 Found 886 hits with Last Name = 'gerhardt' and Initial = 's'
Found 886 hits with Last Name = 'gerhardt' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337733
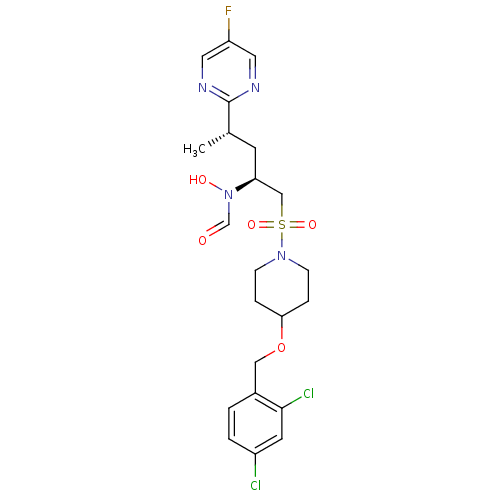
(CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H27Cl2FN4O5S/c1-15(22-26-10-18(25)11-27-22)8-19(29(31)14-30)13-35(32,33)28-6-4-20(5-7-28)34-12-16-2-3-17(23)9-21(16)24/h2-3,9-11,14-15,19-20,31H,4-8,12-13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337721
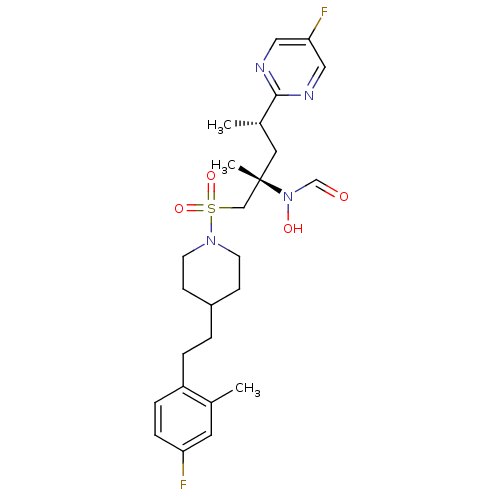
(CHEMBL1683454 | N-((2S,4S)-1-(4-(4-fluoro-2-methyl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34F2N4O4S/c1-18-12-22(26)7-6-21(18)5-4-20-8-10-30(11-9-20)36(34,35)16-25(3,31(33)17-32)13-19(2)24-28-14-23(27)15-29-24/h6-7,12,14-15,17,19-20,33H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337722
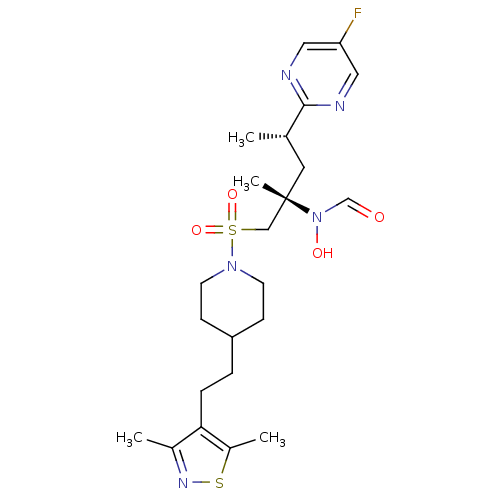
(CHEMBL1683460 | N-((2S,4S)-1-(4-(2-(3,5-dimethylis...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2c(C)nsc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H34FN5O4S2/c1-16(22-25-12-20(24)13-26-22)11-23(4,29(31)15-30)14-35(32,33)28-9-7-19(8-10-28)5-6-21-17(2)27-34-18(21)3/h12-13,15-16,19,31H,5-11,14H2,1-4H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337723
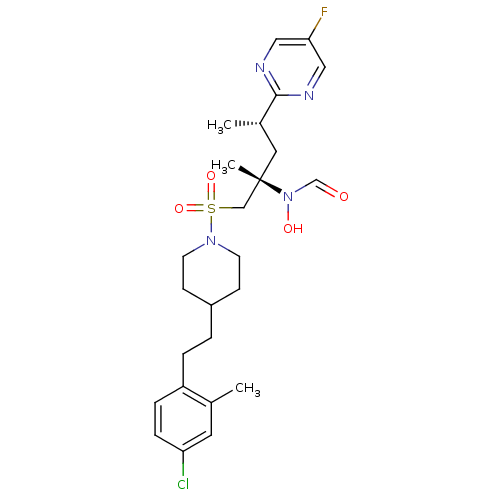
(CHEMBL1683450 | N-((2S,4S)-1-(4-(4-chloro-2-methyl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(Cl)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34ClFN4O4S/c1-18-12-22(26)7-6-21(18)5-4-20-8-10-30(11-9-20)36(34,35)16-25(3,31(33)17-32)13-19(2)24-28-14-23(27)15-29-24/h6-7,12,14-15,17,19-20,33H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337724
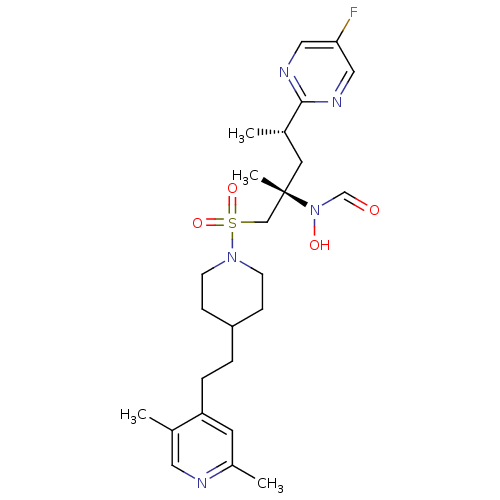
(CHEMBL1683458 | N-((2S,4S)-1-(4-(2-(2,5-dimethylpy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)ncc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H36FN5O4S/c1-18(24-28-14-23(26)15-29-24)12-25(4,31(33)17-32)16-36(34,35)30-9-7-21(8-10-30)5-6-22-11-20(3)27-13-19(22)2/h11,13-15,17-18,21,33H,5-10,12,16H2,1-4H3/t18-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337726
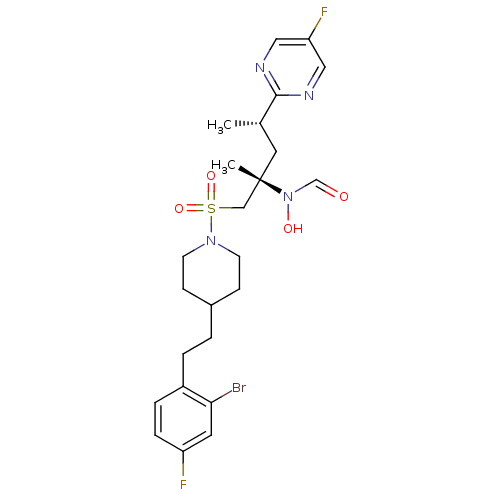
(CHEMBL1683451 | N-((2S,4S)-1-(4-(2-bromo-4-fluorop...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2Br)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H31BrF2N4O4S/c1-17(23-28-13-21(27)14-29-23)12-24(2,31(33)16-32)15-36(34,35)30-9-7-18(8-10-30)3-4-19-5-6-20(26)11-22(19)25/h5-6,11,13-14,16-18,33H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337727
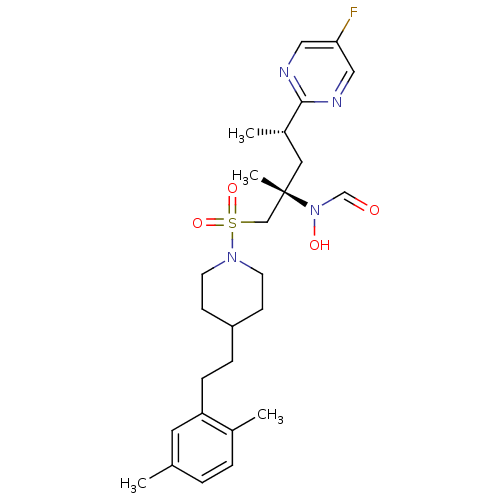
(CHEMBL1683457 | N-((2S,4S)-1-(4-(2,5-dimethylphene...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)ccc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C26H37FN4O4S/c1-19-5-6-20(2)23(13-19)8-7-22-9-11-30(12-10-22)36(34,35)17-26(4,31(33)18-32)14-21(3)25-28-15-24(27)16-29-25/h5-6,13,15-16,18,21-22,33H,7-12,14,17H2,1-4H3/t21-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337725

