

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345134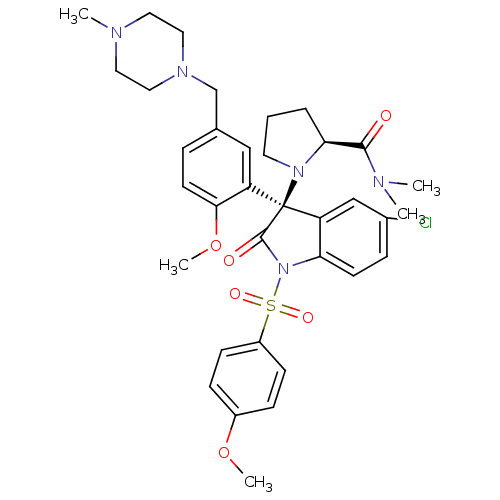 ((S)-1-((R)-5-chloro-3-(2-methoxy-5-((4-methylpiper...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345133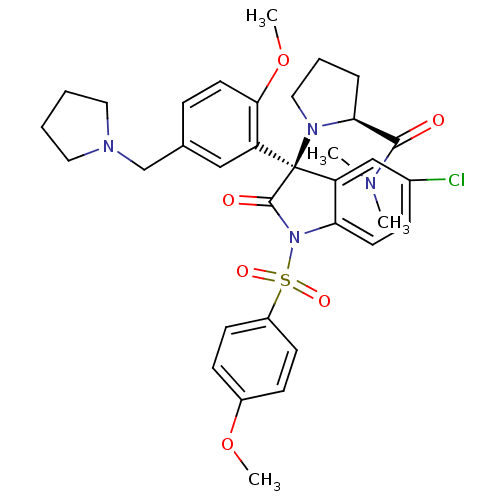 ((S)-1-((R)-5-chloro-3-(2-methoxy-5-(pyrrolidin-1-y...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345131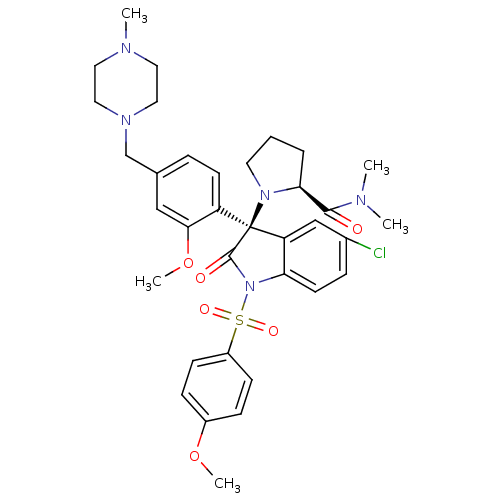 ((S)-1-((R)-5-chloro-3-(2-methoxy-4-((4-methylpiper...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345130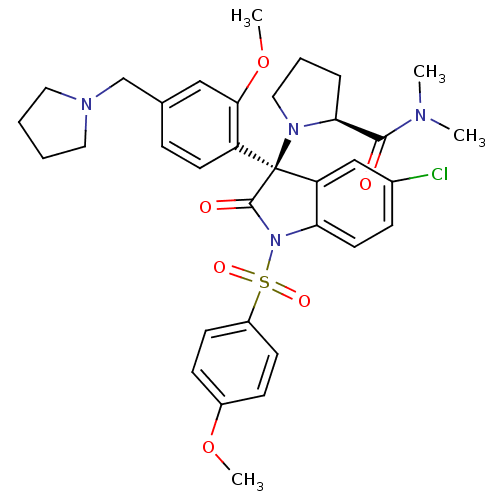 ((S)-1-((R)-5-chloro-3-(2-methoxy-4-(pyrrolidin-1-y...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345132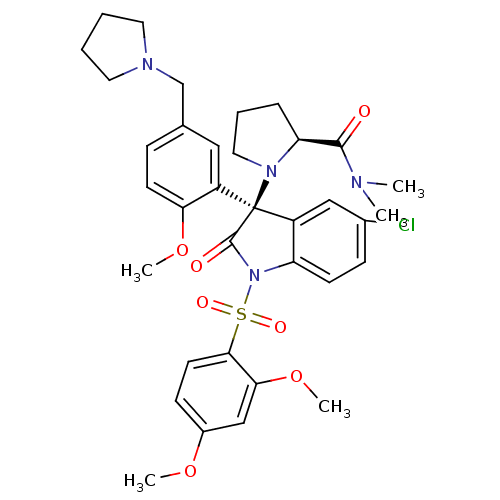 ((S)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulfonyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50176440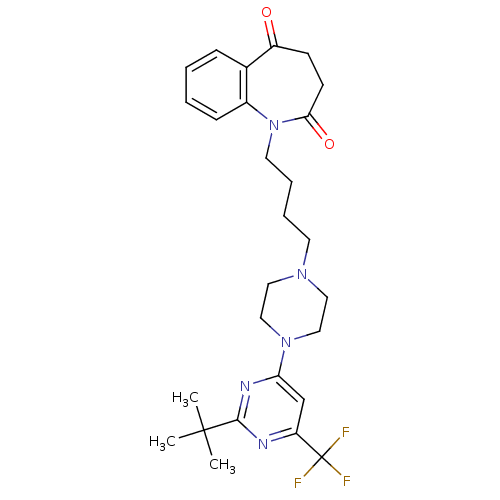 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 658-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.035 BindingDB Entry DOI: 10.7270/Q2K64HN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181170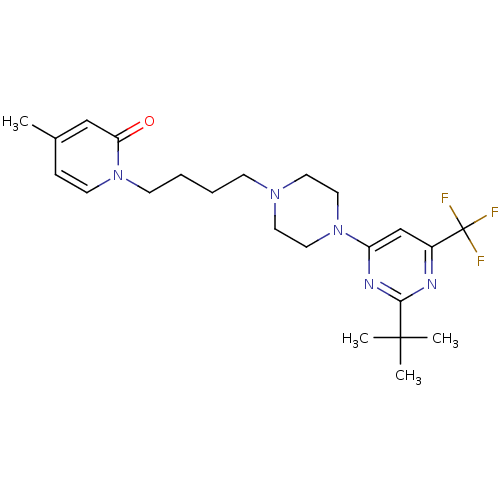 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345129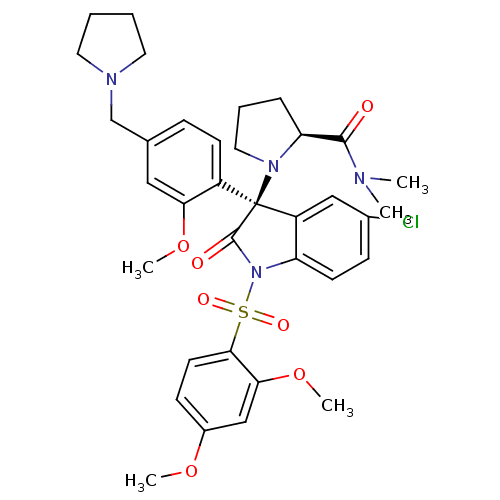 ((S)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulfonyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181161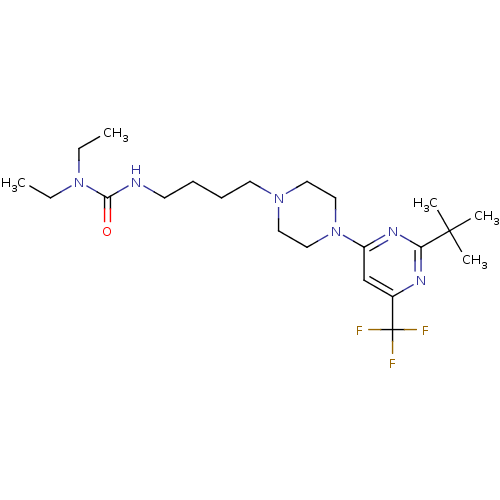 (3-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175212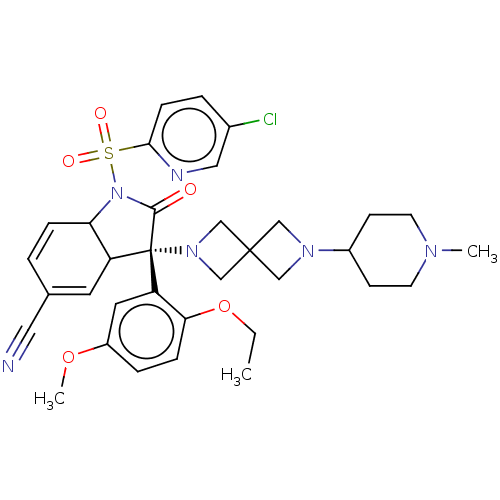 (US9688678, 34 (3S)-1-[(5-chloro-2-pyridyl)sulfonyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175037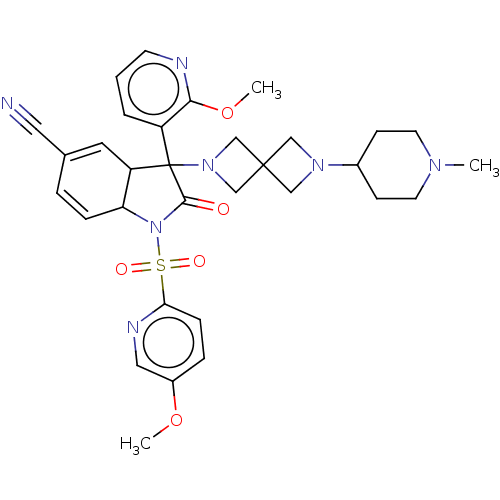 (US9688678, 9A (+)-3-(2-ethoxy-3-pyridyl)-1-[(5-met...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175060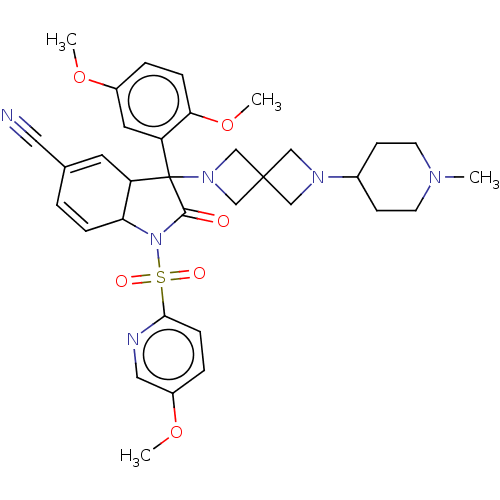 (US9688678, 21 (+-)-3-(2,5-dimethoxyphenyl)-1-[(5-m...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175058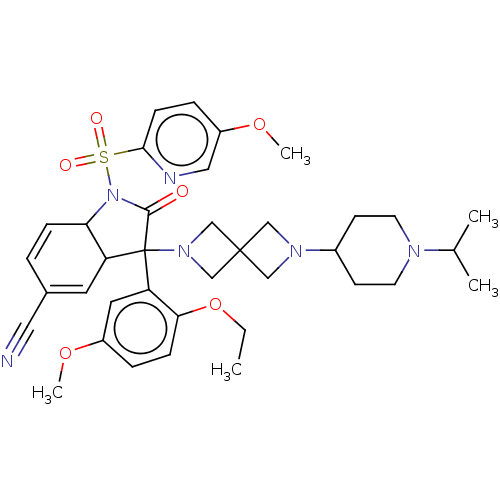 (US9688678, 19 (+-)-3-(2-ethoxy-5-methoxy-phenyl)-3...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM174904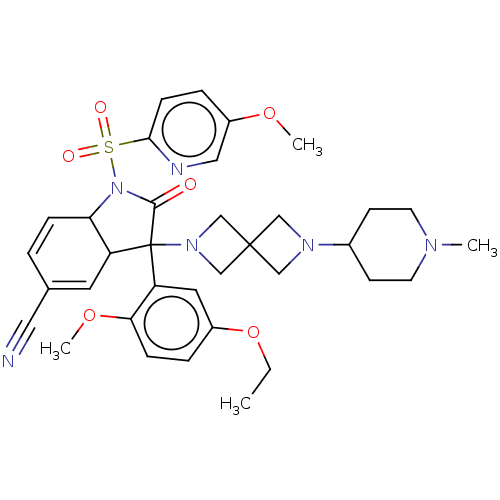 (US9688678, 1A (+)-3-(2-ethoxy-5-methoxy-phenyl)-1-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM316365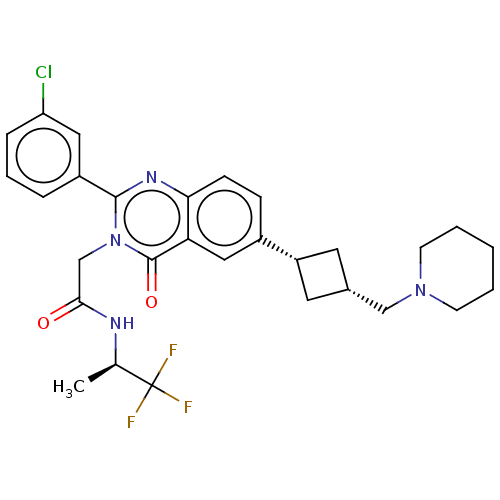 (US9617226, Example 46 | cis-2-[2-(3-Chlorophenyl)-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)).The incub... | US Patent US9617226 (2017) BindingDB Entry DOI: 10.7270/Q2639RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM316345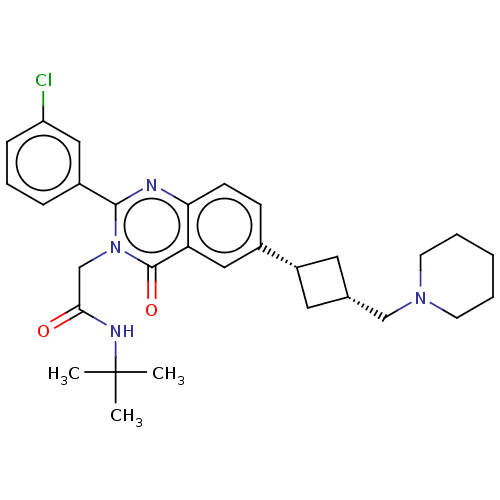 ( cis-N-tert-Butyl-2-[2-(3-chlorophenyl)-4-oxo-6-[3...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)).The incub... | US Patent US9617226 (2017) BindingDB Entry DOI: 10.7270/Q2639RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM316327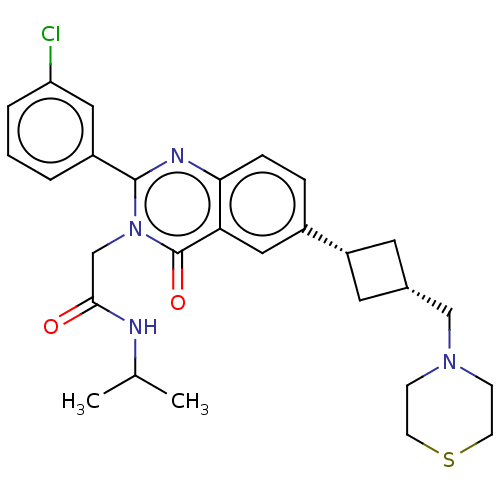 (US9617226, Example 8 | cis-2-[2-(3-Chlorophenyl)-4...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)).The incub... | US Patent US9617226 (2017) BindingDB Entry DOI: 10.7270/Q2639RTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175064 (US9688678, 25 (+)-3-(2-ethoxy-5-methoxy-phenyl)-1-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175039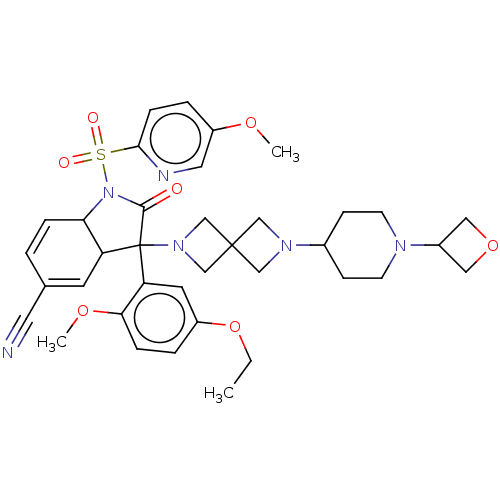 (US9688678, 11 (+)-3-(2-ethoxy-5-methoxyphenyl)-1-(...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175054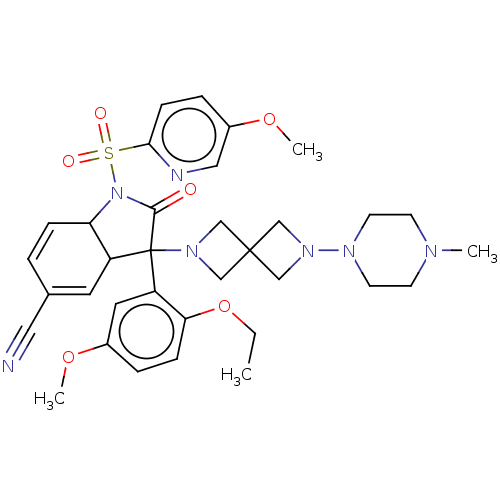 (US9688678, 17 (+-)-3-(2-ethoxy-5-methoxy-phenyl)-1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175055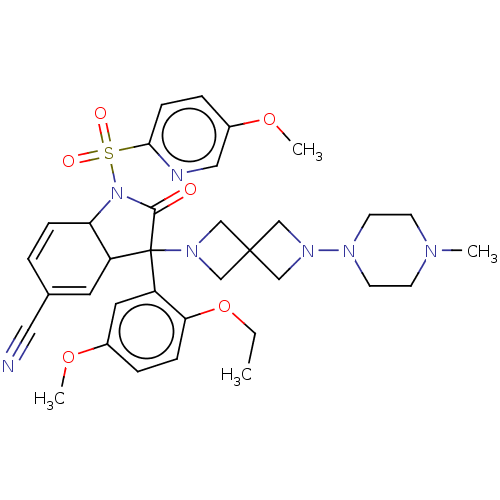 (US9688678, 17B (+)-3-(2-ethoxy-5-methoxy-phenyl)-1...