 Found 215 hits with Last Name = 'gembus' and Initial = 'v'
Found 215 hits with Last Name = 'gembus' and Initial = 'v' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180022
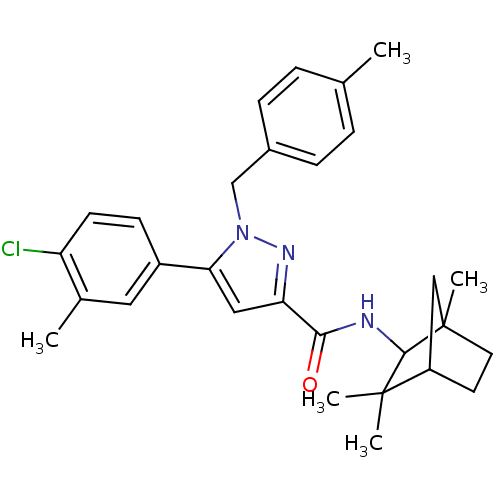
(5-(4-chloro-3-methyl-phenyl)-1-(4-methyl-benzyl)-1...)Show SMILES Cc1ccc(Cn2nc(cc2-c2ccc(Cl)c(C)c2)C(=O)NC2C3(C)CCC(C3)C2(C)C)cc1 |THB:21:22:26.25:28| Show InChI InChI=1S/C29H34ClN3O/c1-18-6-8-20(9-7-18)17-33-25(21-10-11-23(30)19(2)14-21)15-24(32-33)26(34)31-27-28(3,4)22-12-13-29(27,5)16-22/h6-11,14-15,22,27H,12-13,16-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 21.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21281

((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...)Show SMILES Cc1c(C(=O)c2cccc3ccccc23)c2cccc3OC[C@@H](CN4CCOCC4)n1c23 |r| Show InChI InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 52.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392711
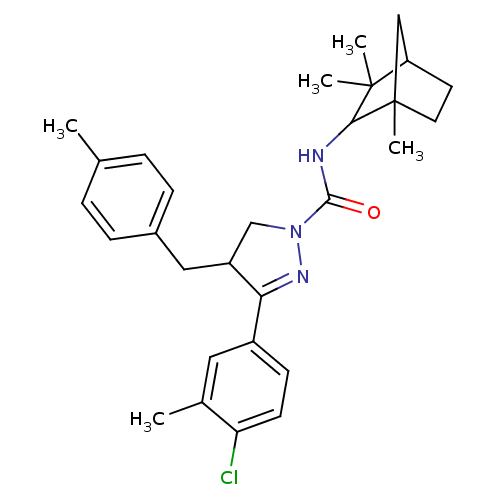
(CHEMBL2151380)Show SMILES Cc1ccc(CC2CN(N=C2c2ccc(Cl)c(C)c2)C(=O)NC2C(C)(C)C3CCC2(C)C3)cc1 |c:9| Show InChI InChI=1S/C29H36ClN3O/c1-18-6-8-20(9-7-18)15-22-17-33(32-25(22)21-10-11-24(30)19(2)14-21)27(34)31-26-28(3,4)23-12-13-29(26,5)16-23/h6-11,14,22-23,26H,12-13,15-17H2,1-5H3,(H,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392710
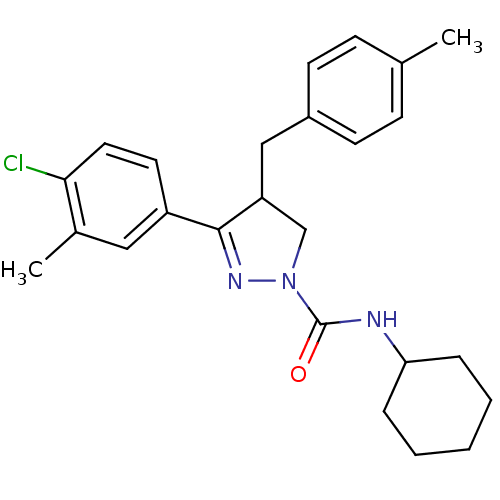
(CHEMBL2151379)Show SMILES Cc1ccc(CC2CN(N=C2c2ccc(Cl)c(C)c2)C(=O)NC2CCCCC2)cc1 |c:9| Show InChI InChI=1S/C25H30ClN3O/c1-17-8-10-19(11-9-17)15-21-16-29(25(30)27-22-6-4-3-5-7-22)28-24(21)20-12-13-23(26)18(2)14-20/h8-14,21-22H,3-7,15-16H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392701
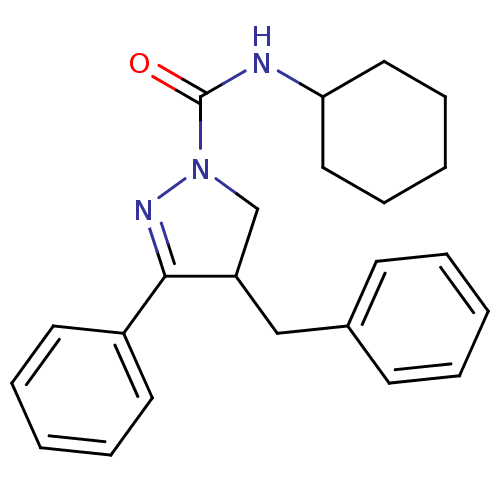
(CHEMBL2151370)Show SMILES O=C(NC1CCCCC1)N1CC(Cc2ccccc2)C(=N1)c1ccccc1 |c:21| Show InChI InChI=1S/C23H27N3O/c27-23(24-21-14-8-3-9-15-21)26-17-20(16-18-10-4-1-5-11-18)22(25-26)19-12-6-2-7-13-19/h1-2,4-7,10-13,20-21H,3,8-9,14-17H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392708
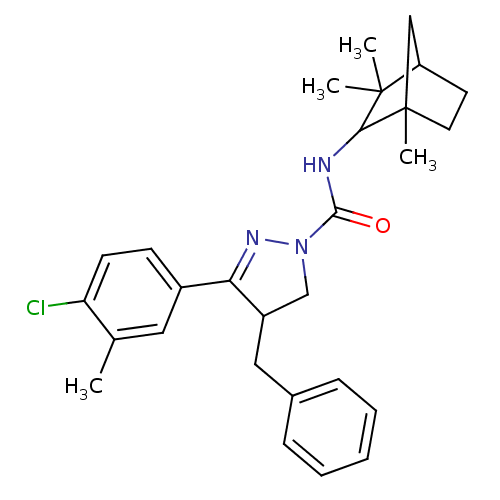
(CHEMBL2151377)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1Cc1ccccc1)C(=O)NC1C(C)(C)C2CCC1(C)C2 |t:9| Show InChI InChI=1S/C28H34ClN3O/c1-18-14-20(10-11-23(18)29)24-21(15-19-8-6-5-7-9-19)17-32(31-24)26(33)30-25-27(2,3)22-12-13-28(25,4)16-22/h5-11,14,21-22,25H,12-13,15-17H2,1-4H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 689 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392708