(CHEMBL1683449 | N-((2S,4S)-1-(4-(2-chloro-4-(trifl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2Cl)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H31ClF4N4O4S/c1-17(23-31-13-21(27)14-32-23)12-24(2,34(36)16-35)15-39(37,38)33-9-7-18(8-10-33)3-4-19-5-6-20(11-22(19)26)25(28,29)30/h5-6,11,13-14,16-18,36H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337736
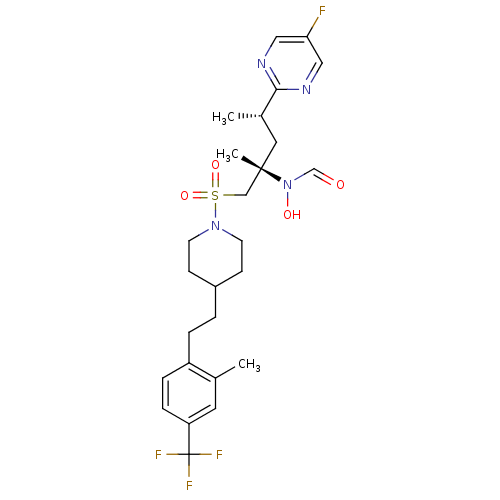
(CHEMBL1683455 | N-((2S,4S)-4-(5-fluoropyrimidin-2-...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2C)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C26H34F4N4O4S/c1-18-12-22(26(28,29)30)7-6-21(18)5-4-20-8-10-33(11-9-20)39(37,38)16-25(3,34(36)17-35)13-19(2)24-31-14-23(27)15-32-24/h6-7,12,14-15,17,19-20,36H,4-5,8-11,13,16H2,1-3H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337731
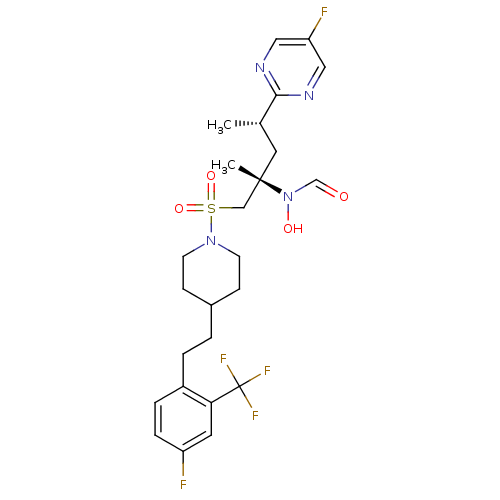
(CHEMBL1683453 | N-((2S,4S)-1-(4-(4-fluoro-2-(trifl...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(F)cc2C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H31F5N4O4S/c1-17(23-31-13-21(27)14-32-23)12-24(2,34(36)16-35)15-39(37,38)33-9-7-18(8-10-33)3-4-19-5-6-20(26)11-22(19)25(28,29)30/h5-6,11,13-14,16-18,36H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337734
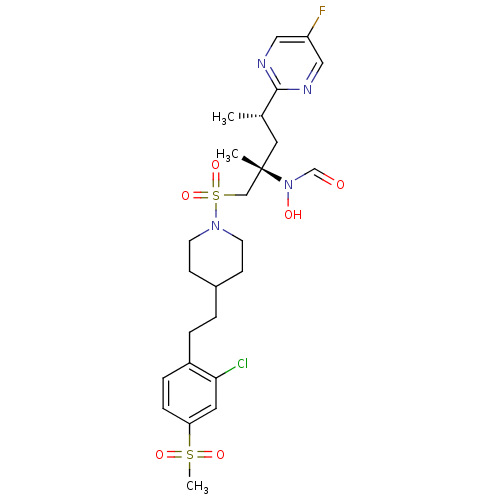
(CHEMBL1683464 | N-((2S,4S)-1-(4-(2-chloro-4-(methy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2Cl)S(C)(=O)=O)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H34ClFN4O6S2/c1-18(24-28-14-21(27)15-29-24)13-25(2,31(33)17-32)16-39(36,37)30-10-8-19(9-11-30)4-5-20-6-7-22(12-23(20)26)38(3,34)35/h6-7,12,14-15,17-19,33H,4-5,8-11,13,16H2,1-3H3/t18-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337739

