 Found 618 hits with Last Name = 'gemkow' and Initial = 'mj'
Found 618 hits with Last Name = 'gemkow' and Initial = 'mj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50234380

(CHEMBL245876 | quinolin-8-yl 4-methyl-3-(piperidin...)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCCCC1)C(=O)Oc1cccc2cccnc12 Show InChI InChI=1S/C22H22N2O4S/c1-16-10-11-18(15-20(16)29(26,27)24-13-3-2-4-14-24)22(25)28-19-9-5-7-17-8-6-12-23-21(17)19/h5-12,15H,2-4,13-14H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 1725-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.042
BindingDB Entry DOI: 10.7270/Q2X92B1F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50234380

(CHEMBL245876 | quinolin-8-yl 4-methyl-3-(piperidin...)Show SMILES Cc1ccc(cc1S(=O)(=O)N1CCCCC1)C(=O)Oc1cccc2cccnc12 Show InChI InChI=1S/C22H22N2O4S/c1-16-10-11-18(15-20(16)29(26,27)24-13-3-2-4-14-24)22(25)28-19-9-5-7-17-8-6-12-23-21(17)19/h5-12,15H,2-4,13-14H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd.
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK cells |
Bioorg Med Chem Lett 18: 1725-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.042
BindingDB Entry DOI: 10.7270/Q2X92B1F |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50257851

((S)-4-((2',3'-dichlorobiphenyl-4-yl)methyl)-2-phen...)Show SMILES Clc1cccc(c1Cl)-c1ccc(CN2CCO[C@H](C2)c2ccccc2)cc1 |r| Show InChI InChI=1S/C23H21Cl2NO/c24-21-8-4-7-20(23(21)25)18-11-9-17(10-12-18)15-26-13-14-27-22(16-26)19-5-2-1-3-6-19/h1-12,22H,13-16H2/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Win55212 form human CB2 receptor transfected in HEK cells |
Bioorg Med Chem Lett 19: 1604-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.033
BindingDB Entry DOI: 10.7270/Q2K937DD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50257851

((S)-4-((2',3'-dichlorobiphenyl-4-yl)methyl)-2-phen...)Show SMILES Clc1cccc(c1Cl)-c1ccc(CN2CCO[C@H](C2)c2ccccc2)cc1 |r| Show InChI InChI=1S/C23H21Cl2NO/c24-21-8-4-7-20(23(21)25)18-11-9-17(10-12-18)15-26-13-14-27-22(16-26)19-5-2-1-3-6-19/h1-12,22H,13-16H2/t22-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 312 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 form human CB2 receptor transfected in HEK cells |
Bioorg Med Chem Lett 19: 1604-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.033
BindingDB Entry DOI: 10.7270/Q2K937DD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50258007
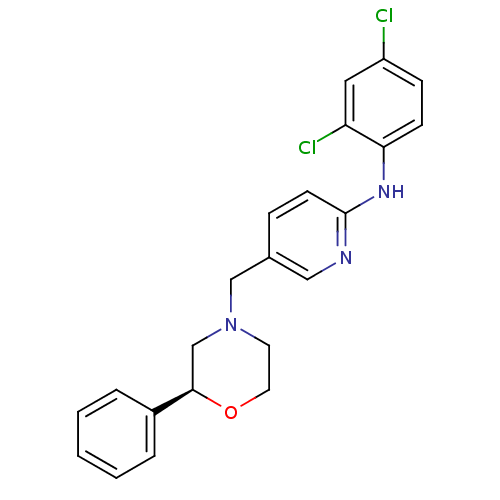
((S)-N-(2,4-dichlorophenyl)-5-((2-phenylmorpholino)...)Show SMILES Clc1ccc(Nc2ccc(CN3CCO[C@H](C3)c3ccccc3)cn2)c(Cl)c1 |r| Show InChI InChI=1S/C22H21Cl2N3O/c23-18-7-8-20(19(24)12-18)26-22-9-6-16(13-25-22)14-27-10-11-28-21(15-27)17-4-2-1-3-5-17/h1-9,12-13,21H,10-11,14-15H2,(H,25,26)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Win55212 form human CB2 receptor transfected in HEK cells |
Bioorg Med Chem Lett 19: 1604-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.033
BindingDB Entry DOI: 10.7270/Q2K937DD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50211843