(CHEMBL2151377)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1Cc1ccccc1)C(=O)NC1C(C)(C)C2CCC1(C)C2 |t:9| Show InChI InChI=1S/C28H34ClN3O/c1-18-14-20(10-11-23(18)29)24-21(15-19-8-6-5-7-9-19)17-32(31-24)26(33)30-25-27(2,3)22-12-13-28(25,4)16-22/h5-11,14,21-22,25H,12-13,15-17H2,1-4H3,(H,30,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392709
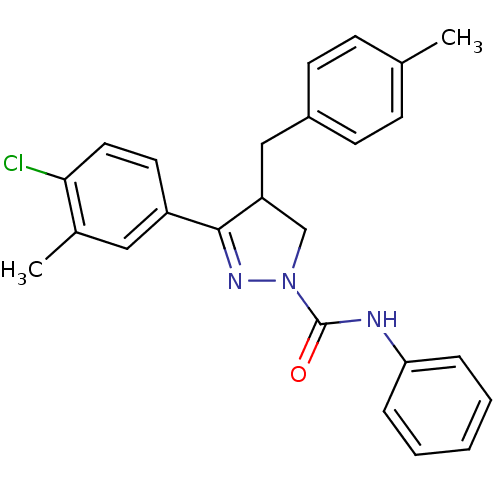
(CHEMBL2151378)Show SMILES Cc1ccc(CC2CN(N=C2c2ccc(Cl)c(C)c2)C(=O)Nc2ccccc2)cc1 |c:9| Show InChI InChI=1S/C25H24ClN3O/c1-17-8-10-19(11-9-17)15-21-16-29(25(30)27-22-6-4-3-5-7-22)28-24(21)20-12-13-23(26)18(2)14-20/h3-14,21H,15-16H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392707
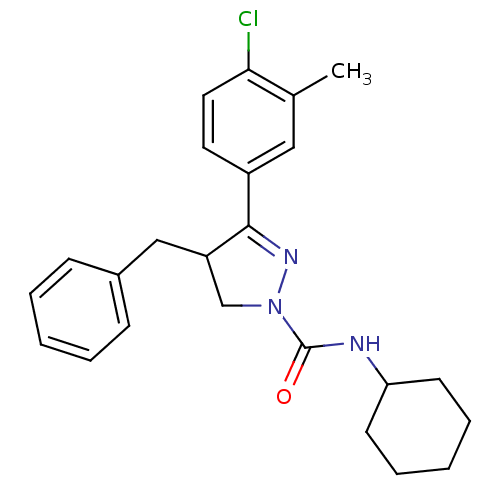
(CHEMBL2151376)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1Cc1ccccc1)C(=O)NC1CCCCC1 |t:9| Show InChI InChI=1S/C24H28ClN3O/c1-17-14-19(12-13-22(17)25)23-20(15-18-8-4-2-5-9-18)16-28(27-23)24(29)26-21-10-6-3-7-11-21/h2,4-5,8-9,12-14,20-21H,3,6-7,10-11,15-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392711