(CHEMBL1683461 | N-((2S,4S)-1-(4-(2-(4,6-dimethylpy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cnc(C)cc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H36FN5O4S/c1-18-11-20(3)27-13-22(18)6-5-21-7-9-30(10-8-21)36(34,35)16-25(4,31(33)17-32)12-19(2)24-28-14-23(26)15-29-24/h11,13-15,17,19,21,33H,5-10,12,16H2,1-4H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337735
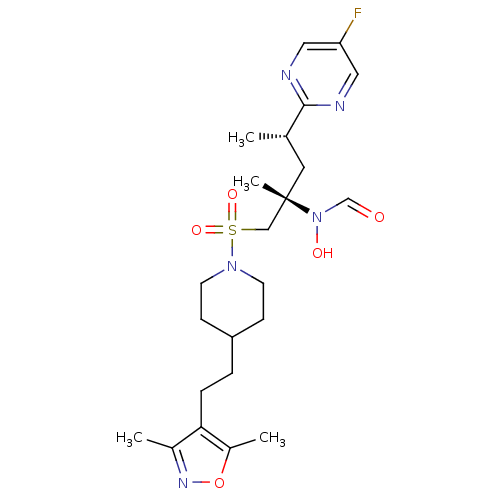
(CHEMBL1615187 | N-[(2S,4S)-1-({4-[2-(3,5-dimethyl-...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2c(C)noc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H34FN5O5S/c1-16(22-25-12-20(24)13-26-22)11-23(4,29(31)15-30)14-35(32,33)28-9-7-19(8-10-28)5-6-21-17(2)27-34-18(21)3/h12-13,15-16,19,31H,5-11,14H2,1-4H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337743
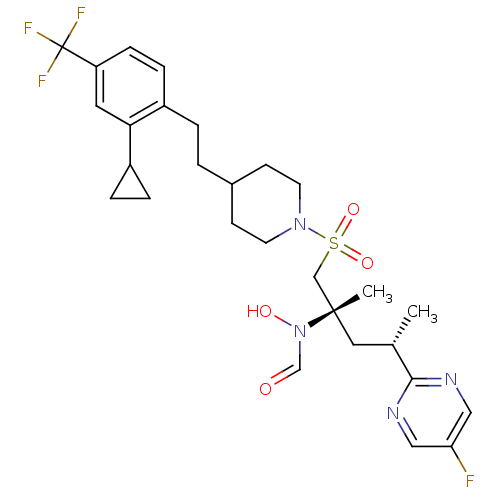
(CHEMBL1683456 | N-((2S,4S)-1-(4-(2-cyclopropyl-4-(...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(cc2C2CC2)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C28H36F4N4O4S/c1-19(26-33-15-24(29)16-34-26)14-27(2,36(38)18-37)17-41(39,40)35-11-9-20(10-12-35)3-4-21-7-8-23(28(30,31)32)13-25(21)22-5-6-22/h7-8,13,15-16,18-20,22,38H,3-6,9-12,14,17H2,1-2H3/t19-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337733

(CHEMBL1683444 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C22H27Cl2FN4O5S/c1-15(22-26-10-18(25)11-27-22)8-19(29(31)14-30)13-35(32,33)28-6-4-20(5-7-28)34-12-16-2-3-17(23)9-21(16)24/h2-3,9-11,14-15,19-20,31H,4-8,12-13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50148829
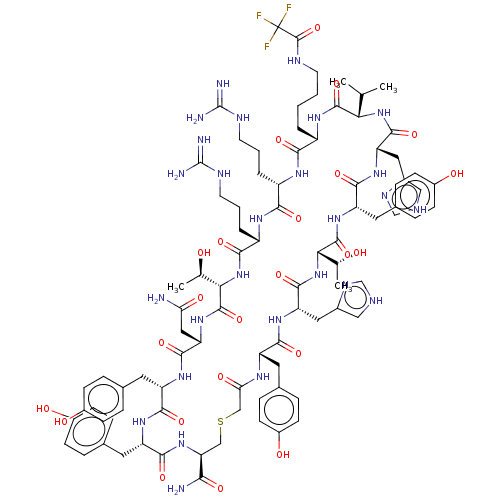
(CHEMBL3769432)Show SMILES [H][C@]1(NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CCCCNC(=O)C(F)(F)F)NC(=O)[C@@H](NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@]([H])(NC(=O)[C@H](Cc2c[nH]cn2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CSC[C@H](NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CC(N)=O)NC1=O)C(N)=O)[C@@H](C)O)C(C)C)[C@@H](C)O |r| Show InChI InChI=1S/C90H122F3N27O23S/c1-44(2)70-84(140)110-57(9-5-6-28-102-87(143)90(91,92)93)74(130)108-58(10-7-29-103-88(96)97)75(131)109-59(11-8-30-104-89(98)99)76(132)119-71(45(3)121)86(142)116-66(37-68(94)127)81(137)112-61(32-48-14-22-54(124)23-15-48)78(134)111-62(33-49-16-24-55(125)25-17-49)80(136)117-67(73(95)129)40-144-41-69(128)107-60(31-47-12-20-53(123)21-13-47)77(133)113-65(36-52-39-101-43-106-52)83(139)120-72(46(4)122)85(141)115-63(34-50-18-26-56(126)27-19-50)79(135)114-64(82(138)118-70)35-51-38-100-42-105-51/h12-27,38-39,42-46,57-67,70-72,121-126H,5-11,28-37,40-41H2,1-4H3,(H2,94,127)(H2,95,129)(H,100,105)(H,101,106)(H,102,143)(H,107,128)(H,108,130)(H,109,131)(H,110,140)(H,111,134)(H,112,137)(H,113,133)(H,114,135)(H,115,141)(H,116,142)(H,117,136)(H,118,138)(H,119,132)(H,120,139)(H4,96,97,103)(H4,98,99,104)/t45-,46-,57+,58+,59+,60+,61+,62+,63+,64+,65+,66+,67+,70+,71+,72+/m1/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Albert-Ludwigs-University Freiburg
Curated by ChEMBL
| Assay Description
Inhibition of human SIRT2 |
J Med Chem 59: 1599-612 (2016)
Article DOI: 10.1021/acs.jmedchem.5b01517
BindingDB Entry DOI: 10.7270/Q2HX1FH2 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337744
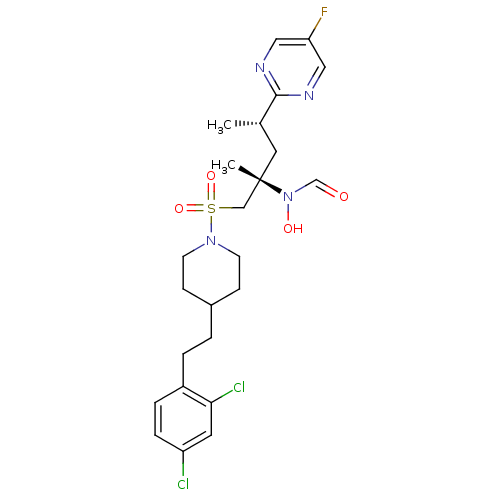
(CHEMBL1683447 | N-((2S,4S)-1-(4-(2,4-dichlorophene...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ccc(Cl)cc2Cl)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H31Cl2FN4O4S/c1-17(23-28-13-21(27)14-29-23)12-24(2,31(33)16-32)15-36(34,35)30-9-7-18(8-10-30)3-4-19-5-6-20(25)11-22(19)26/h5-6,11,13-14,16-18,33H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414644
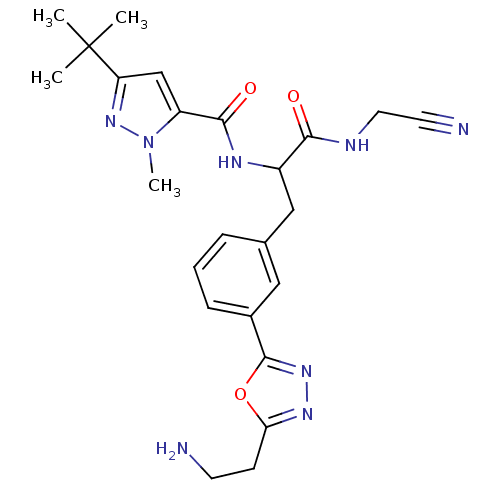
(CHEMBL555122)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CCN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C24H30N8O3/c1-24(2,3)19-14-18(32(4)31-19)22(34)28-17(21(33)27-11-10-26)13-15-6-5-7-16(12-15)23-30-29-20(35-23)8-9-25/h5-7,12,14,17H,8-9,11,13,25H2,1-4H3,(H,27,33)(H,28,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337730
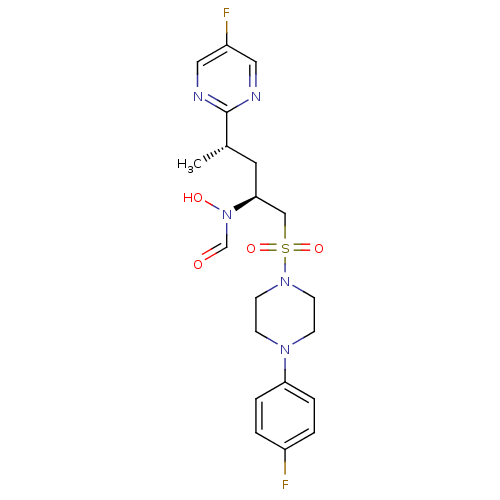
(CHEMBL1683443 | N-((2S,4S)-1-(4-(4-fluorophenyl)pi...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccc(F)cc1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C20H25F2N5O4S/c1-15(20-23-11-17(22)12-24-20)10-19(27(29)14-28)13-32(30,31)26-8-6-25(7-9-26)18-4-2-16(21)3-5-18/h2-5,11-12,14-15,19,29H,6-10,13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50337730