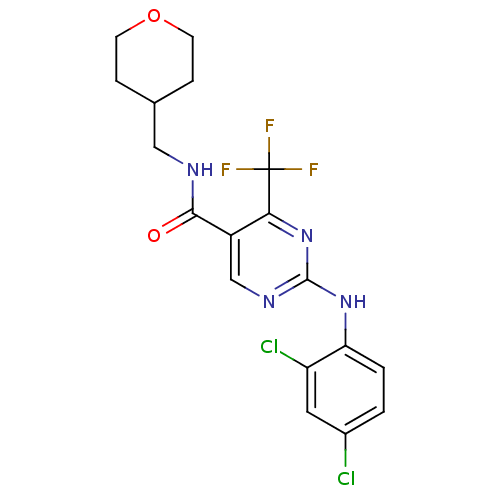
(2-(2,4-dichlorophenylamino)-4-trifluoromethyl-pyri...)Show SMILES FC(F)(F)c1nc(Nc2ccc(Cl)cc2Cl)ncc1C(=O)NCC1CCOCC1 Show InChI InChI=1S/C18H17Cl2F3N4O2/c19-11-1-2-14(13(20)7-11)26-17-25-9-12(15(27-17)18(21,22)23)16(28)24-8-10-3-5-29-6-4-10/h1-2,7,9-10H,3-6,8H2,(H,24,28)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 form human CB2 receptor transfected in HEK cells |
Bioorg Med Chem Lett 19: 1604-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.033
BindingDB Entry DOI: 10.7270/Q2K937DD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50258007

((S)-N-(2,4-dichlorophenyl)-5-((2-phenylmorpholino)...)Show SMILES Clc1ccc(Nc2ccc(CN3CCO[C@H](C3)c3ccccc3)cn2)c(Cl)c1 |r| Show InChI InChI=1S/C22H21Cl2N3O/c23-18-7-8-20(19(24)12-18)26-22-9-6-16(13-25-22)14-27-10-11-28-21(15-27)17-4-2-1-3-5-17/h1-9,12-13,21H,10-11,14-15H2,(H,25,26)/t21-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 form human CB2 receptor transfected in HEK cells |
Bioorg Med Chem Lett 19: 1604-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.033
BindingDB Entry DOI: 10.7270/Q2K937DD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50211843

(2-(2,4-dichlorophenylamino)-4-trifluoromethyl-pyri...)Show SMILES FC(F)(F)c1nc(Nc2ccc(Cl)cc2Cl)ncc1C(=O)NCC1CCOCC1 Show InChI InChI=1S/C18H17Cl2F3N4O2/c19-11-1-2-14(13(20)7-11)26-17-25-9-12(15(27-17)18(21,22)23)16(28)24-8-10-3-5-29-6-4-10/h1-2,7,9-10H,3-6,8H2,(H,24,28)(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]Win55212 form human CB2 receptor transfected in HEK cells |
Bioorg Med Chem Lett 19: 1604-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.033
BindingDB Entry DOI: 10.7270/Q2K937DD |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325090
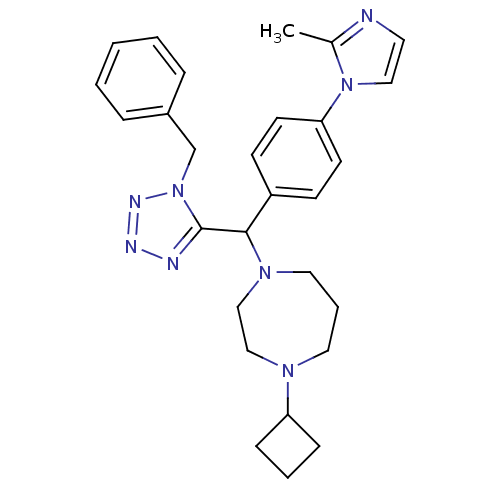
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(2-methyl-1H-imid...)Show SMILES Cc1nccn1-c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C28H34N8/c1-22-29-15-18-35(22)26-13-11-24(12-14-26)27(34-17-6-16-33(19-20-34)25-9-5-10-25)28-30-31-32-36(28)21-23-7-3-2-4-8-23/h2-4,7-8,11-15,18,25,27H,5-6,9-10,16-17,19-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325082
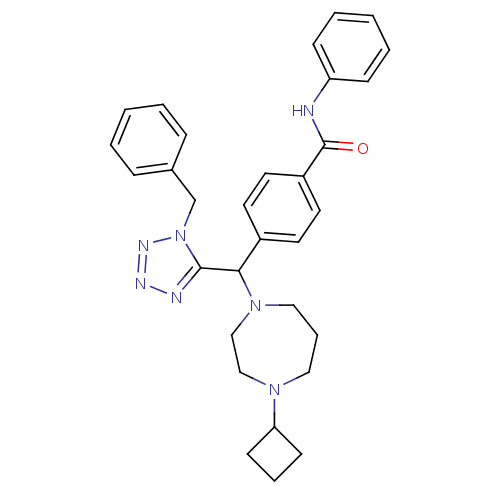
(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES O=C(Nc1ccccc1)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C31H35N7O/c39-31(32-27-11-5-2-6-12-27)26-17-15-25(16-18-26)29(37-20-8-19-36(21-22-37)28-13-7-14-28)30-33-34-35-38(30)23-24-9-3-1-4-10-24/h1-6,9-12,15-18,28-29H,7-8,13-14,19-23H2,(H,32,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325092
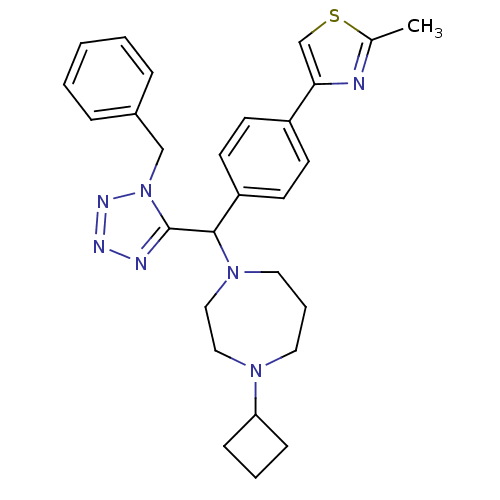
(4-(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4...)Show SMILES Cc1nc(cs1)-c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C28H33N7S/c1-21-29-26(20-36-21)23-11-13-24(14-12-23)27(34-16-6-15-33(17-18-34)25-9-5-10-25)28-30-31-32-35(28)19-22-7-3-2-4-8-22/h2-4,7-8,11-14,20,25,27H,5-6,9-10,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325065