(CHEMBL2151380)Show SMILES Cc1ccc(CC2CN(N=C2c2ccc(Cl)c(C)c2)C(=O)NC2C(C)(C)C3CCC2(C)C3)cc1 |c:9| Show InChI InChI=1S/C29H36ClN3O/c1-18-6-8-20(9-7-18)15-22-17-33(32-25(22)21-10-11-24(30)19(2)14-21)27(34)31-26-28(3,4)23-12-13-29(26,5)16-23/h6-11,14,22-23,26H,12-13,15-17H2,1-5H3,(H,31,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392699
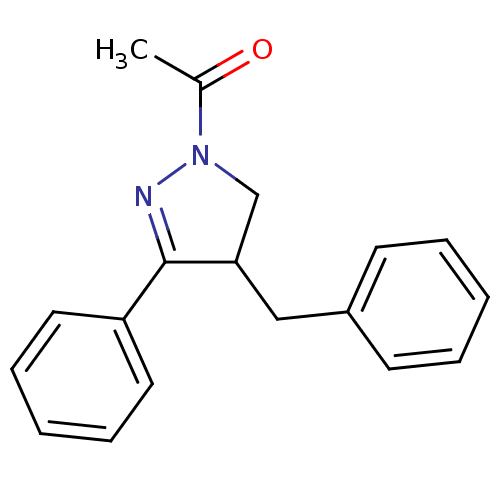
(CHEMBL2151368)Show InChI InChI=1S/C18H18N2O/c1-14(21)20-13-17(12-15-8-4-2-5-9-15)18(19-20)16-10-6-3-7-11-16/h2-11,17H,12-13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392700
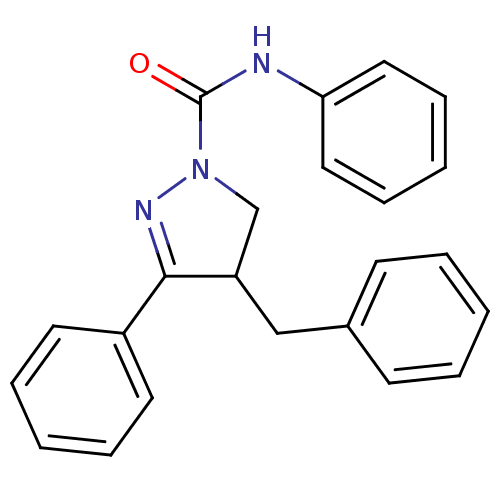
(CHEMBL2151369)Show SMILES O=C(Nc1ccccc1)N1CC(Cc2ccccc2)C(=N1)c1ccccc1 |c:21| Show InChI InChI=1S/C23H21N3O/c27-23(24-21-14-8-3-9-15-21)26-17-20(16-18-10-4-1-5-11-18)22(25-26)19-12-6-2-7-13-19/h1-15,20H,16-17H2,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392702
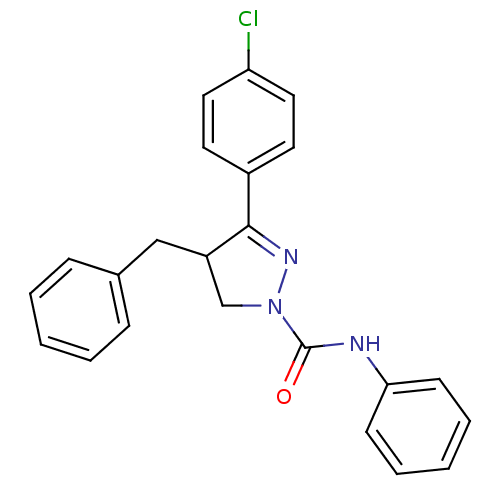
(CHEMBL2151371)Show SMILES Clc1ccc(cc1)C1=NN(CC1Cc1ccccc1)C(=O)Nc1ccccc1 |t:8| Show InChI InChI=1S/C23H20ClN3O/c24-20-13-11-18(12-14-20)22-19(15-17-7-3-1-4-8-17)16-27(26-22)23(28)25-21-9-5-2-6-10-21/h1-14,19H,15-16H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392703
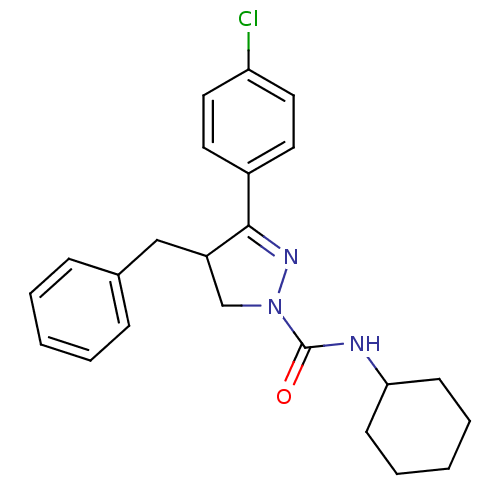
(CHEMBL2151372)Show SMILES Clc1ccc(cc1)C1=NN(CC1Cc1ccccc1)C(=O)NC1CCCCC1 |t:8| Show InChI InChI=1S/C23H26ClN3O/c24-20-13-11-18(12-14-20)22-19(15-17-7-3-1-4-8-17)16-27(26-22)23(28)25-21-9-5-2-6-10-21/h1,3-4,7-8,11-14,19,21H,2,5-6,9-10,15-16H2,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392704
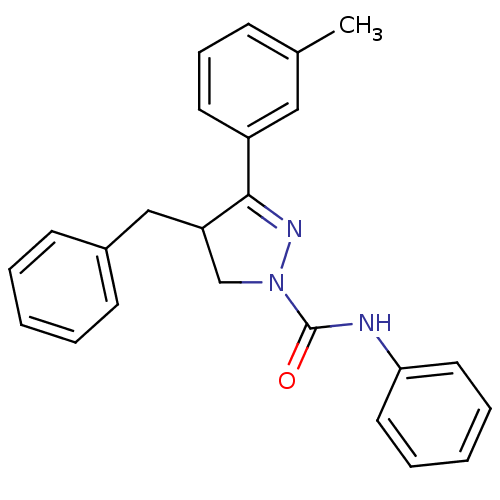
(CHEMBL2151373)Show SMILES Cc1cccc(c1)C1=NN(CC1Cc1ccccc1)C(=O)Nc1ccccc1 |t:8| Show InChI InChI=1S/C24H23N3O/c1-18-9-8-12-20(15-18)23-21(16-19-10-4-2-5-11-19)17-27(26-23)24(28)25-22-13-6-3-7-14-22/h2-15,21H,16-17H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392705
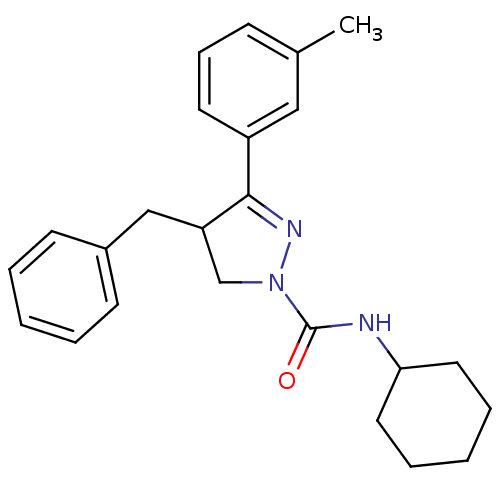
(CHEMBL2151374)Show SMILES Cc1cccc(c1)C1=NN(CC1Cc1ccccc1)C(=O)NC1CCCCC1 |t:8| Show InChI InChI=1S/C24H29N3O/c1-18-9-8-12-20(15-18)23-21(16-19-10-4-2-5-11-19)17-27(26-23)24(28)25-22-13-6-3-7-14-22/h2,4-5,8-12,15,21-22H,3,6-7,13-14,16-17H2,1H3,(H,25,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392712
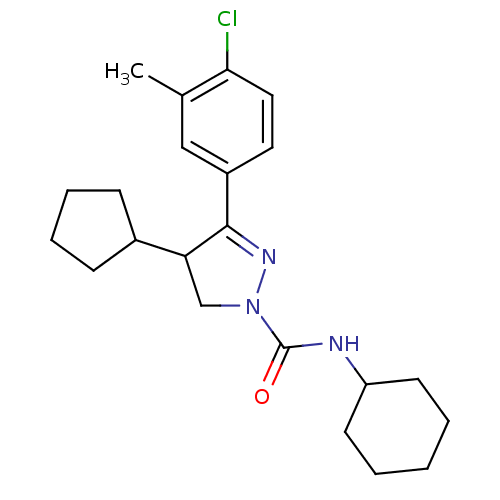
(CHEMBL2151381)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1C1CCCC1)C(=O)NC1CCCCC1 |t:9| Show InChI InChI=1S/C22H30ClN3O/c1-15-13-17(11-12-20(15)23)21-19(16-7-5-6-8-16)14-26(25-21)22(27)24-18-9-3-2-4-10-18/h11-13,16,18-19H,2-10,14H2,1H3,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392710