(CHEMBL1683443 | N-((2S,4S)-1-(4-(4-fluorophenyl)pi...)Show SMILES C[C@@H](C[C@@H](CS(=O)(=O)N1CCN(CC1)c1ccc(F)cc1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C20H25F2N5O4S/c1-15(20-23-11-17(22)12-24-20)10-19(27(29)14-28)13-32(30,31)26-8-6-25(7-9-26)18-4-2-16(21)3-5-18/h2-5,11-12,14-15,19,29H,6-10,13H2,1H3/t15-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337737
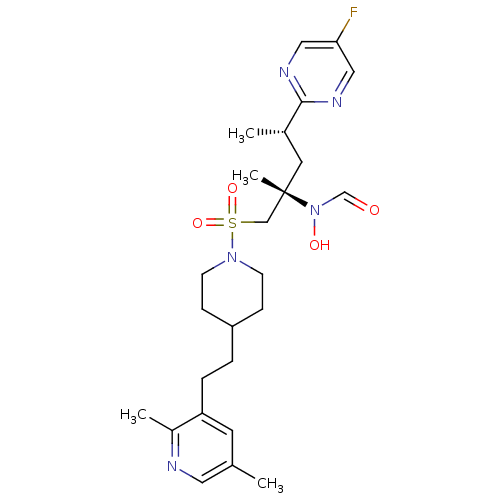
(CHEMBL1683459 | N-((2S,4S)-1-(4-(2-(2,5-dimethylpy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)cnc2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H36FN5O4S/c1-18-11-22(20(3)27-13-18)6-5-21-7-9-30(10-8-21)36(34,35)16-25(4,31(33)17-32)12-19(2)24-28-14-23(26)15-29-24/h11,13-15,17,19,21,33H,5-10,12,16H2,1-4H3/t19-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399335

(CHEMBL2177609)Show SMILES CC(C)(C)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(26.01,-25.71,;26.64,-24.31,;28.17,-24.15,;27.4,-25.64,;25.74,-23.06,;26.22,-21.6,;24.97,-20.69,;23.72,-21.6,;24.2,-23.06,;23.29,-24.31,;21.76,-24.13,;20.85,-25.38,;21.48,-26.79,;23.02,-26.95,;23.91,-25.7,;20.57,-28.04,;19.04,-27.88,;21.2,-29.44,;27.68,-21.12,;28,-19.62,;28.82,-22.16,;30.29,-21.68,;31.49,-20.41,;32.81,-20.9,;34.21,-20.55,;34.22,-19.02,;32.82,-18.44,;31.48,-18.92,;31.79,-19.67,;31.79,-21.26,;33.2,-21.83,)| Show InChI InChI=1S/C25H31N3O3/c1-25(2,3)22-20(13-26-28(22)19-6-4-16(5-7-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h4-7,13-15,17-18,21H,8-12H2,1-3H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human adipocytes assessed as cortisone to cortisol conversion by scintillation counting method |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216
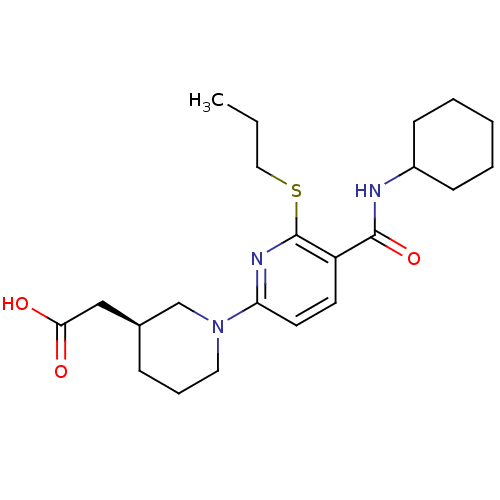
(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human adipocytes assessed as cortisone to cortisol conversion by scintillation counting method |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414641
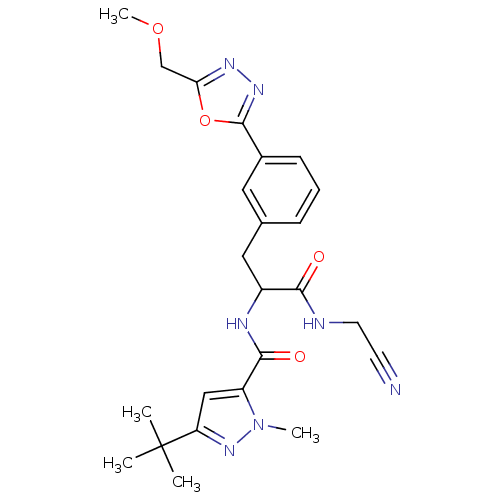
(CHEMBL554065)Show SMILES COCc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O4/c1-24(2,3)19-13-18(31(4)30-19)22(33)27-17(21(32)26-10-9-25)12-15-7-6-8-16(11-15)23-29-28-20(35-23)14-34-5/h6-8,11,13,17H,10,12,14H2,1-5H3,(H,26,32)(H,27,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414643