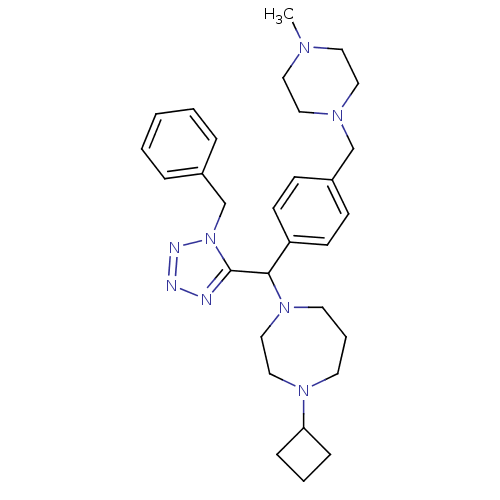
(1-((1-benzyl-1H-tetrazol-5-yl)(4-((4-methylpiperaz...)Show SMILES CN1CCN(Cc2ccc(cc2)C(N2CCCN(CC2)C2CCC2)c2nnnn2Cc2ccccc2)CC1 Show InChI InChI=1S/C30H42N8/c1-34-17-19-35(20-18-34)23-26-11-13-27(14-12-26)29(37-16-6-15-36(21-22-37)28-9-5-10-28)30-31-32-33-38(30)24-25-7-3-2-4-8-25/h2-4,7-8,11-14,28-29H,5-6,9-10,15-24H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50325065

(1-((1-benzyl-1H-tetrazol-5-yl)(4-((4-methylpiperaz...)Show SMILES CN1CCN(Cc2ccc(cc2)C(N2CCCN(CC2)C2CCC2)c2nnnn2Cc2ccccc2)CC1 Show InChI InChI=1S/C30H42N8/c1-34-17-19-35(20-18-34)23-26-11-13-27(14-12-26)29(37-16-6-15-36(21-22-37)28-9-5-10-28)30-31-32-33-38(30)24-25-7-3-2-4-8-25/h2-4,7-8,11-14,28-29H,5-6,9-10,15-24H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325087
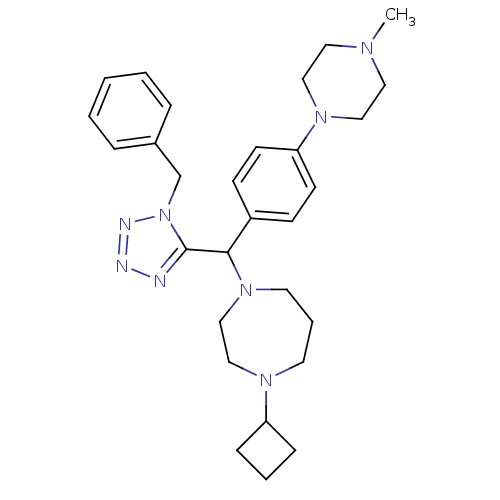
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(4-methylpiperazi...)Show SMILES CN1CCN(CC1)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C29H40N8/c1-33-17-19-35(20-18-33)27-13-11-25(12-14-27)28(36-16-6-15-34(21-22-36)26-9-5-10-26)29-30-31-32-37(29)23-24-7-3-2-4-8-24/h2-4,7-8,11-14,26,28H,5-6,9-10,15-23H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325086
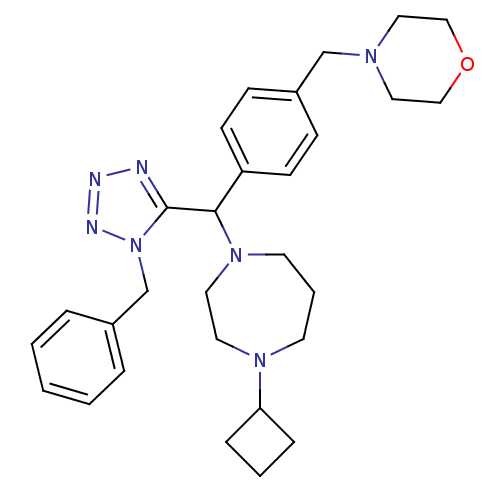
(4-(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4...)Show SMILES C(N1CCOCC1)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C29H39N7O/c1-2-6-24(7-3-1)23-36-29(30-31-32-36)28(35-15-5-14-34(16-17-35)27-8-4-9-27)26-12-10-25(11-13-26)22-33-18-20-37-21-19-33/h1-3,6-7,10-13,27-28H,4-5,8-9,14-23H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325094
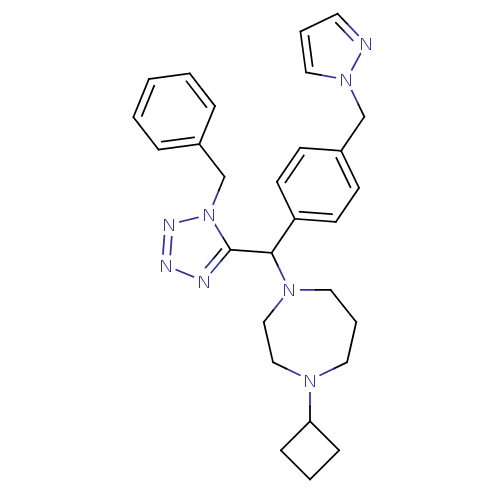
(1-((4-((1H-pyrazol-1-yl)methyl)phenyl)(1-benzyl-1H...)Show SMILES C(c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1)n1cccn1 Show InChI InChI=1S/C28H34N8/c1-2-7-23(8-3-1)22-36-28(30-31-32-36)27(34-17-6-16-33(19-20-34)26-9-4-10-26)25-13-11-24(12-14-25)21-35-18-5-15-29-35/h1-3,5,7-8,11-15,18,26-27H,4,6,9-10,16-17,19-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325096
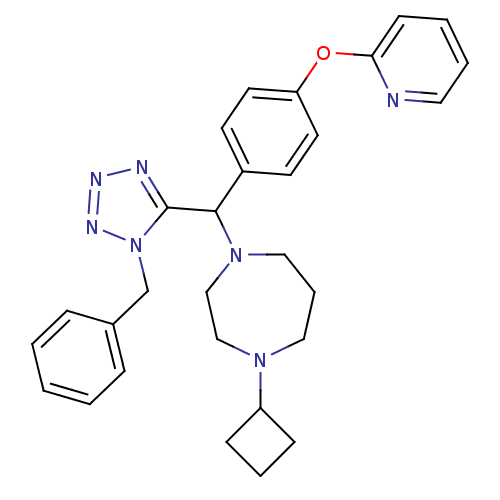
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(pyridin-2-yloxy)...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(Oc2ccccn2)cc1 Show InChI InChI=1S/C29H33N7O/c1-2-8-23(9-3-1)22-36-29(31-32-33-36)28(35-19-7-18-34(20-21-35)25-10-6-11-25)24-13-15-26(16-14-24)37-27-12-4-5-17-30-27/h1-5,8-9,12-17,25,28H,6-7,10-11,18-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325089
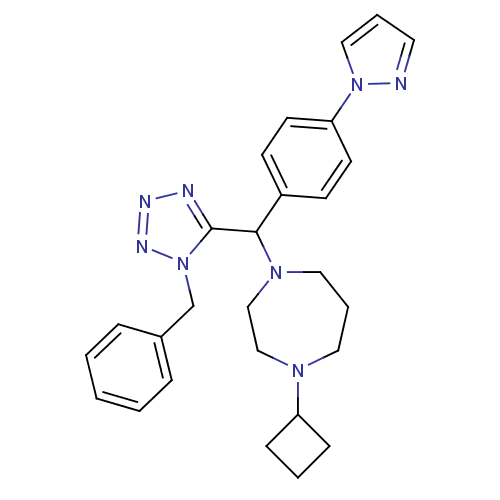
(1-((4-(1H-pyrazol-1-yl)phenyl)(1-benzyl-1H-tetrazo...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(cc1)-n1cccn1 Show InChI InChI=1S/C27H32N8/c1-2-7-22(8-3-1)21-35-27(29-30-31-35)26(23-11-13-25(14-12-23)34-18-5-15-28-34)33-17-6-16-32(19-20-33)24-9-4-10-24/h1-3,5,7-8,11-15,18,24,26H,4,6,9-10,16-17,19-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325064
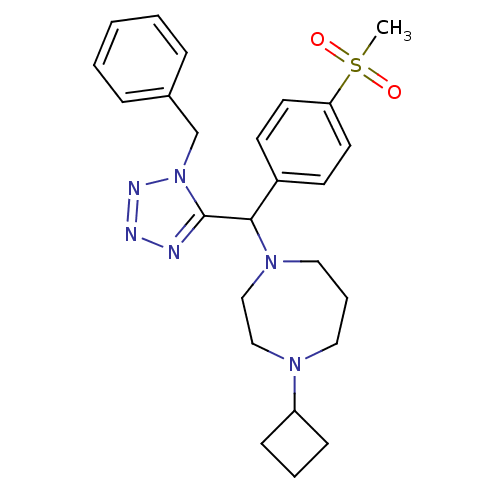