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175056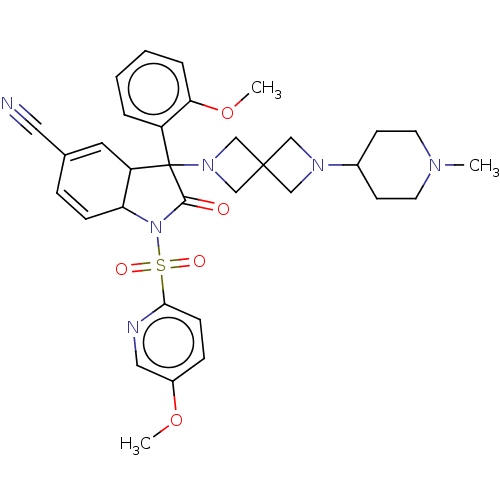 (US9688678, 18 (+-)3-(2-methoxyphenyl)-1-[(5-methox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM175057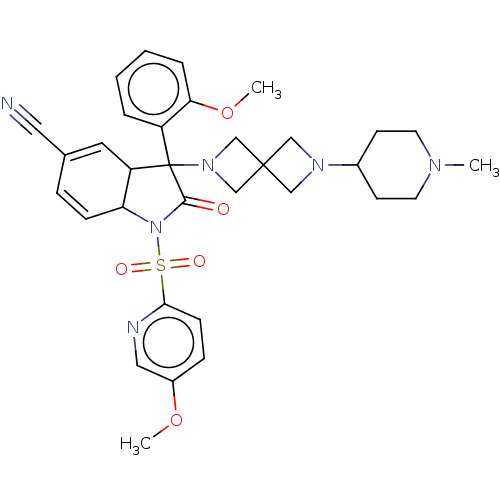 (US9688678, 18A (+)-3-(2-methoxyphenyl)-1-[(5-metho...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | <-51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
AbbVie Deutschland GmbH & Co. KG US Patent | Assay Description The binding assay was carried out by the method based on that of Tahara et al. (Tahara A et al., Brit. J. Pharmacol. 125, 1463-1470 (1998)). The incu... | US Patent US9688678 (2017) BindingDB Entry DOI: 10.7270/Q2SN0740 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50466882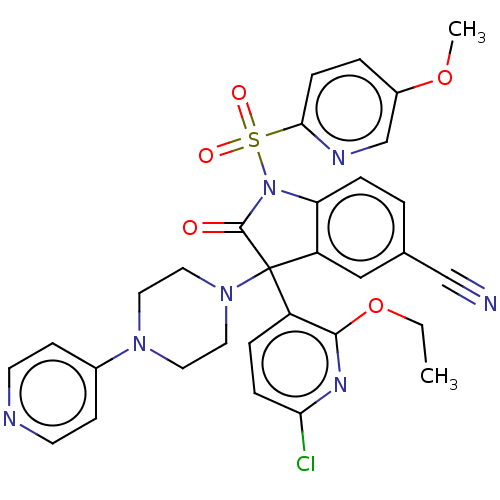 (CHEMBL4280894) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cell membranes after 60 mins by TopCount microplate scintillation counti... | Bioorg Med Chem Lett 28: 3260-3264 (2018) Article DOI: 10.1016/j.bmcl.2018.07.043 BindingDB Entry DOI: 10.7270/Q2CC13CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181164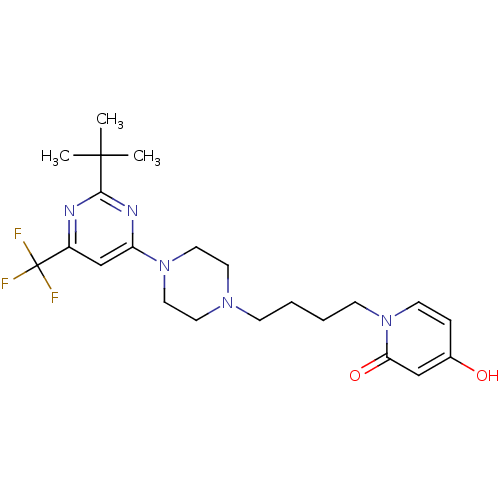 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345139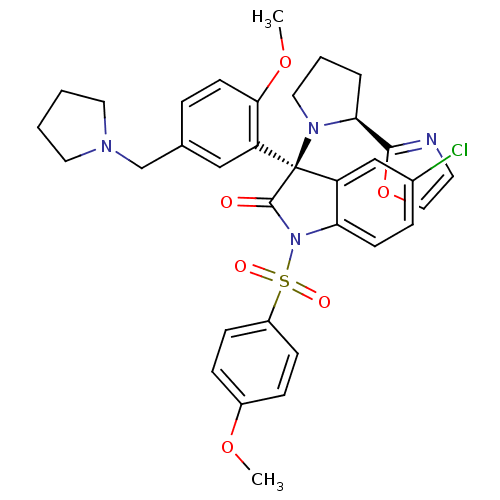 ((R)-5-chloro-3-(2-methoxy-5-(pyrrolidin-1-ylmethyl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50466872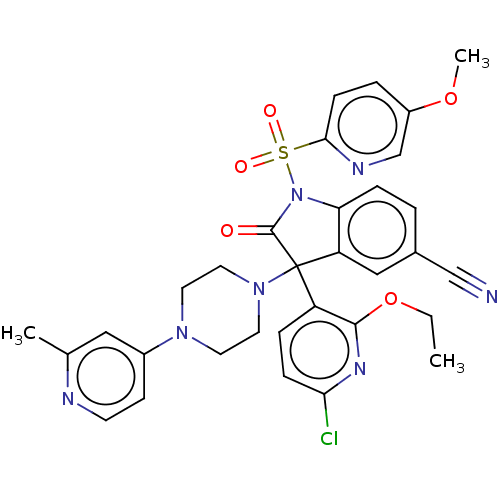 (CHEMBL4281098) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cell membranes after 60 mins by TopCount microplate scintillation counti... | Bioorg Med Chem Lett 28: 3260-3264 (2018) Article DOI: 10.1016/j.bmcl.2018.07.043 BindingDB Entry DOI: 10.7270/Q2CC13CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50466872 (CHEMBL4281098) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cell membranes after 60 mins by TopCount microplate scintillation counti... | Bioorg Med Chem Lett 28: 3260-3264 (2018) Article DOI: 10.1016/j.bmcl.2018.07.043 BindingDB Entry DOI: 10.7270/Q2CC13CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345143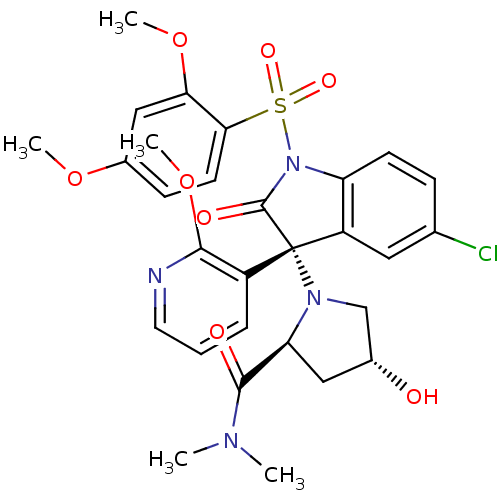 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345142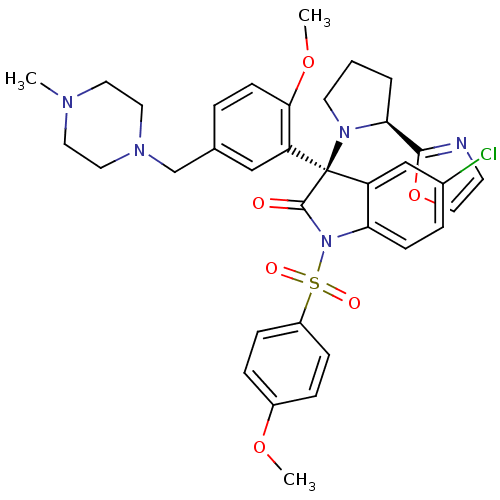 ((R)-5-chloro-3-(2-methoxy-5-((4-methylpiperazin-1-...