(CHEMBL2151379)Show SMILES Cc1ccc(CC2CN(N=C2c2ccc(Cl)c(C)c2)C(=O)NC2CCCCC2)cc1 |c:9| Show InChI InChI=1S/C25H30ClN3O/c1-17-8-10-19(11-9-17)15-21-16-29(25(30)27-22-6-4-3-5-7-22)28-24(21)20-12-13-23(26)18(2)14-20/h8-14,21-22H,3-7,15-16H2,1-2H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392709

(CHEMBL2151378)Show SMILES Cc1ccc(CC2CN(N=C2c2ccc(Cl)c(C)c2)C(=O)Nc2ccccc2)cc1 |c:9| Show InChI InChI=1S/C25H24ClN3O/c1-17-8-10-19(11-9-17)15-21-16-29(25(30)27-22-6-4-3-5-7-22)28-24(21)20-12-13-23(26)18(2)14-20/h3-14,21H,15-16H2,1-2H3,(H,27,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392707

(CHEMBL2151376)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1Cc1ccccc1)C(=O)NC1CCCCC1 |t:9| Show InChI InChI=1S/C24H28ClN3O/c1-17-14-19(12-13-22(17)25)23-20(15-18-8-4-2-5-9-18)16-28(27-23)24(29)26-21-10-6-3-7-11-21/h2,4-5,8-9,12-14,20-21H,3,6-7,10-11,15-16H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392706
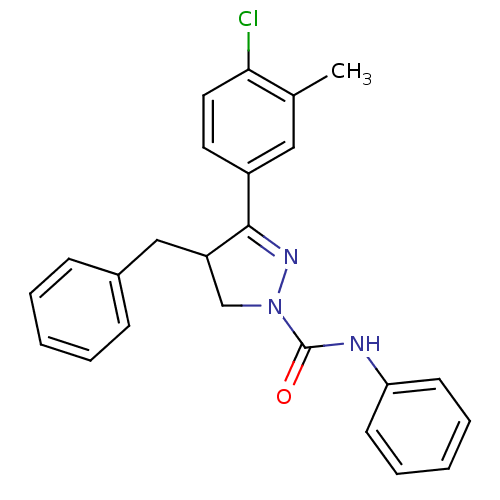
(CHEMBL2151375)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1Cc1ccccc1)C(=O)Nc1ccccc1 |t:9| Show InChI InChI=1S/C24H22ClN3O/c1-17-14-19(12-13-22(17)25)23-20(15-18-8-4-2-5-9-18)16-28(27-23)24(29)26-21-10-6-3-7-11-21/h2-14,20H,15-16H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392705

(CHEMBL2151374)Show SMILES Cc1cccc(c1)C1=NN(CC1Cc1ccccc1)C(=O)NC1CCCCC1 |t:8| Show InChI InChI=1S/C24H29N3O/c1-18-9-8-12-20(15-18)23-21(16-19-10-4-2-5-11-19)17-27(26-23)24(28)25-22-13-6-3-7-14-22/h2,4-5,8-12,15,21-22H,3,6-7,13-14,16-17H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392704

(CHEMBL2151373)Show SMILES Cc1cccc(c1)C1=NN(CC1Cc1ccccc1)C(=O)Nc1ccccc1 |t:8| Show InChI InChI=1S/C24H23N3O/c1-18-9-8-12-20(15-18)23-21(16-19-10-4-2-5-11-19)17-27(26-23)24(28)25-22-13-6-3-7-14-22/h2-15,21H,16-17H2,1H3,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392703

(CHEMBL2151372)Show SMILES Clc1ccc(cc1)C1=NN(CC1Cc1ccccc1)C(=O)NC1CCCCC1 |t:8| Show InChI InChI=1S/C23H26ClN3O/c24-20-13-11-18(12-14-20)22-19(15-17-7-3-1-4-8-17)16-27(26-22)23(28)25-21-9-5-2-6-10-21/h1,3-4,7-8,11-14,19,21H,2,5-6,9-10,15-16H2,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392702

(CHEMBL2151371)Show SMILES Clc1ccc(cc1)C1=NN(CC1Cc1ccccc1)C(=O)Nc1ccccc1 |t:8| Show InChI InChI=1S/C23H20ClN3O/c24-20-13-11-18(12-14-20)22-19(15-17-7-3-1-4-8-17)16-27(26-22)23(28)25-21-9-5-2-6-10-21/h1-14,19H,15-16H2,(H,25,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392701

(CHEMBL2151370)Show SMILES O=C(NC1CCCCC1)N1CC(Cc2ccccc2)C(=N1)c1ccccc1 |c:21| Show InChI InChI=1S/C23H27N3O/c27-23(24-21-14-8-3-9-15-21)26-17-20(16-18-10-4-1-5-11-18)22(25-26)19-12-6-2-7-13-19/h1-2,4-7,10-13,20-21H,3,8-9,14-17H2,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392699