(CHEMBL557455)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CN)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H28N8O3/c1-23(2,3)18-12-17(31(4)30-18)21(33)27-16(20(32)26-9-8-24)11-14-6-5-7-15(10-14)22-29-28-19(13-25)34-22/h5-7,10,12,16H,9,11,13,25H2,1-4H3,(H,26,32)(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216

(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of 11betaHSD1 in human isolated adipocytes using [3H]cortisone as substrate after 6 hrs by flow scintillation analysis |
J Med Chem 55: 5951-64 (2012)
Article DOI: 10.1021/jm300592r
BindingDB Entry DOI: 10.7270/Q24F1RTK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337740
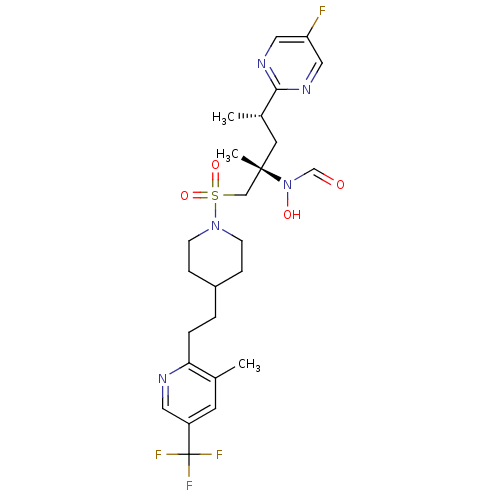
(CHEMBL1683463 | N-((2S,4S)-4-(5-fluoropyrimidin-2-...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ncc(cc2C)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C25H33F4N5O4S/c1-17-10-20(25(27,28)29)12-30-22(17)5-4-19-6-8-33(9-7-19)39(37,38)15-24(3,34(36)16-35)11-18(2)23-31-13-21(26)14-32-23/h10,12-14,16,18-19,36H,4-9,11,15H2,1-3H3/t18-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50414636
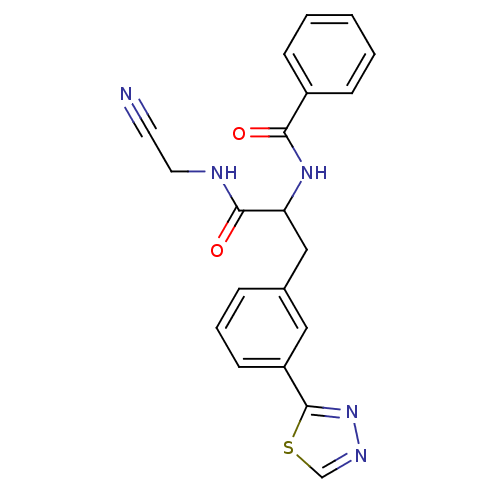
(CHEMBL559880)Show SMILES O=C(NCC#N)C(Cc1cccc(c1)-c1nncs1)NC(=O)c1ccccc1 Show InChI InChI=1S/C20H17N5O2S/c21-9-10-22-19(27)17(24-18(26)15-6-2-1-3-7-15)12-14-5-4-8-16(11-14)20-25-23-13-28-20/h1-8,11,13,17H,10,12H2,(H,22,27)(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin S assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337732
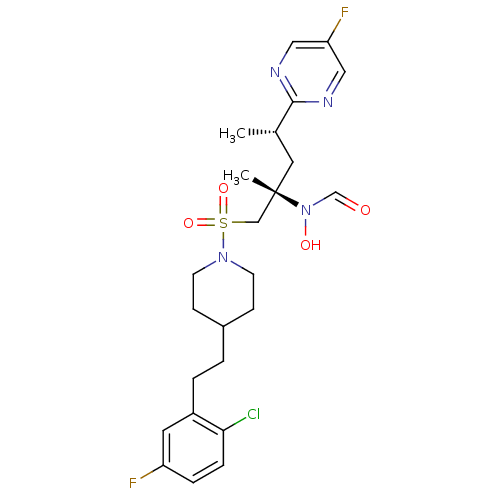
(CHEMBL1683452 | N-((2S,4S)-1-(4-(2-chloro-5-fluoro...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(F)ccc2Cl)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H31ClF2N4O4S/c1-17(23-28-13-21(27)14-29-23)12-24(2,31(33)16-32)15-36(34,35)30-9-7-18(8-10-30)3-4-19-11-20(26)5-6-22(19)25/h5-6,11,13-14,16-18,33H,3-4,7-10,12,15H2,1-2H3/t17-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399353
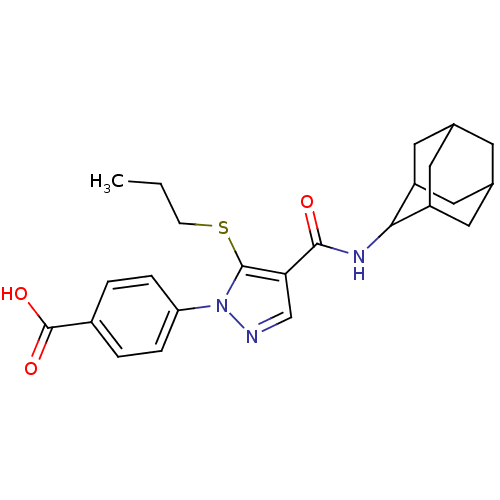
(CHEMBL2177615)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:30:29:27:23.24.25,20:21:23.30.24:28.26.27,THB:30:24:21.29.28:27,20:21:27:23.24.25,25:24:21:28.26.27,25:26:21:23.30.24,(42.49,-16.8,;40.96,-16.96,;40.05,-15.71,;40.68,-14.31,;39.78,-13.06,;40.26,-11.6,;39.01,-10.69,;37.76,-11.6,;38.24,-13.06,;37.33,-14.3,;35.8,-14.13,;34.89,-15.38,;35.51,-16.79,;37.05,-16.94,;37.95,-15.7,;34.61,-18.03,;33.08,-17.87,;35.24,-19.44,;41.72,-11.12,;42.04,-9.62,;42.86,-12.15,;44.33,-11.68,;45.53,-10.4,;46.85,-10.89,;48.25,-10.55,;48.26,-9.02,;46.86,-8.44,;45.52,-8.92,;45.83,-9.67,;45.83,-11.26,;47.24,-11.82,)| Show InChI InChI=1S/C24H29N3O3S/c1-2-7-31-23-20(13-25-27(23)19-5-3-16(4-6-19)24(29)30)22(28)26-21-17-9-14-8-15(11-17)12-18(21)10-14/h3-6,13-15,17-18,21H,2,7-12H2,1H3,(H,26,28)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414640

