.131
BindingDB Entry DOI: 10.7270/Q2X92BDC |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324835

((R)-4-hydroxy-7-(1-hydroxy-2-(3-(methyl(phenyl)ami...)Show SMILES CN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12)c1ccccc1 |r| Show InChI InChI=1S/C19H23N3O3S/c1-22(13-6-3-2-4-7-13)11-5-10-20-12-16(24)14-8-9-15(23)17-18(14)26-19(25)21-17/h2-4,6-9,16,20,23-24H,5,10-12H2,1H3,(H,21,25)/t16-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151718
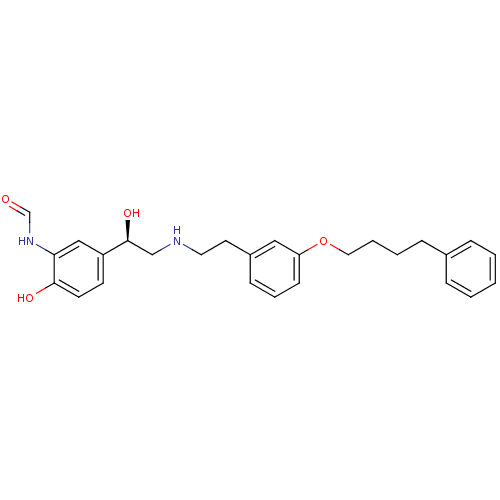
(CHEMBL440561 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{2-...)Show SMILES O[C@@H](CNCCc1cccc(OCCCCc2ccccc2)c1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C27H32N2O4/c30-20-29-25-18-23(12-13-26(25)31)27(32)19-28-15-14-22-10-6-11-24(17-22)33-16-5-4-9-21-7-2-1-3-8-21/h1-3,6-8,10-13,17-18,20,27-28,31-32H,4-5,9,14-16,19H2,(H,29,30)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324842

((R)-4-hydroxy-7-(1-hydroxy-2-(2,4,4-trimethylpenta...)Show SMILES CC(C)(C)CC(C)(C)NC[C@H](O)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C17H26N2O3S/c1-16(2,3)9-17(4,5)18-8-12(21)10-6-7-11(20)13-14(10)23-15(22)19-13/h6-7,12,18,20-21H,8-9H2,1-5H3,(H,19,22)/t12-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta-1 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50151723
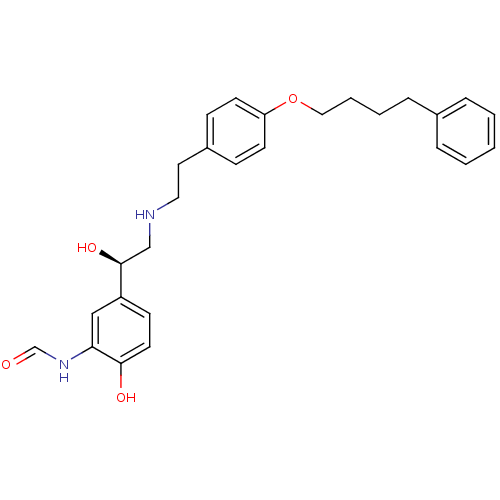
(CHEMBL185052 | N-[2-Hydroxy-5-((R)-1-hydroxy-2-{2-...)Show SMILES O[C@@H](CNCCc1ccc(OCCCCc2ccccc2)cc1)c1ccc(O)c(NC=O)c1 Show InChI InChI=1S/C27H32N2O4/c30-20-29-25-18-23(11-14-26(25)31)27(32)19-28-16-15-22-9-12-24(13-10-22)33-17-5-4-8-21-6-2-1-3-7-21/h1-3,6-7,9-14,18,20,27-28,31-32H,4-5,8,15-17,19H2,(H,29,30)/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Horsham Research Centre
Curated by ChEMBL
| Assay Description
Binding affinity for human beta-2 adrenergic receptor |
Bioorg Med Chem Lett 14: 4705-10 (2004)
Article DOI: 10.1016/j.bmcl.2004.06.086
BindingDB Entry DOI: 10.7270/Q2416XSZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50324848
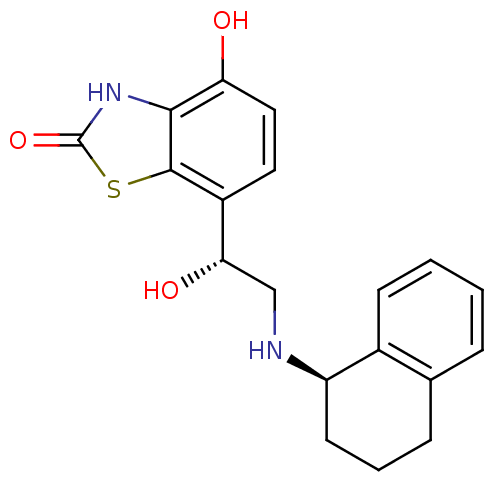
(4-hydroxy-7-((R)-1-hydroxy-2-((R)-1,2,3,4-tetrahyd...)Show SMILES O[C@@H](CN[C@@H]1CCCc2ccccc12)c1ccc(O)c2[nH]c(=O)sc12 |r| Show InChI InChI=1S/C19H20N2O3S/c22-15-9-8-13(18-17(15)21-19(24)25-18)16(23)10-20-14-7-3-5-11-4-1-2-6-12(11)14/h1-2,4,6,8-9,14,16,20,22-23H,3,5,7,10H2,(H,21,24)/t14-,16+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]CGP12177 from human beta2 adrenoceptor |
Bioorg Med Chem Lett 20: 5302-7 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.136
BindingDB Entry DOI: 10.7270/Q2HD7VVM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data