(CHEMBL2151368)Show InChI InChI=1S/C18H18N2O/c1-14(21)20-13-17(12-15-8-4-2-5-9-15)18(19-20)16-10-6-3-7-11-16/h2-11,17H,12-13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50392700

(CHEMBL2151369)Show SMILES O=C(Nc1ccccc1)N1CC(Cc2ccccc2)C(=N1)c1ccccc1 |c:21| Show InChI InChI=1S/C23H21N3O/c27-23(24-21-14-8-3-9-15-21)26-17-20(16-18-10-4-1-5-11-18)22(25-26)19-12-6-2-7-13-19/h1-15,20H,16-17H2,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392706

(CHEMBL2151375)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1Cc1ccccc1)C(=O)Nc1ccccc1 |t:9| Show InChI InChI=1S/C24H22ClN3O/c1-17-14-19(12-13-22(17)25)23-20(15-18-8-4-2-5-9-18)16-28(27-23)24(29)26-21-10-6-3-7-11-21/h2-14,20H,15-16H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50392712

(CHEMBL2151381)Show SMILES Cc1cc(ccc1Cl)C1=NN(CC1C1CCCC1)C(=O)NC1CCCCC1 |t:9| Show InChI InChI=1S/C22H30ClN3O/c1-15-13-17(11-12-20(15)23)21-19(16-7-5-6-8-16)14-26(25-21)22(27)24-18-9-3-2-4-10-18/h11-13,16,18-19H,2-10,14H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Avenue de l'Universit£
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO cell membranes after 1 hr by scintillation counting |
Eur J Med Chem 58: 396-404 (2012)
Article DOI: 10.1016/j.ejmech.2012.10.031
BindingDB Entry DOI: 10.7270/Q2474BZG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50291665
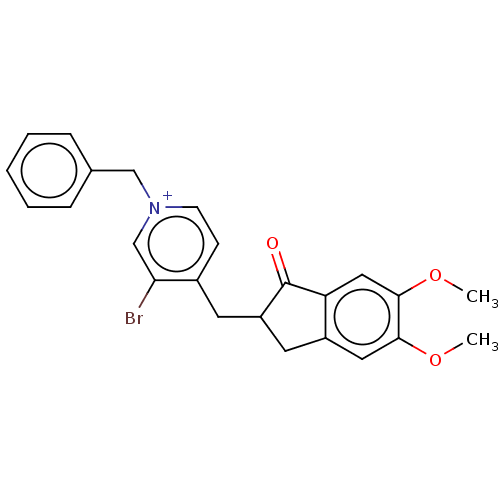
(CHEMBL4165327)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4ccccc4)cc3Br)C(=O)c2cc1OC Show InChI InChI=1S/C24H23BrNO3.BrH/c1-28-22-12-18-11-19(24(27)20(18)13-23(22)29-2)10-17-8-9-26(15-21(17)25)14-16-6-4-3-5-7-16;/h3-9,12-13,15,19H,10-11,14H2,1-2H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50291432
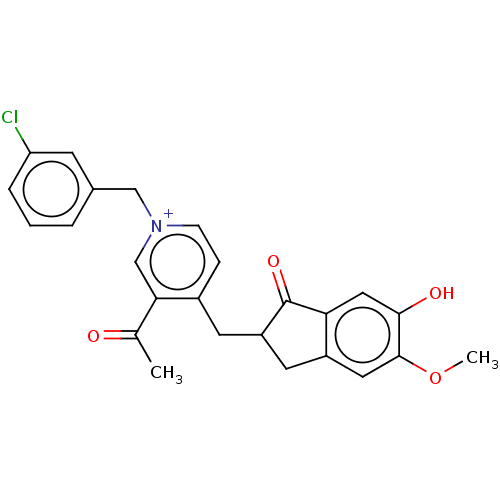
(CHEMBL4159705)Show SMILES COc1cc2CC(Cc3cc[n+](Cc4cccc(Cl)c4)cc3C(C)=O)C(=O)c2cc1O Show InChI InChI=1S/C25H22ClNO4/c1-15(28)22-14-27(13-16-4-3-5-20(26)8-16)7-6-17(22)9-19-10-18-11-24(31-2)23(29)12-21(18)25(19)30/h3-8,11-12,14,19H,9-10,13H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238227