(CHEMBL562844)Show SMILES Cc1nnc(s1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O2S/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337729
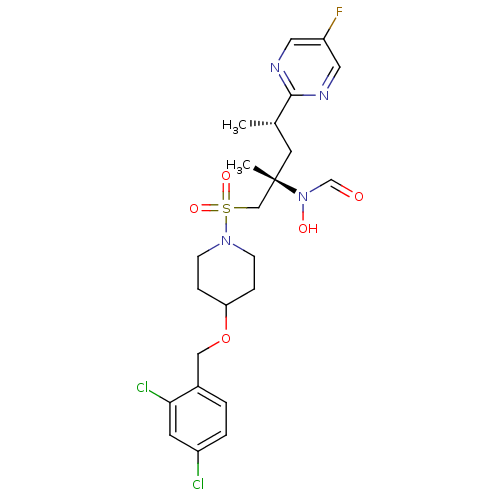
(CHEMBL1615186 | N-((2S,4S)-1-(4-(2,4-dichlorobenzy...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CC1)OCc1ccc(Cl)cc1Cl)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H29Cl2FN4O5S/c1-16(22-27-11-19(26)12-28-22)10-23(2,30(32)15-31)14-36(33,34)29-7-5-20(6-8-29)35-13-17-3-4-18(24)9-21(17)25/h3-4,9,11-12,15-16,20,32H,5-8,10,13-14H2,1-2H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337745
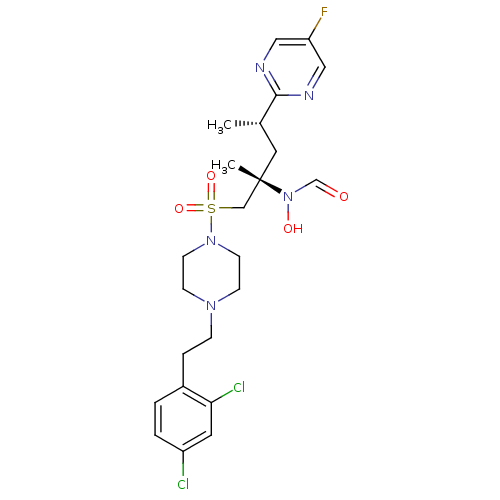
(CHEMBL1683446 | N-((2S,4S)-1-(4-(2,4-dichlorophene...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCN(CCc2ccc(Cl)cc2Cl)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H30Cl2FN5O4S/c1-17(22-27-13-20(26)14-28-22)12-23(2,31(33)16-32)15-36(34,35)30-9-7-29(8-10-30)6-5-18-3-4-19(24)11-21(18)25/h3-4,11,13-14,16-17,33H,5-10,12,15H2,1-2H3/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414641

(CHEMBL554065)Show SMILES COCc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C24H29N7O4/c1-24(2,3)19-13-18(31(4)30-19)22(33)27-17(21(32)26-10-9-25)12-15-7-6-8-16(11-15)23-29-28-20(35-23)14-34-5/h6-8,11,13,17H,10,12,14H2,1-5H3,(H,26,32)(H,27,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399348
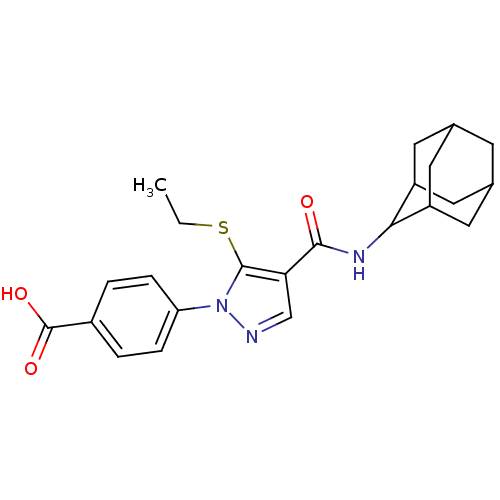
(CHEMBL2177620)Show SMILES CCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:19:20:22.29.23:27.25.26,29:28:26:22.23.24,THB:19:20:26:22.23.24,29:23:20.28.27:26,24:23:20:27.25.26,24:25:20:22.29.23,(-1.15,-44.09,;-2.05,-42.84,;-1.42,-41.44,;-2.34,-40.19,;-1.85,-38.73,;-3.11,-37.83,;-4.34,-38.73,;-3.88,-40.19,;-4.77,-41.44,;-6.3,-41.27,;-7.22,-42.51,;-6.58,-43.92,;-5.04,-44.07,;-4.15,-42.83,;-7.49,-45.16,;-9.03,-45,;-6.86,-46.57,;-.38,-38.26,;-.07,-36.76,;.76,-39.29,;2.22,-38.82,;3.42,-37.54,;4.75,-38.03,;6.14,-37.69,;6.15,-36.16,;4.76,-35.58,;3.41,-36.06,;3.72,-36.81,;3.72,-38.4,;5.13,-38.96,)| Show InChI InChI=1S/C23H27N3O3S/c1-2-30-22-19(12-24-26(22)18-5-3-15(4-6-18)23(28)29)21(27)25-20-16-8-13-7-14(10-16)11-17(20)9-13/h3-6,12-14,16-17,20H,2,7-11H2,1H3,(H,25,27)(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337741
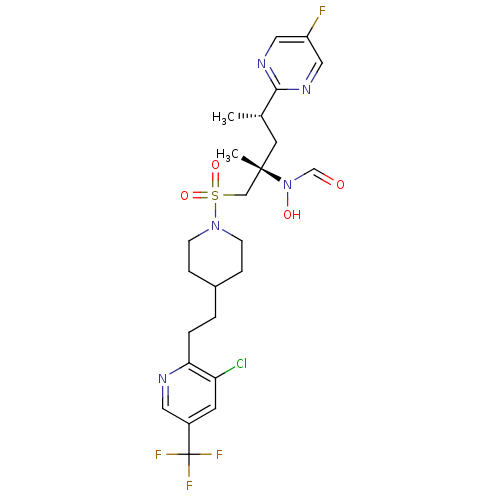
(CHEMBL1683462 | N-((2S,4S)-1-(4-(2-(3-chloro-5-(tr...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2ncc(cc2Cl)C(F)(F)F)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C24H30ClF4N5O4S/c1-16(22-31-12-19(26)13-32-22)10-23(2,34(36)15-35)14-39(37,38)33-7-5-17(6-8-33)3-4-21-20(25)9-18(11-30-21)24(27,28)29/h9,11-13,15-17,36H,3-8,10,14H2,1-2H3/t16-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50384389
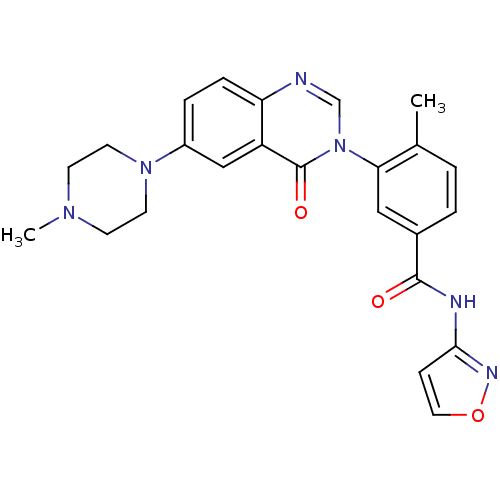
(CHEMBL2031461)Show SMILES CN1CCN(CC1)c1ccc2ncn(-c3cc(ccc3C)C(=O)Nc3ccon3)c(=O)c2c1 Show InChI InChI=1S/C24H24N6O3/c1-16-3-4-17(23(31)26-22-7-12-33-27-22)13-21(16)30-15-25-20-6-5-18(14-19(20)24(30)32)29-10-8-28(2)9-11-29/h3-7,12-15H,8-11H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4.68 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha-mediated TNFalpha secretion in LPS-stimulated human whole blood assessed as IC50 equals to free drug concentration at human wh... |
Bioorg Med Chem Lett 22: 3879-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.116
BindingDB Entry DOI: 10.7270/Q2VH5PWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399346