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177356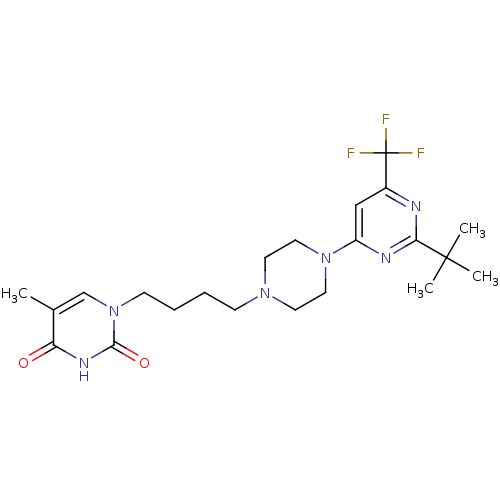 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177350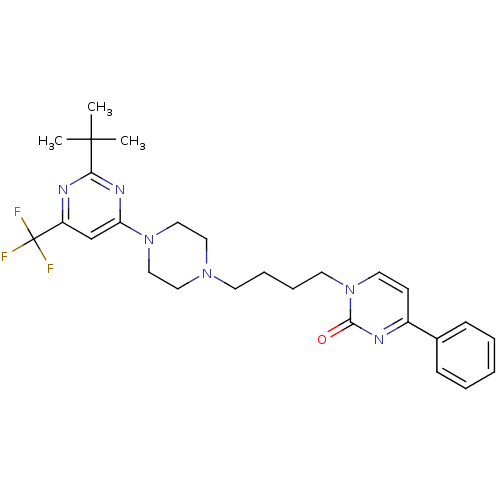 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177350 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181169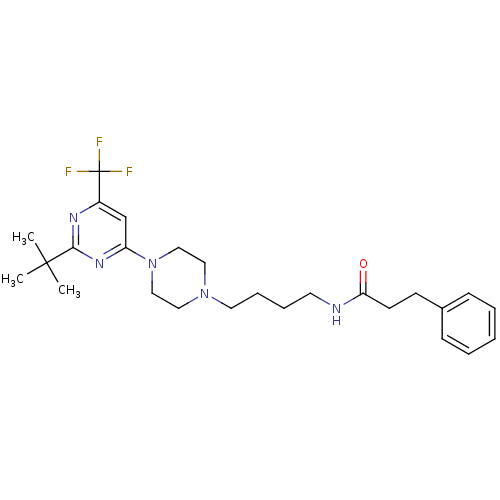 (CHEMBL207871 | N-(4-(4-(2-tert-butyl-6-(trifluorom...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177357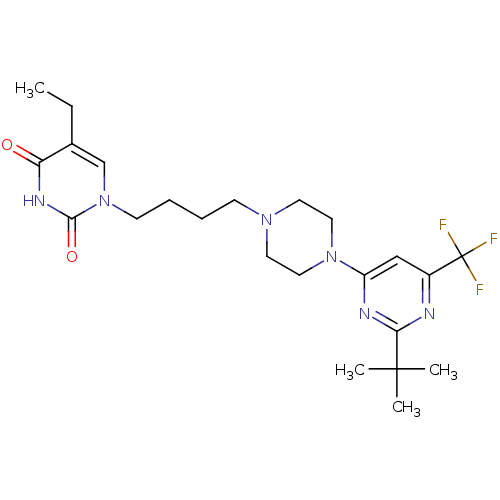 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177349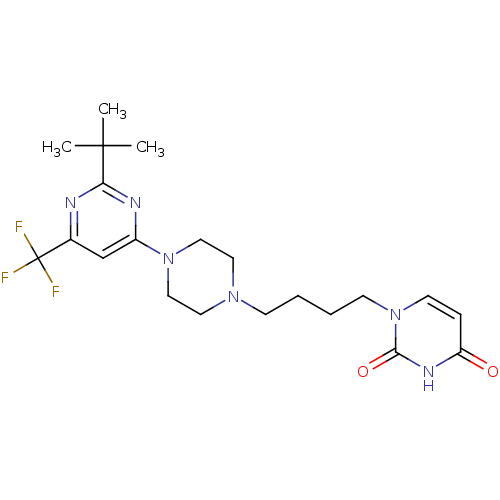 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177349 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50466873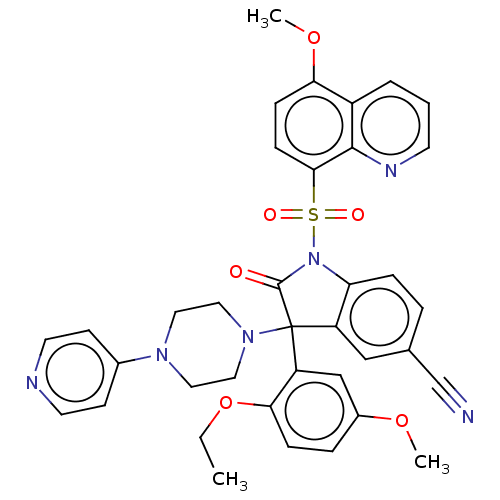 (CHEMBL4292610) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cell membranes after 60 mins by TopCount microplate scintillation counti... | Bioorg Med Chem Lett 28: 3260-3264 (2018) Article DOI: 10.1016/j.bmcl.2018.07.043 BindingDB Entry DOI: 10.7270/Q2CC13CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50466884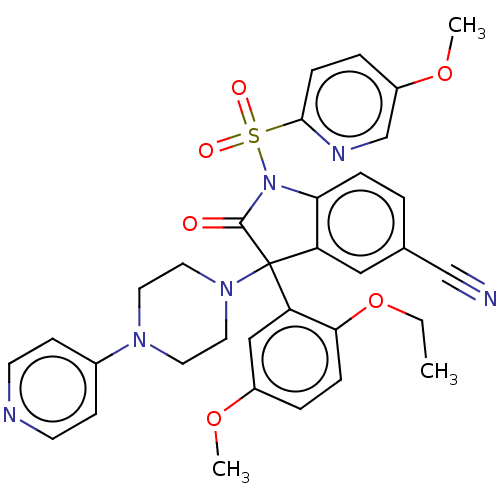 (CHEMBL4289186) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AbbVie Deutschland GmbH & Co. KG Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cell membranes after 60 mins by TopCount microplate scintillation counti... | Bioorg