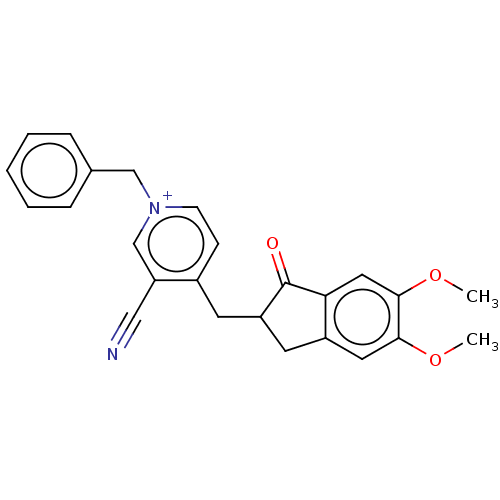
(CHEMBL4071377)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4ccccc4)cc3C#N)C(=O)c2cc1OC Show InChI InChI=1S/C25H23N2O3.BrH/c1-29-23-12-19-11-20(25(28)22(19)13-24(23)30-2)10-18-8-9-27(16-21(18)14-26)15-17-6-4-3-5-7-17;/h3-9,12-13,16,20H,10-11,15H2,1-2H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cholinesterase
(Equus caballus (Horse)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured every mi... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238223
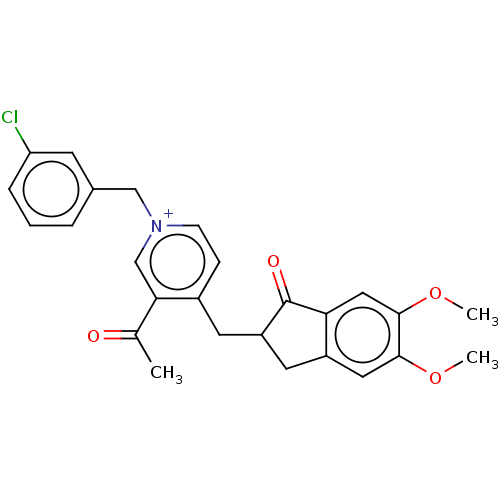
(CHEMBL4102333)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4cccc(Cl)c4)cc3C(C)=O)C(=O)c2cc1OC Show InChI InChI=1S/C26H25ClNO4.BrH/c1-16(29)23-15-28(14-17-5-4-6-21(27)9-17)8-7-18(23)10-20-11-19-12-24(31-2)25(32-3)13-22(19)26(20)30;/h4-9,12-13,15,20H,10-11,14H2,1-3H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238216
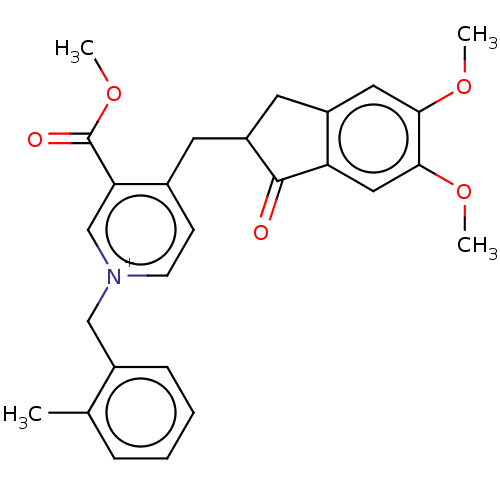
(CHEMBL4095535)Show SMILES [Br-].COC(=O)c1c[n+](Cc2ccccc2C)ccc1CC1Cc2cc(OC)c(OC)cc2C1=O Show InChI InChI=1S/C27H28NO5.BrH/c1-17-7-5-6-8-19(17)15-28-10-9-18(23(16-28)27(30)33-4)11-21-12-20-13-24(31-2)25(32-3)14-22(20)26(21)29;/h5-10,13-14,16,21H,11-12,15H2,1-4H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Bos taurus (bovine)) | BDBM50449479
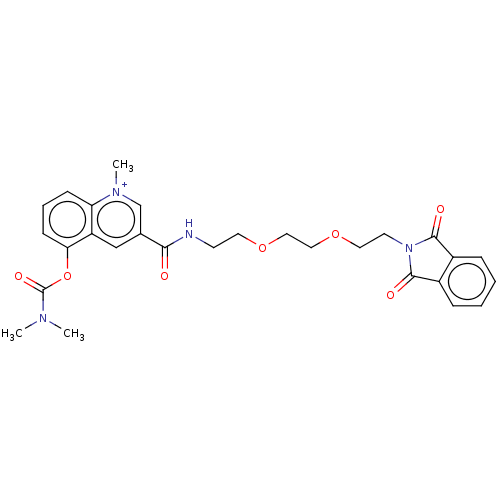
(CHEMBL4168520)Show SMILES [O-]S(=O)(=O)C(F)(F)F.CN(C)C(=O)Oc1cccc2[n+](C)cc(cc12)C(=O)NCCOCCOCCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C28H30N4O7/c1-30(2)28(36)39-24-10-6-9-23-22(24)17-19(18-31(23)3)25(33)29-11-13-37-15-16-38-14-12-32-26(34)20-7-4-5-8-21(20)27(32)35/h4-10,17-18H,11-16H2,1-3H3/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of bovine erythrocytes AChE using acetylthiocholine iodide as substrate by Ellman's method |
Eur J Med Chem 155: 171-182 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.057
BindingDB Entry DOI: 10.7270/Q2Q52S5C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50449479