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C25H32N6O2S/c1-34(32,33)23-13-11-21(12-14-23)24(30-16-6-15-29(17-18-30)22-9-5-10-22)25-26-27-28-31(25)19-20-7-3-2-4-8-20/h2-4,7-8,11-14,22,24H,5-6,9-10,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325066
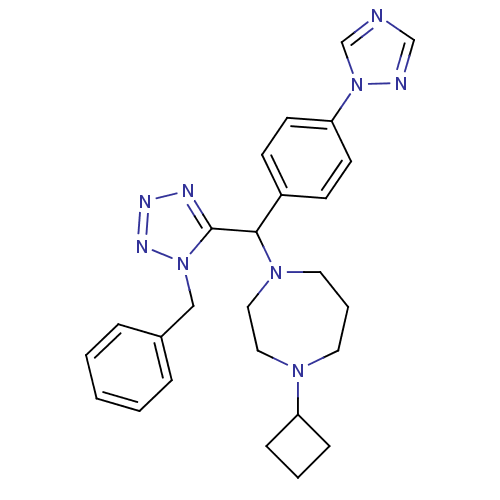
(1-((4-(1H-1,2,4-triazol-1-yl)phenyl)(1-benzyl-1H-t...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(cc1)-n1cncn1 Show InChI InChI=1S/C26H31N9/c1-2-6-21(7-3-1)18-34-26(29-30-31-34)25(22-10-12-24(13-11-22)35-20-27-19-28-35)33-15-5-14-32(16-17-33)23-8-4-9-23/h1-3,6-7,10-13,19-20,23,25H,4-5,8-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325051
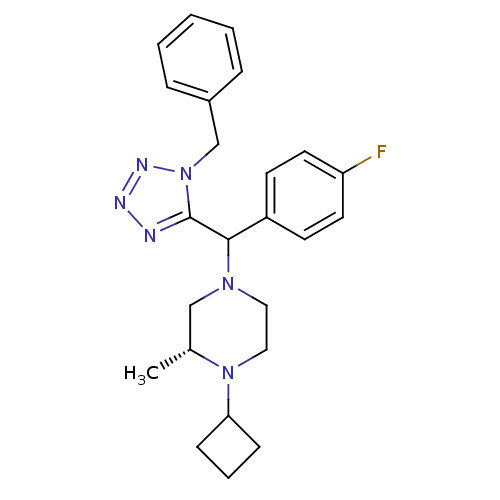
((2R)-4-((1-benzyl-1H-tetrazol-5-yl)(4-fluorophenyl...)Show SMILES C[C@@H]1CN(CCN1C1CCC1)C(c1nnnn1Cc1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H29FN6/c1-18-16-29(14-15-30(18)22-8-5-9-22)23(20-10-12-21(25)13-11-20)24-26-27-28-31(24)17-19-6-3-2-4-7-19/h2-4,6-7,10-13,18,22-23H,5,8-9,14-17H2,1H3/t18-,23?/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325063
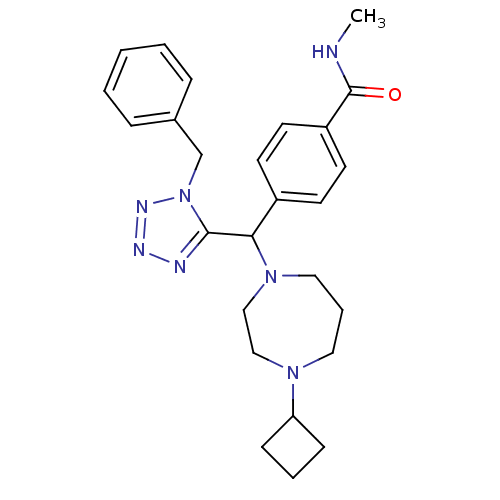
(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES CNC(=O)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C26H33N7O/c1-27-26(34)22-13-11-21(12-14-22)24(32-16-6-15-31(17-18-32)23-9-5-10-23)25-28-29-30-33(25)19-20-7-3-2-4-8-20/h2-4,7-8,11-14,23-24H,5-6,9-10,15-19H2,1H3,(H,27,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325052
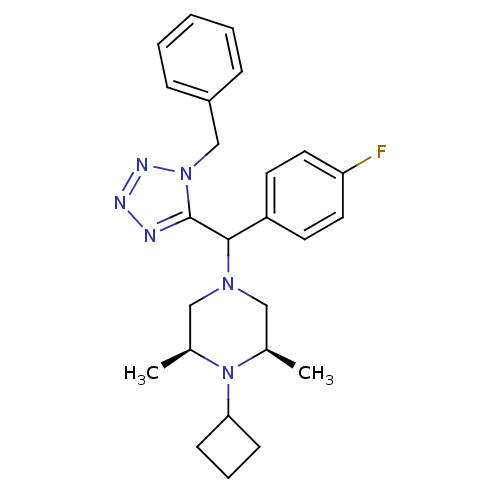
((2R,6S)-4-((1-benzyl-1H-tetrazol-5-yl)(4-fluorophe...)Show SMILES C[C@H]1CN(C[C@@H](C)N1C1CCC1)C(c1nnnn1Cc1ccccc1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C25H31FN6/c1-18-15-30(16-19(2)32(18)23-9-6-10-23)24(21-11-13-22(26)14-12-21)25-27-28-29-31(25)17-20-7-4-3-5-8-20/h3-5,7-8,11-14,18-19,23-24H,6,9-10,15-17H2,1-2H3/t18-,19+,24? | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325093
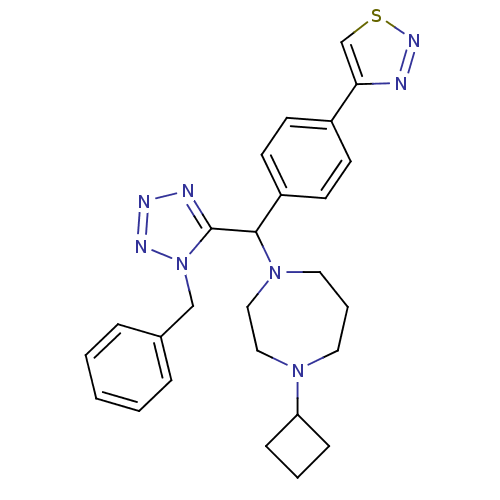
(4-(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(cc1)-c1csnn1 Show InChI InChI=1S/C26H30N8S/c1-2-6-20(7-3-1)18-34-26(28-29-30-34)25(22-12-10-21(11-13-22)24-19-35-31-27-24)33-15-5-14-32(16-17-33)23-8-4-9-23/h1-3,6-7,10-13,19,23,25H,4-5,8-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325067
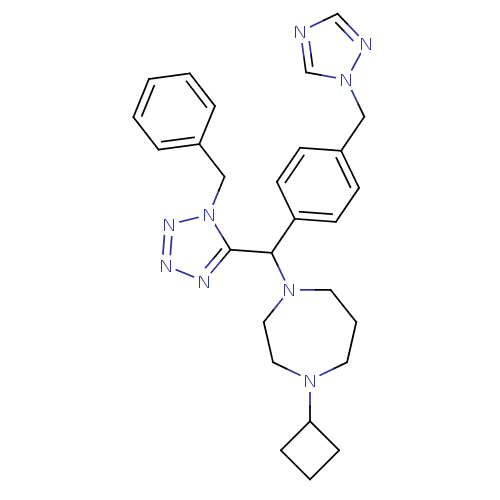
(1-((4-((1H-1,2,4-triazol-1-yl)methyl)phenyl)(1-ben...)Show SMILES C(c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1)n1cncn1 Show InChI InChI=1S/C27H33N9/c1-2-6-22(7-3-1)19-36-27(30-31-32-36)26(34-15-5-14-33(16-17-34)25-8-4-9-25)24-12-10-23(11-13-24)18-35-21-28-20-29-35/h1-3,6-7,10-13,20-21,25-26H,4-5,8-9,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325095
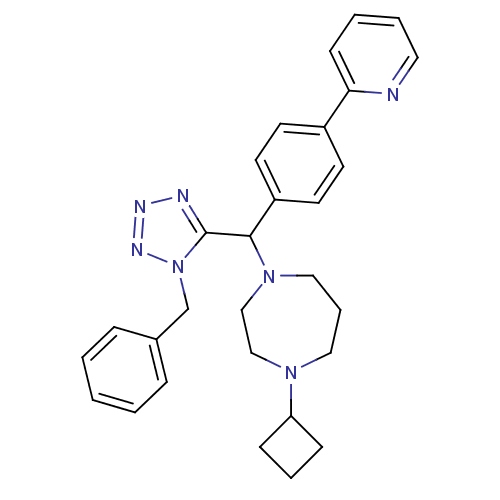
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(pyridin-2-yl)phe...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(cc1)-c1ccccn1 Show InChI InChI=1S/C29H33N7/c1-2-8-23(9-3-1)22-36-29(31-32-33-36)28(35-19-7-18-34(20-21-35)26-10-6-11-26)25-15-13-24(14-16-25)27-12-4-5-17-30-27/h1-5,8-9,12-17,26,28H,6-7,10-11,18-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325088