(CHEMBL2180883)Show SMILES OC(=O)c1ccc(cc1)-n1ncc(C(=O)NC2C3CC4CC(C3)CC2C4)c1SC1CCCC1 |TLB:15:16:18.25.19:23.21.22,25:24:22:18.19.20,THB:15:16:22:18.19.20,25:19:16.24.23:22,20:19:16:23.21.22,20:21:16:18.25.19,(27.7,-42.8,;29.23,-42.96,;29.85,-44.37,;30.13,-41.71,;29.51,-40.3,;30.41,-39.06,;31.95,-39.23,;32.57,-40.63,;31.67,-41.87,;32.85,-37.99,;32.38,-36.52,;33.62,-35.61,;34.87,-36.52,;36.34,-36.05,;36.66,-34.54,;37.48,-37.08,;38.95,-36.61,;40.14,-35.33,;41.47,-35.82,;42.87,-35.47,;42.88,-33.95,;41.48,-33.37,;40.13,-33.85,;40.44,-34.6,;40.45,-36.19,;41.85,-36.75,;34.39,-37.99,;35.3,-39.23,;34.67,-40.64,;33.17,-40.96,;33,-42.49,;34.41,-43.12,;35.44,-41.97,)| Show InChI InChI=1S/C26H31N3O3S/c30-24(28-23-18-10-15-9-16(12-18)13-19(23)11-15)22-14-27-29(25(22)33-21-3-1-2-4-21)20-7-5-17(6-8-20)26(31)32/h5-8,14-16,18-19,21,23H,1-4,9-13H2,(H,28,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399351
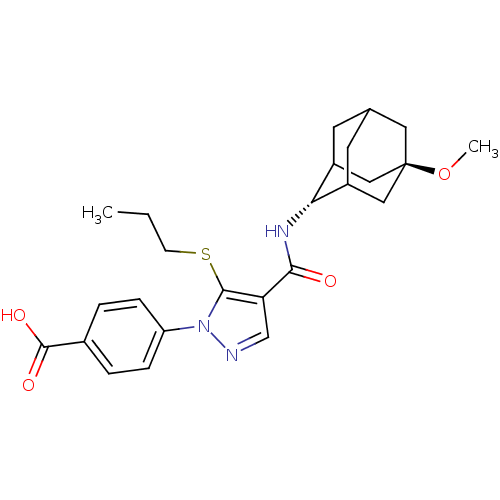
(CHEMBL2177617)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)N[C@H]1C2CC3CC1C[C@](C3)(C2)OC |r,wU:21.22,wD:28.35,TLB:20:21:30.27.28:25.24.23,20:21:23:30.28.29,31:28:21.26.25:23,THB:27:26:23:30.28.29,27:28:21.26.25:23,29:28:21:25.24.23,29:24:21:30.27.28,31:28:21:25.24.23,(6.35,-29.86,;4.82,-30.02,;3.92,-28.78,;4.54,-27.37,;3.64,-26.12,;4.12,-24.66,;2.87,-23.75,;1.63,-24.66,;2.1,-26.12,;1.19,-27.37,;-.35,-27.2,;-1.26,-28.44,;-.62,-29.85,;.92,-30.01,;1.82,-28.76,;-1.54,-31.1,;-3.07,-30.94,;-.9,-32.51,;5.58,-24.19,;5.91,-22.68,;6.73,-25.22,;8.19,-24.75,;9.39,-23.47,;9.38,-21.98,;10.73,-21.51,;9.69,-22.73,;9.69,-24.32,;11.1,-24.89,;12.11,-23.61,;12.12,-22.08,;10.72,-23.96,;13.65,-23.55,;14.47,-24.85,)| Show InChI InChI=1S/C25H31N3O4S/c1-3-8-33-23-20(14-26-28(23)19-6-4-16(5-7-19)24(30)31)22(29)27-21-17-9-15-10-18(21)13-25(11-15,12-17)32-2/h4-7,14-15,17-18,21H,3,8-13H2,1-2H3,(H,27,29)(H,30,31)/t15?,17?,18?,21-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414639
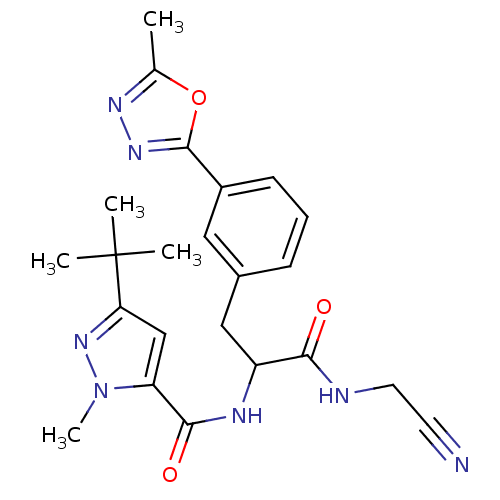
(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414638
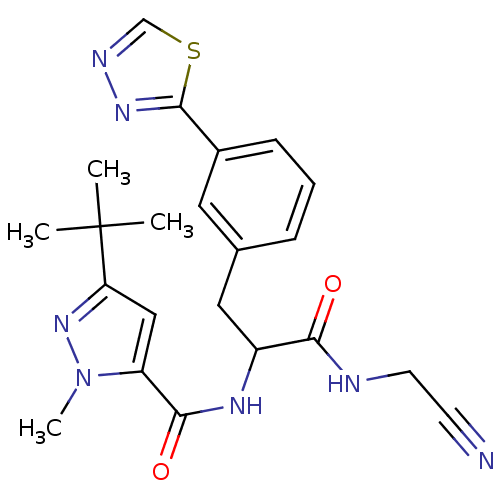
(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
A disintegrin and metalloproteinase with thrombospondin motifs 4
(Homo sapiens (Human)) | BDBM50337742
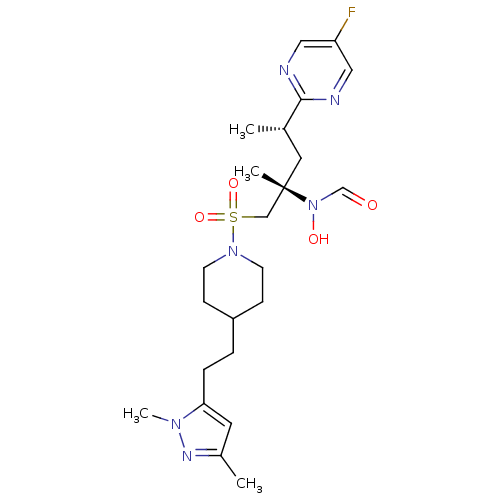
(CHEMBL1683465 | N-((2S,4S)-1-(4-(2-(1,3-dimethyl-1...)Show SMILES C[C@@H](C[C@@](C)(CS(=O)(=O)N1CCC(CCc2cc(C)nn2C)CC1)N(O)C=O)c1ncc(F)cn1 |r| Show InChI InChI=1S/C23H35FN6O4S/c1-17(22-25-13-20(24)14-26-22)12-23(3,30(32)16-31)15-35(33,34)29-9-7-19(8-10-29)5-6-21-11-18(2)27-28(21)4/h11,13-14,16-17,19,32H,5-10,12,15H2,1-4H3/t17-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of ADAMTS-4 assessed as substrate cleavage by measuring increase in fluorescence after 16 hrs |
Bioorg Med Chem Lett 21: 1376-81 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.036
BindingDB Entry DOI: 10.7270/Q2DV1K5M |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399336