(CHEMBL4168520)Show SMILES [O-]S(=O)(=O)C(F)(F)F.CN(C)C(=O)Oc1cccc2[n+](C)cc(cc12)C(=O)NCCOCCOCCN1C(=O)c2ccccc2C1=O Show InChI InChI=1S/C28H30N4O7/c1-30(2)28(36)39-24-10-6-9-23-22(24)17-19(18-31(23)3)25(33)29-11-13-37-15-16-38-14-12-32-26(34)20-7-4-5-8-21(20)27(32)35/h4-10,17-18H,11-16H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured eve... |
Eur J Med Chem 155: 171-182 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.057
BindingDB Entry DOI: 10.7270/Q2Q52S5C |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238235
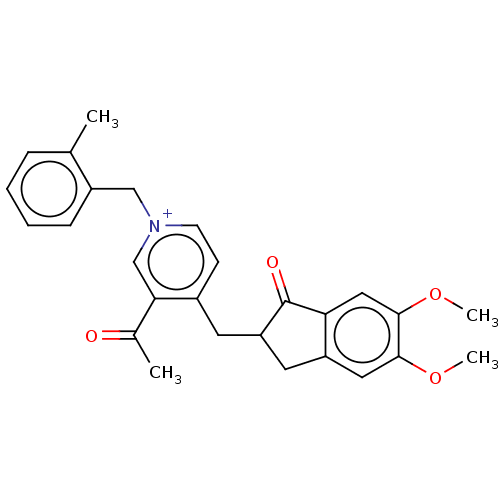
(CHEMBL4081915)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4ccccc4C)cc3C(C)=O)C(=O)c2cc1OC Show InChI InChI=1S/C27H28NO4.BrH/c1-17-7-5-6-8-20(17)15-28-10-9-19(24(16-28)18(2)29)11-22-12-21-13-25(31-3)26(32-4)14-23(21)27(22)30;/h5-10,13-14,16,22H,11-12,15H2,1-4H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50291438
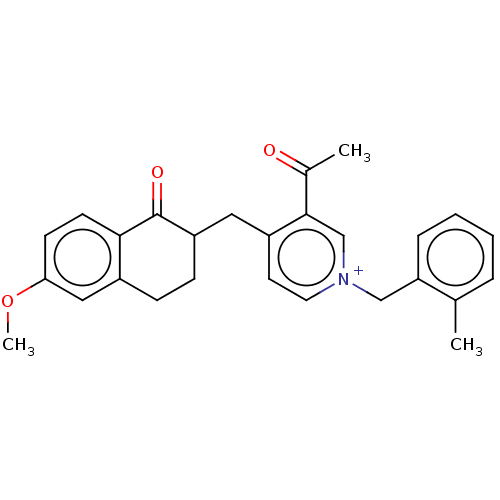
(CHEMBL4161065)Show SMILES [Br-].COc1ccc2C(=O)C(Cc3cc[n+](Cc4ccccc4C)cc3C(C)=O)CCc2c1 Show InChI InChI=1S/C27H28NO3.BrH/c1-18-6-4-5-7-23(18)16-28-13-12-21(26(17-28)19(2)29)14-22-9-8-20-15-24(31-3)10-11-25(20)27(22)30;/h4-7,10-13,15,17,22H,8-9,14,16H2,1-3H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50291612
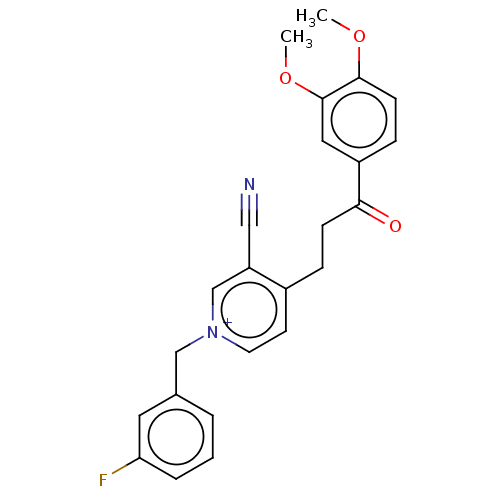
(CHEMBL4163678)Show SMILES [Br-].COc1ccc(cc1OC)C(=O)CCc1cc[n+](Cc2cccc(F)c2)cc1C#N Show InChI InChI=1S/C24H22FN2O3/c1-29-23-9-7-19(13-24(23)30-2)22(28)8-6-18-10-11-27(16-20(18)14-26)15-17-4-3-5-21(25)12-17/h3-5,7,9-13,16H,6,8,15H2,1-2H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238224
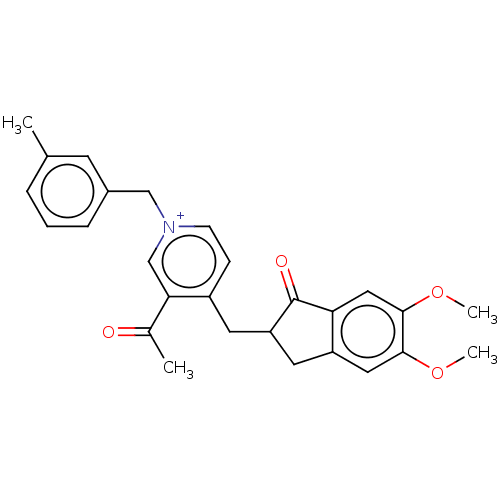
(CHEMBL4094081)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4cccc(C)c4)cc3C(C)=O)C(=O)c2cc1OC Show InChI InChI=1S/C27H28NO4.BrH/c1-17-6-5-7-19(10-17)15-28-9-8-20(24(16-28)18(2)29)11-22-12-21-13-25(31-3)26(32-4)14-23(21)27(22)30;/h5-10,13-14,16,22H,11-12,15H2,1-4H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50291664
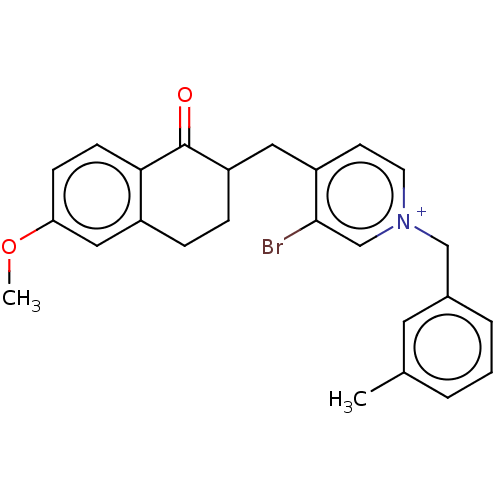
(CHEMBL4173239)Show SMILES [Br-].COc1ccc2C(=O)C(Cc3cc[n+](Cc4cccc(C)c4)cc3Br)CCc2c1 Show InChI InChI=1S/C25H25BrNO2.BrH/c1-17-4-3-5-18(12-17)15-27-11-10-20(24(26)16-27)13-21-7-6-19-14-22(29-2)8-9-23(19)25(21)28;/h3-5,8-12,14,16,21H,6-7,13,15H2,1-2H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50291633
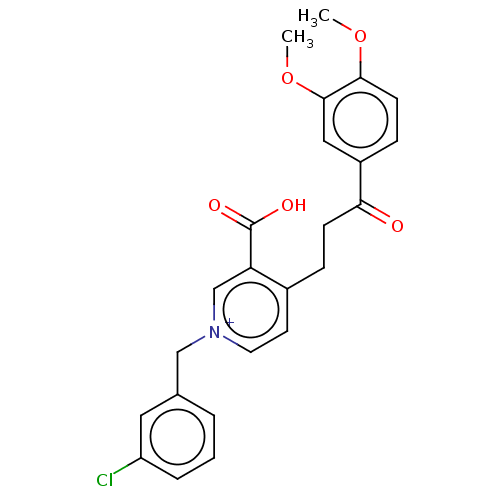