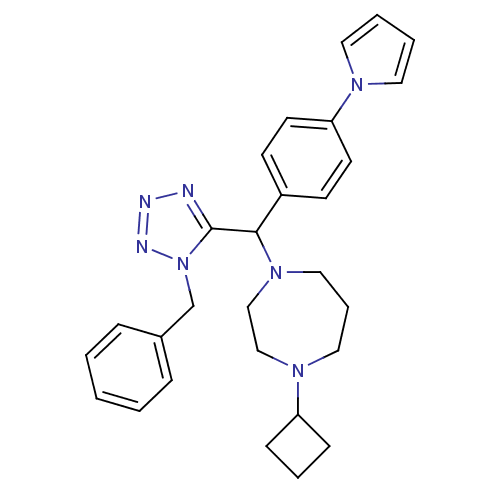
(1-((4-(1H-pyrrol-1-yl)phenyl)(1-benzyl-1H-tetrazol...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(cc1)-n1cccc1 Show InChI InChI=1S/C28H33N7/c1-2-8-23(9-3-1)22-35-28(29-30-31-35)27(24-12-14-26(15-13-24)32-16-4-5-17-32)34-19-7-18-33(20-21-34)25-10-6-11-25/h1-5,8-9,12-17,25,27H,6-7,10-11,18-22H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
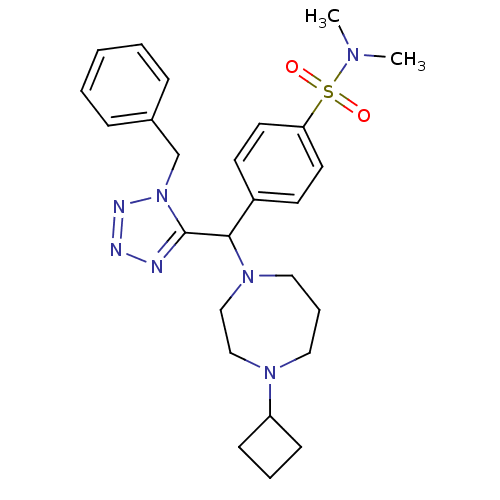
(Homo sapiens (Human)) | BDBM50325084
(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C26H35N7O2S/c1-30(2)36(34,35)24-14-12-22(13-15-24)25(32-17-7-16-31(18-19-32)23-10-6-11-23)26-27-28-29-33(26)20-21-8-4-3-5-9-21/h3-5,8-9,12-15,23,25H,6-7,10-11,16-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50325066

(1-((4-(1H-1,2,4-triazol-1-yl)phenyl)(1-benzyl-1H-t...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(cc1)-n1cncn1 Show InChI InChI=1S/C26H31N9/c1-2-6-21(7-3-1)18-34-26(29-30-31-34)25(22-10-12-24(13-11-22)35-20-27-19-28-35)33-15-5-14-32(16-17-33)23-8-4-9-23/h1-3,6-7,10-13,19-20,23,25H,4-5,8-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325091
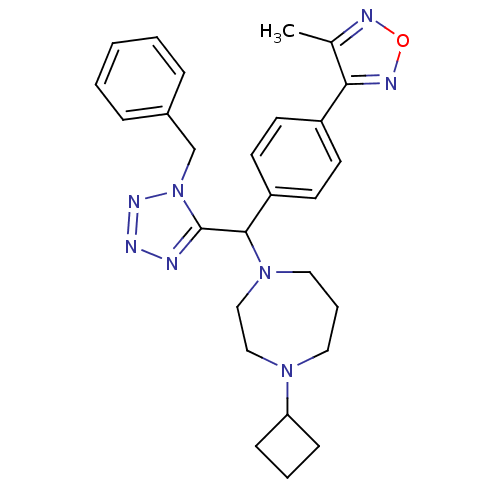
(3-(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4...)Show SMILES Cc1nonc1-c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C27H32N8O/c1-20-25(30-36-29-20)22-11-13-23(14-12-22)26(34-16-6-15-33(17-18-34)24-9-5-10-24)27-28-31-32-35(27)19-21-7-3-2-4-8-21/h2-4,7-8,11-14,24,26H,5-6,9-10,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50355111
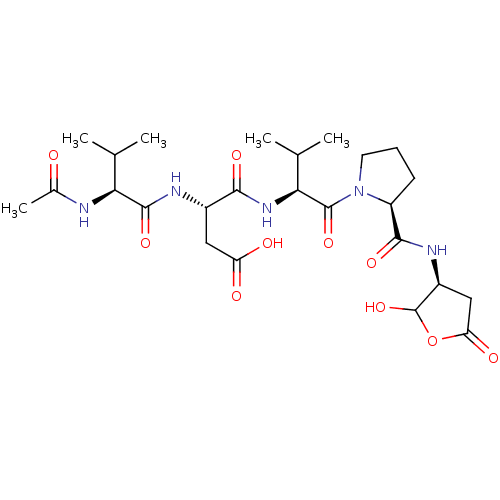
(CHEMBL1835210)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCC[C@H]1C(=O)N[C@H]1CC(=O)OC1O |r| Show InChI InChI=1S/C25H39N5O10/c1-11(2)19(26-13(5)31)23(37)27-14(9-17(32)33)21(35)29-20(12(3)4)24(38)30-8-6-7-16(30)22(36)28-15-10-18(34)40-25(15)39/h11-12,14-16,19-20,25,39H,6-10H2,1-5H3,(H,26,31)(H,27,37)(H,28,36)(H,29,35)(H,32,33)/t14-,15-,16-,19-,20-,25?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal histidine-tagged caspase-3 using Ac-DEVD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis |
Bioorg Med Chem 19: 5833-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.020
BindingDB Entry DOI: 10.7270/Q20V8D6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50325063

(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES CNC(=O)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C26H33N7O/c1-27-26(34)22-13-11-21(12-14-22)24(32-16-6-15-31(17-18-32)23-9-5-10-23)25-28-29-30-33(25)19-20-7-3-2-4-8-20/h2-4,7-8,11-14,23-24H,5-6,9-10,15-19H2,1H3,(H,27,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325097
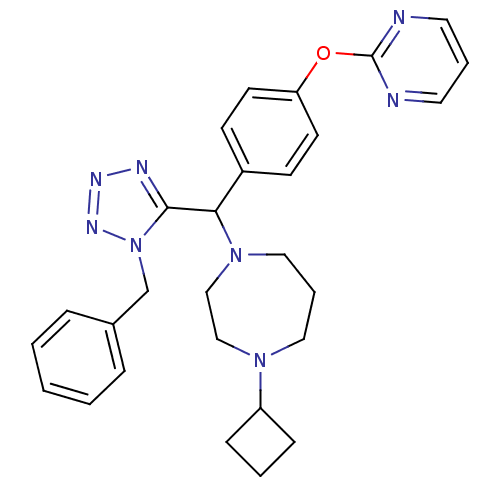
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(pyrimidin-2-ylox...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc(Oc2ncccn2)cc1 Show InChI InChI=1S/C28H32N8O/c1-2-7-22(8-3-1)21-36-27(31-32-33-36)26(35-18-6-17-34(19-20-35)24-9-4-10-24)23-11-13-25(14-12-23)37-28-29-15-5-16-30-28/h1-3,5,7-8,11-16,24,26H,4,6,9-10,17-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325076