Med Chem Lett 28: 3260-3264 (2018) Article DOI: 10.1016/j.bmcl.2018.07.043 BindingDB Entry DOI: 10.7270/Q2CC13CC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345140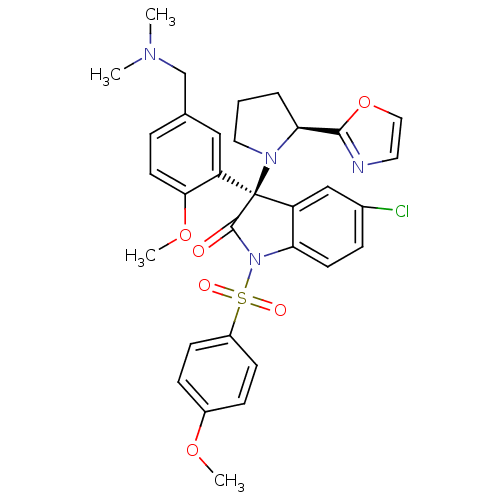 ((R)-5-chloro-3-(5-((dimethylamino)methyl)-2-methox...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50345145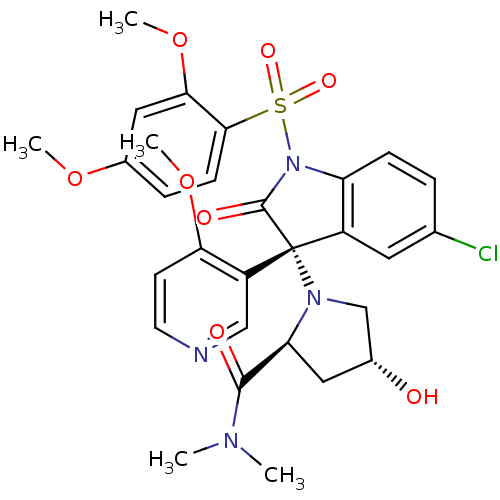 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Curated by ChEMBL | Assay Description Displacement of [3H]AVP from human vasopressin V1b receptor expressed in CHO cells by scintillation counting | Bioorg Med Chem Lett 21: 3828-31 (2011) Article DOI: 10.1016/j.bmcl.2011.03.012 BindingDB Entry DOI: 10.7270/Q20P10BC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181165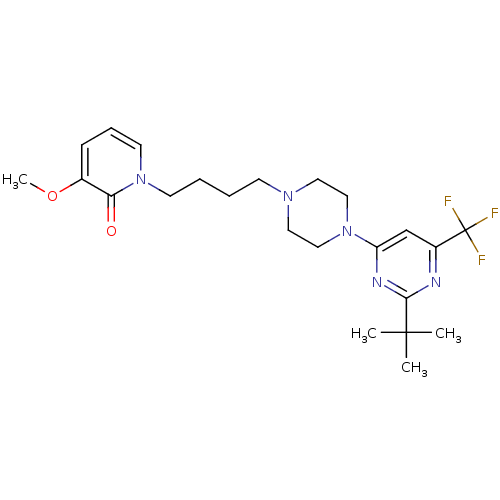 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181172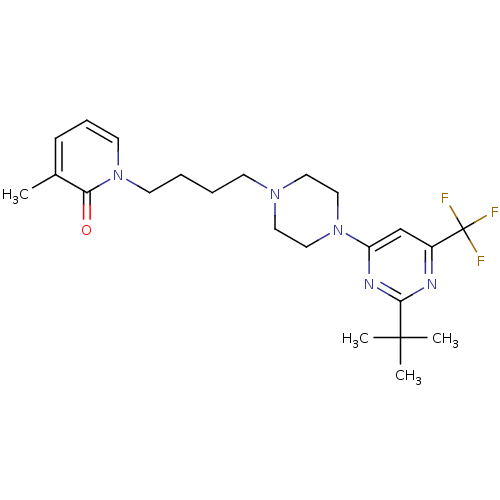 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177347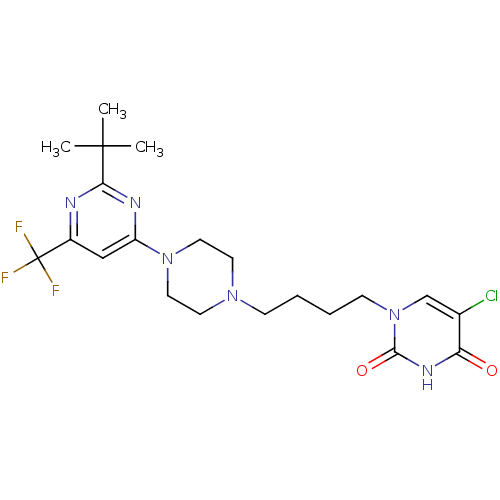 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50176451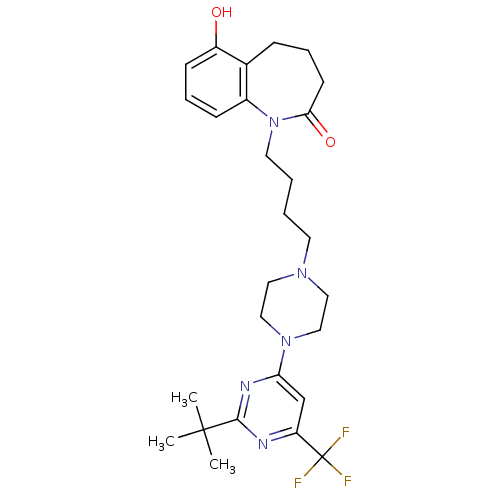 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 658-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.035 