(CHEMBL4165973)Show SMILES [Br-].COc1ccc(cc1OC)C(=O)CCc1cc[n+](Cc2cccc(Cl)c2)cc1C(O)=O Show InChI InChI=1S/C24H22ClNO5/c1-30-22-9-7-18(13-23(22)31-2)21(27)8-6-17-10-11-26(15-20(17)24(28)29)14-16-4-3-5-19(25)12-16/h3-5,7,9-13,15H,6,8,14H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ever... |
Eur J Med Chem 145: 165-190 (2018)
Article DOI: 10.1016/j.ejmech.2017.12.084
BindingDB Entry DOI: 10.7270/Q2FN18RR |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238213
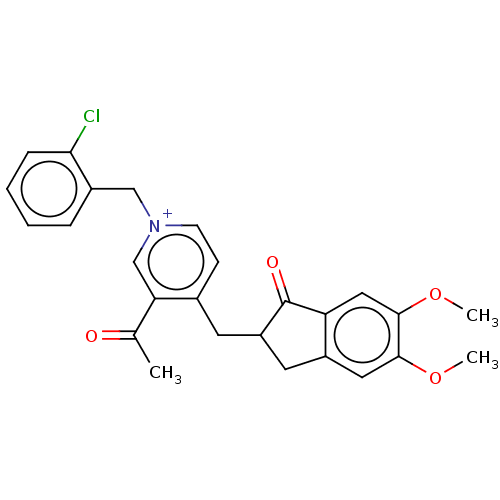
(CHEMBL4066548)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4ccccc4Cl)cc3C(C)=O)C(=O)c2cc1OC Show InChI InChI=1S/C26H25ClNO4.BrH/c1-16(29)22-15-28(14-18-6-4-5-7-23(18)27)9-8-17(22)10-20-11-19-12-24(31-2)25(32-3)13-21(19)26(20)30;/h4-9,12-13,15,20H,10-11,14H2,1-3H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238225
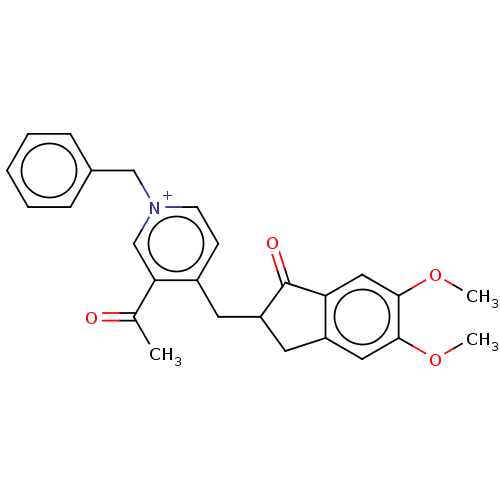
(CHEMBL4060011)Show SMILES [Br-].COc1cc2CC(Cc3cc[n+](Cc4ccccc4)cc3C(C)=O)C(=O)c2cc1OC Show InChI InChI=1S/C26H26NO4.BrH/c1-17(28)23-16-27(15-18-7-5-4-6-8-18)10-9-19(23)11-21-12-20-13-24(30-2)25(31-3)14-22(20)26(21)29;/h4-10,13-14,16,21H,11-12,15H2,1-3H3;1H/q+1;/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE in human erythrocytes using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured ... |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50238212
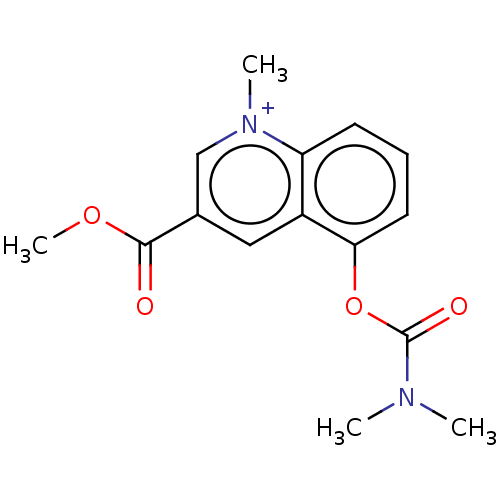
(CHEMBL4077952)Show InChI InChI=1S/C15H17N2O4/c1-16(2)15(19)21-13-7-5-6-12-11(13)8-10(9-17(12)3)14(18)20-4/h5-9H,1-4H3/q+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
VFP Therapies
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
J Med Chem 60: 5909-5926 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00702
BindingDB Entry DOI: 10.7270/Q22V2JC5 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50449485
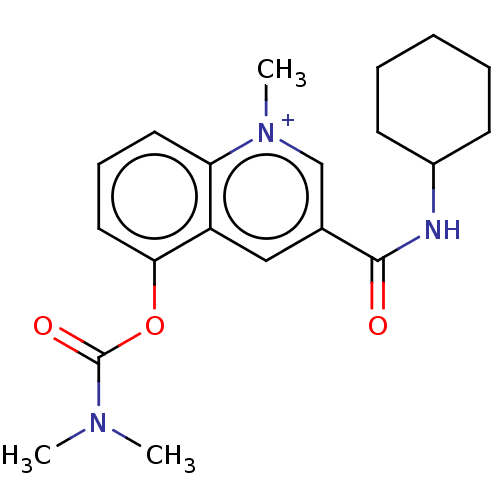
(CHEMBL4160280)Show SMILES [I-].CN(C)C(=O)Oc1cccc2[n+](C)cc(cc12)C(=O)NC1CCCCC1 Show InChI InChI=1S/C20H25N3O3/c1-22(2)20(25)26-18-11-7-10-17-16(18)12-14(13-23(17)3)19(24)21-15-8-5-4-6-9-15/h7,10-13,15H,4-6,8-9H2,1-3H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocytes AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured eve... |
Eur J Med Chem 155: 171-182 (2018)
Article DOI: 10.1016/j.ejmech.2018.05.057
BindingDB Entry DOI: 10.7270/Q2Q52S5C |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data