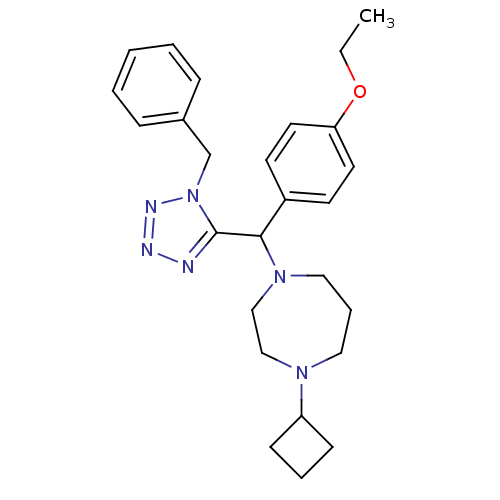
(1-((1-benzyl-1H-tetrazol-5-yl)(4-ethoxyphenyl)meth...)Show SMILES CCOc1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C26H34N6O/c1-2-33-24-14-12-22(13-15-24)25(31-17-7-16-30(18-19-31)23-10-6-11-23)26-27-28-29-32(26)20-21-8-4-3-5-9-21/h3-5,8-9,12-15,23,25H,2,6-7,10-11,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50355109
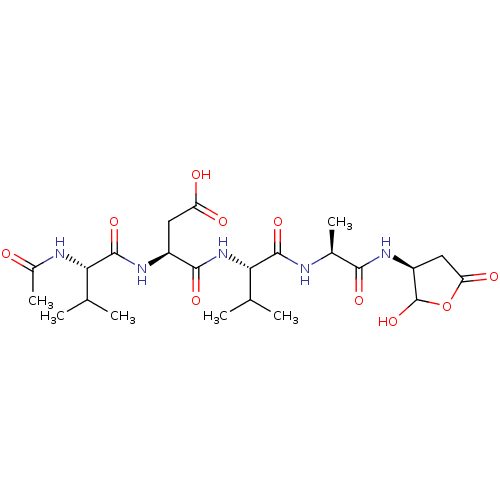
(CHEMBL1835209)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](C)C(=O)N[C@H]1CC(=O)OC1O |r| Show InChI InChI=1S/C23H37N5O10/c1-9(2)17(25-12(6)29)22(36)26-13(7-15(30)31)20(34)28-18(10(3)4)21(35)24-11(5)19(33)27-14-8-16(32)38-23(14)37/h9-11,13-14,17-18,23,37H,7-8H2,1-6H3,(H,24,35)(H,25,29)(H,26,36)(H,27,33)(H,28,34)(H,30,31)/t11-,13-,14-,17-,18-,23?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal histidine-tagged caspase-3 using Ac-DEVD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis |
Bioorg Med Chem 19: 5833-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.020
BindingDB Entry DOI: 10.7270/Q20V8D6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50325067

(1-((4-((1H-1,2,4-triazol-1-yl)methyl)phenyl)(1-ben...)Show SMILES C(c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1)n1cncn1 Show InChI InChI=1S/C27H33N9/c1-2-6-22(7-3-1)19-36-27(30-31-32-36)26(34-15-5-14-33(16-17-34)25-8-4-9-25)24-12-10-23(11-13-24)18-35-21-28-20-29-35/h1-3,6-7,10-13,20-21,25-26H,4-5,8-9,14-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Rattus norvegicus (rat)) | BDBM50325064

(1-((1-benzyl-1H-tetrazol-5-yl)(4-(methylsulfonyl)p...)Show SMILES CS(=O)(=O)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C25H32N6O2S/c1-34(32,33)23-13-11-21(12-14-23)24(30-16-6-15-29(17-18-30)22-9-5-10-22)25-26-27-28-31(25)19-20-7-3-2-4-8-20/h2-4,7-8,11-14,22,24H,5-6,9-10,15-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at rat histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325073
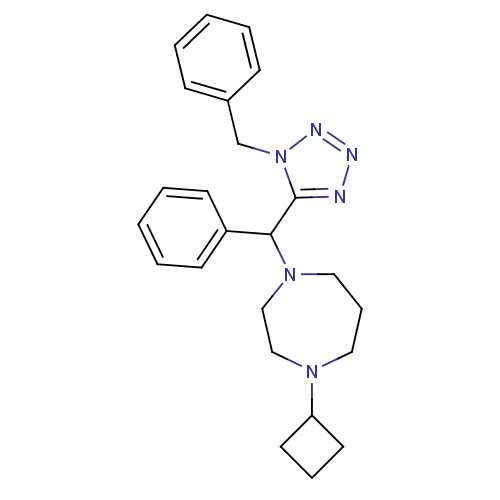
(1-((1-benzyl-1H-tetrazol-5-yl)(phenyl)methyl)-4-cy...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccccc1 Show InChI InChI=1S/C24H30N6/c1-3-9-20(10-4-1)19-30-24(25-26-27-30)23(21-11-5-2-6-12-21)29-16-8-15-28(17-18-29)22-13-7-14-22/h1-6,9-12,22-23H,7-8,13-19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325081
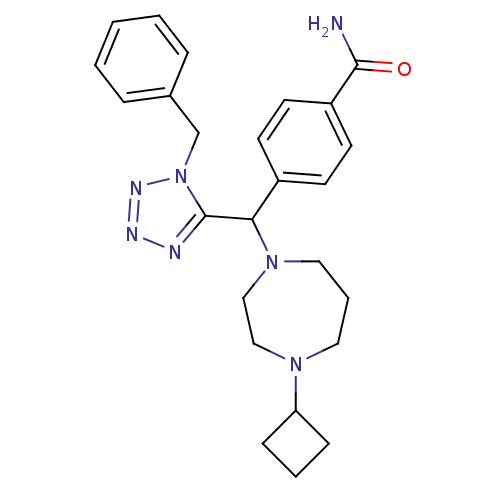
(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES NC(=O)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C25H31N7O/c26-24(33)21-12-10-20(11-13-21)23(31-15-5-14-30(16-17-31)22-8-4-9-22)25-27-28-29-32(25)18-19-6-2-1-3-7-19/h1-3,6-7,10-13,22-23H,4-5,8-9,14-18H2,(H2,26,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325083
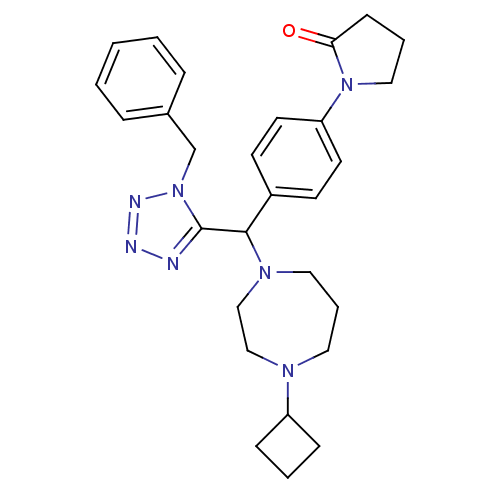