BindingDB Entry DOI: 10.7270/Q2K64HN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177344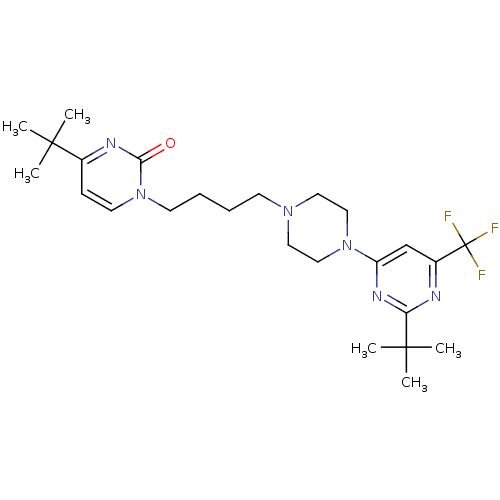 (4-tert-butyl-1-(4-(4-(2-tert-butyl-6-(trifluoromet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50181156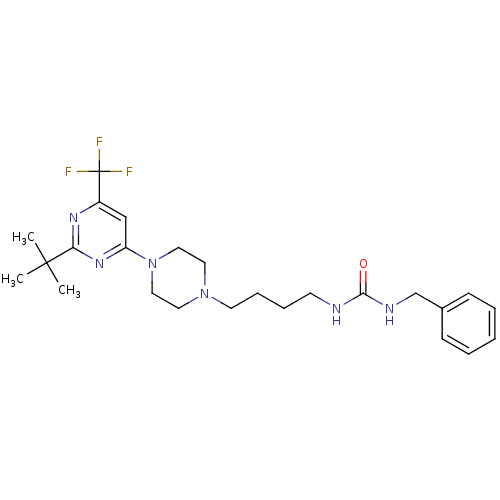 (1-benzyl-3-(4-(4-(2-tert-butyl-6-(trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to cloned human dopamine D3 receptor | Bioorg Med Chem Lett 16: 1934-7 (2006) Article DOI: 10.1016/j.bmcl.2005.12.079 BindingDB Entry DOI: 10.7270/Q29S1QMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177354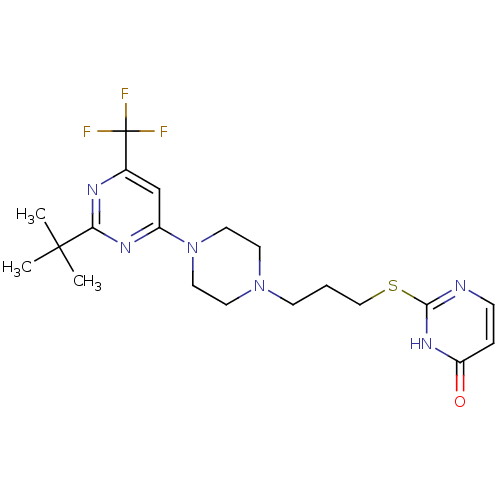 (2-(3-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(3) dopamine receptor (Homo sapiens (Human)) | BDBM50177343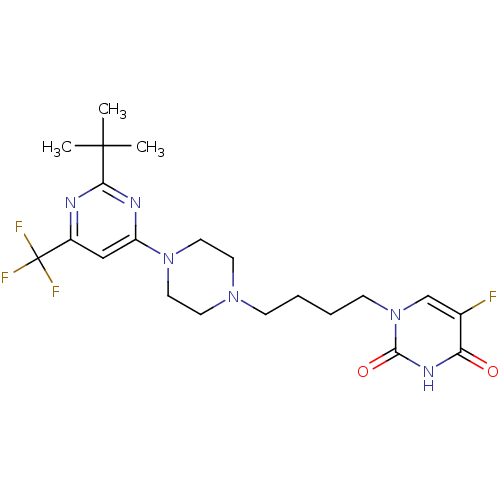 (1-(4-(4-(2-tert-butyl-6-(trifluoromethyl)pyrimidin...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott GmbH & Co. KG Curated by ChEMBL | Assay Description Binding affinity to human cloned dopamine D3 receptor | Bioorg Med Chem Lett 16: 490-4 (2005) Article DOI: 10.1016/j.bmcl.2005.10.068 BindingDB Entry DOI: 10.7270/Q2TH8M89 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 653 total ) | Next | Last >> |