(1-(4-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4...)Show SMILES O=C1CCCN1c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C28H35N7O/c36-26-11-5-18-34(26)25-14-12-23(13-15-25)27(33-17-6-16-32(19-20-33)24-9-4-10-24)28-29-30-31-35(28)21-22-7-2-1-3-8-22/h1-3,7-8,12-15,24,27H,4-6,9-11,16-21H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Caspase-2
(Homo sapiens (Human)) | BDBM50355101
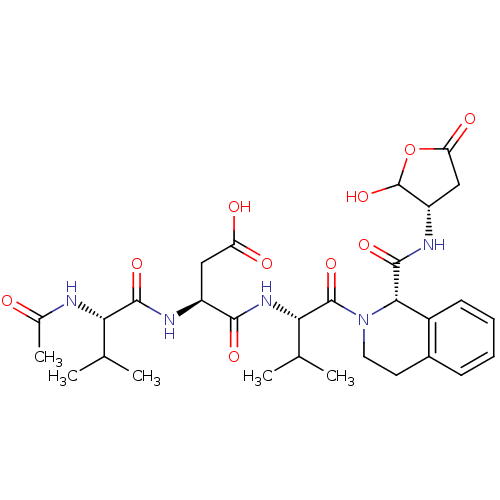
(CHEMBL1835324)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCc2ccccc2[C@H]1C(=O)N[C@H]1CC(=O)OC1O |r| Show InChI InChI=1S/C30H41N5O10/c1-14(2)23(31-16(5)36)27(41)32-19(12-21(37)38)26(40)34-24(15(3)4)29(43)35-11-10-17-8-6-7-9-18(17)25(35)28(42)33-20-13-22(39)45-30(20)44/h6-9,14-15,19-20,23-25,30,44H,10-13H2,1-5H3,(H,31,36)(H,32,41)(H,33,42)(H,34,40)(H,37,38)/t19-,20-,23-,24-,25-,30?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of caspase-2 (amino acids 170 to 452) using Ac-VDVAD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis |
Bioorg Med Chem 19: 5833-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.020
BindingDB Entry DOI: 10.7270/Q20V8D6X |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50355099
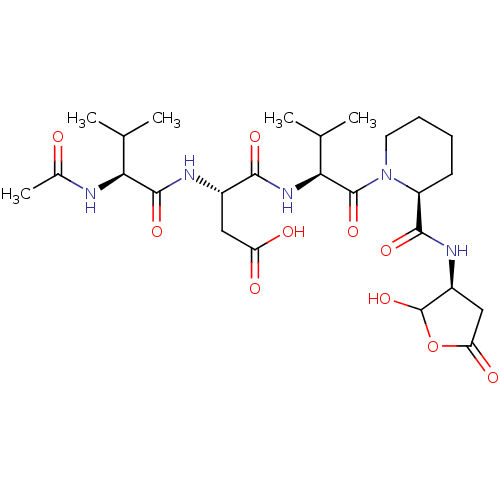
(CHEMBL1835322)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCCC[C@H]1C(=O)N[C@H]1CC(=O)OC1O |r| Show InChI InChI=1S/C26H41N5O10/c1-12(2)20(27-14(5)32)24(38)28-15(10-18(33)34)22(36)30-21(13(3)4)25(39)31-9-7-6-8-17(31)23(37)29-16-11-19(35)41-26(16)40/h12-13,15-17,20-21,26,40H,6-11H2,1-5H3,(H,27,32)(H,28,38)(H,29,37)(H,30,36)(H,33,34)/t15-,16-,17-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal FLAG-tagged human caspase-3 expressed in HEK293 T17 cells using Ac-DEVD-AMC coumarin-120 as substrate pre-incubated for 2 hr... |
Bioorg Med Chem 19: 5833-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.020
BindingDB Entry DOI: 10.7270/Q20V8D6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325044
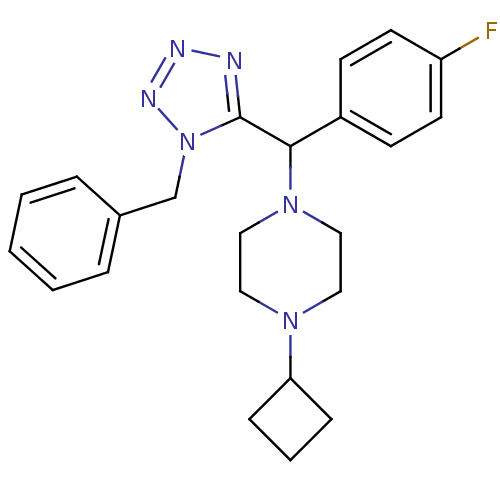
(1-((1-benzyl-1H-tetrazol-5-yl)(4-fluorophenyl)meth...)Show SMILES Fc1ccc(cc1)C(N1CCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C23H27FN6/c24-20-11-9-19(10-12-20)22(29-15-13-28(14-16-29)21-7-4-8-21)23-25-26-27-30(23)17-18-5-2-1-3-6-18/h1-3,5-6,9-12,21-22H,4,7-8,13-17H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50355099

(CHEMBL1835322)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N1CCCC[C@H]1C(=O)N[C@H]1CC(=O)OC1O |r| Show InChI InChI=1S/C26H41N5O10/c1-12(2)20(27-14(5)32)24(38)28-15(10-18(33)34)22(36)30-21(13(3)4)25(39)31-9-7-6-8-17(31)23(37)29-16-11-19(35)41-26(16)40/h12-13,15-17,20-21,26,40H,6-11H2,1-5H3,(H,27,32)(H,28,38)(H,29,37)(H,30,36)(H,33,34)/t15-,16-,17-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal histidine-tagged caspase-3 using Ac-DEVD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis |
Bioorg Med Chem 19: 5833-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.020
BindingDB Entry DOI: 10.7270/Q20V8D6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325074
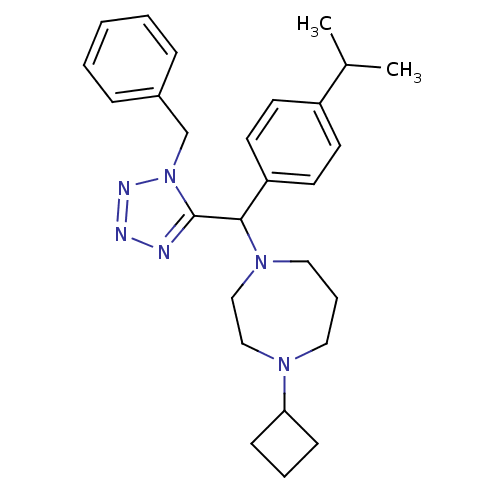
(1-((1-benzyl-1H-tetrazol-5-yl)(4-isopropylphenyl)m...)Show SMILES CC(C)c1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C27H36N6/c1-21(2)23-12-14-24(15-13-23)26(32-17-7-16-31(18-19-32)25-10-6-11-25)27-28-29-30-33(27)20-22-8-4-3-5-9-22/h3-5,8-9,12-15,21,25-26H,6-7,10-11,16-20H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325077
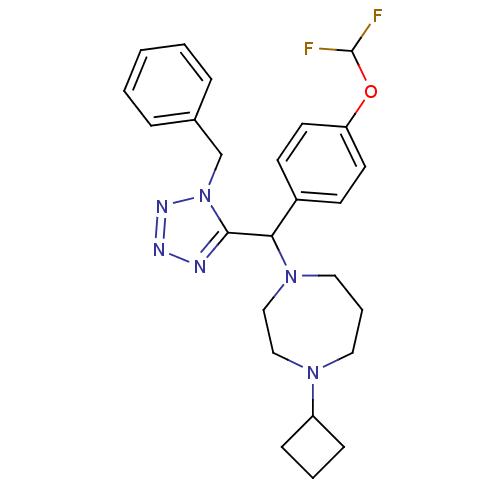
(1-((1-benzyl-1H-tetrazol-5-yl)(4-(difluoromethoxy)...)Show SMILES FC(F)Oc1ccc(cc1)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C25H30F2N6O/c26-25(27)34-22-12-10-20(11-13-22)23(32-15-5-14-31(16-17-32)21-8-4-9-21)24-28-29-30-33(24)18-19-6-2-1-3-7-19/h1-3,6-7,10-13,21,23,25H,4-5,8-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Caspase-3
(Homo sapiens (Human)) | BDBM50355110
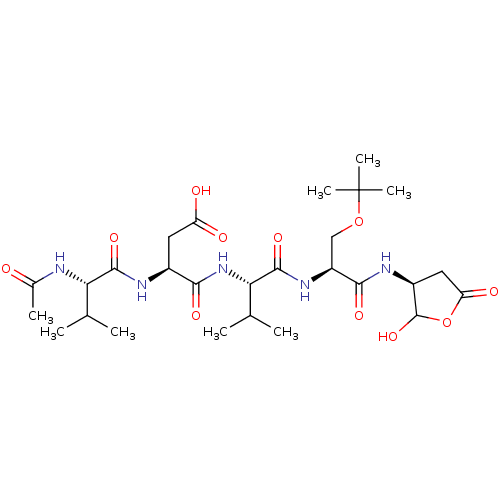
(CHEMBL1835208)Show SMILES CC(C)[C@H](NC(C)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](COC(C)(C)C)C(=O)N[C@H]1CC(=O)OC1O |r| Show InChI InChI=1S/C27H45N5O11/c1-12(2)20(28-14(5)33)24(39)29-15(9-18(34)35)22(37)32-21(13(3)4)25(40)31-17(11-42-27(6,7)8)23(38)30-16-10-19(36)43-26(16)41/h12-13,15-17,20-21,26,41H,9-11H2,1-8H3,(H,28,33)(H,29,39)(H,30,38)(H,31,40)(H,32,37)(H,34,35)/t15-,16-,17-,20-,21-,26?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Management, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal histidine-tagged caspase-3 using Ac-DEVD-AMC coumarin-120 as substrate after 20 mins by fluorometric analysis |
Bioorg Med Chem 19: 5833-51 (2011)
Article DOI: 10.1016/j.bmc.2011.08.020
BindingDB Entry DOI: 10.7270/Q20V8D6X |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325045
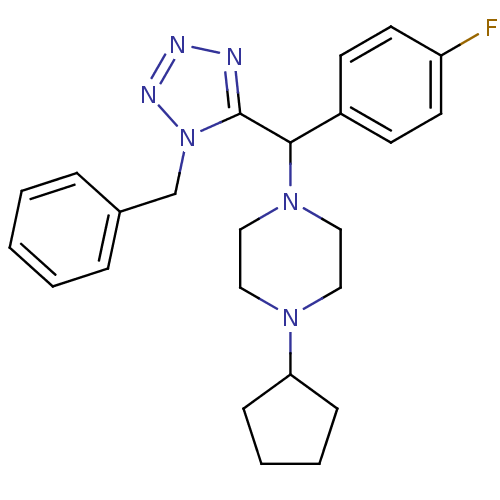
(1-((1-benzyl-1H-tetrazol-5-yl)(4-fluorophenyl)meth...)Show SMILES Fc1ccc(cc1)C(N1CCN(CC1)C1CCCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C24H29FN6/c25-21-12-10-20(11-13-21)23(30-16-14-29(15-17-30)22-8-4-5-9-22)24-26-27-28-31(24)18-19-6-2-1-3-7-19/h1-3,6-7,10-13,22-23H,4-5,8-9,14-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325101
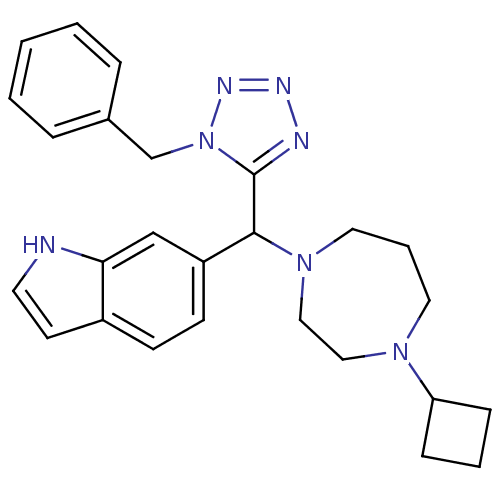
(6-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES C(c1ccccc1)n1nnnc1C(N1CCCN(CC1)C1CCC1)c1ccc2cc[nH]c2c1 Show InChI InChI=1S/C26H31N7/c1-2-6-20(7-3-1)19-33-26(28-29-30-33)25(22-11-10-21-12-13-27-24(21)18-22)32-15-5-14-31(16-17-32)23-8-4-9-23/h1-3,6-7,10-13,18,23,25,27H,4-5,8-9,14-17,19H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50325102
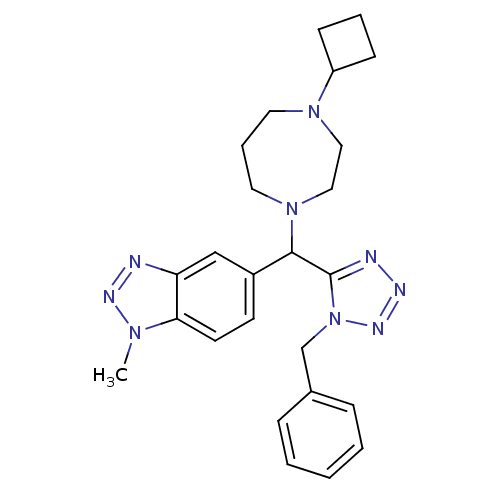
(5-((1-benzyl-1H-tetrazol-5-yl)(4-cyclobutyl-1,4-di...)Show SMILES Cn1nnc2cc(ccc12)C(N1CCCN(CC1)C1CCC1)c1nnnn1Cc1ccccc1 Show InChI InChI=1S/C25H31N9/c1-31-23-12-11-20(17-22(23)26-29-31)24(33-14-6-13-32(15-16-33)21-9-5-10-21)25-27-28-30-34(25)18-19-7-3-2-4-8-19/h2-4,7-8,11-12,17,21,24H,5-6,9-10,13-16,18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Evotec (UK) Ltd
Curated by ChEMBL
| Assay Description
Antagonist activity at human histamine H3 receptor |
Bioorg Med Chem Lett 20: 5165-9 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.009
BindingDB Entry DOI: 10.7270/Q2TQ61RV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data