(CHEMBL2177608)Show SMILES CC1(CC1)c1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)NC1C2CC3CC(C2)CC1C3 |TLB:20:21:23.30.24:28.26.27,30:29:27:23.24.25,THB:20:21:27:23.24.25,30:24:21.29.28:27,25:24:21:28.26.27,25:26:21:23.30.24,(10.13,-23.34,;8.59,-23.5,;8.59,-25.04,;7.26,-24.26,;7.69,-22.25,;8.17,-20.79,;6.92,-19.88,;5.68,-20.79,;6.15,-22.25,;5.25,-23.5,;3.71,-23.33,;2.81,-24.57,;3.43,-25.98,;4.97,-26.14,;5.87,-24.89,;2.53,-27.23,;.99,-27.07,;3.15,-28.63,;9.64,-20.31,;9.96,-18.81,;10.78,-21.35,;12.24,-20.87,;13.44,-19.6,;14.77,-20.09,;16.17,-19.74,;16.18,-18.21,;14.78,-17.63,;13.43,-18.11,;13.74,-18.86,;13.75,-20.45,;15.15,-21.02,)| Show InChI InChI=1S/C25H29N3O3/c1-25(6-7-25)22-20(13-26-28(22)19-4-2-16(3-5-19)24(30)31)23(29)27-21-17-9-14-8-15(11-17)12-18(21)10-14/h2-5,13-15,17-18,21H,6-12H2,1H3,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50399352

(CHEMBL2177616)Show SMILES CCCSc1c(cnn1-c1ccc(cc1)C(O)=O)C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:21.22,wD:28.31,TLB:20:21:31.27.28:25.24.23,20:21:23:31.28.30,29:28:21.26.25:23,THB:27:26:23:31.28.30,27:28:21.26.25:23,30:28:21:25.24.23,30:24:21:31.27.28,29:28:21:25.24.23,(58.75,-15.24,;57.22,-15.4,;56.32,-14.15,;56.94,-12.74,;56.04,-11.5,;56.52,-10.03,;55.27,-9.12,;54.03,-10.03,;54.5,-11.5,;53.59,-12.74,;52.06,-12.57,;51.15,-13.81,;51.78,-15.22,;53.32,-15.38,;54.22,-14.14,;50.87,-16.47,;49.34,-16.31,;51.5,-17.88,;57.98,-9.56,;58.31,-8.05,;59.13,-10.59,;60.59,-10.12,;61.79,-8.84,;61.78,-7.36,;63.13,-6.88,;62.09,-8.11,;62.09,-9.7,;63.5,-10.26,;64.51,-8.98,;66.05,-8.92,;64.52,-7.46,;63.12,-9.33,)| Show InChI InChI=1S/C24H29N3O4S/c1-2-7-32-22-19(13-25-27(22)18-5-3-15(4-6-18)23(29)30)21(28)26-20-16-8-14-9-17(20)12-24(31,10-14)11-16/h3-6,13-14,16-17,20,31H,2,7-12H2,1H3,(H,26,28)(H,29,30)/t14?,16?,17?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414639

(CHEMBL550872)Show SMILES Cc1nnc(o1)-c1cccc(CC(NC(=O)c2cc(nn2C)C(C)(C)C)C(=O)NCC#N)c1 Show InChI InChI=1S/C23H27N7O3/c1-14-27-28-22(33-14)16-8-6-7-15(11-16)12-17(20(31)25-10-9-24)26-21(32)18-13-19(23(2,3)4)29-30(18)5/h6-8,11,13,17H,10,12H2,1-5H3,(H,25,31)(H,26,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Cathepsin L2
(Homo sapiens (Human)) | BDBM50414638

(CHEMBL549378)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nncs1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C22H25N7O2S/c1-22(2,3)18-12-17(29(4)28-18)20(31)26-16(19(30)24-9-8-23)11-14-6-5-7-15(10-14)21-27-25-13-32-21/h5-7,10,12-13,16H,9,11H2,1-4H3,(H,24,30)(H,26,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L2 assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50414642

(CHEMBL549791)Show SMILES Cn1nc(cc1C(=O)NC(Cc1cccc(c1)-c1nnc(CO)o1)C(=O)NCC#N)C(C)(C)C Show InChI InChI=1S/C23H27N7O4/c1-23(2,3)18-12-17(30(4)29-18)21(33)26-16(20(32)25-9-8-24)11-14-6-5-7-15(10-14)22-28-27-19(13-31)34-22/h5-7,10,12,16,31H,9,11,13H2,1-4H3,(H,25,32)(H,26,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin L assessed as inhibition of fluorogenic substrate cleavage |
Bioorg Med Chem Lett 19: 4622-5 (2009)
Article DOI: 10.1016/j.bmcl.2009.06.090
BindingDB Entry DOI: 10.7270/Q2N017S3 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50384389

(CHEMBL2031461)Show SMILES CN1CCN(CC1)c1ccc2ncn(-c3cc(ccc3C)C(=O)Nc3ccon3)c(=O)c2c1 Show InChI InChI=1S/C24H24N6O3/c1-16-3-4-17(23(31)26-22-7-12-33-27-22)13-21(16)30-15-25-20-6-5-18(14-19(20)24(30)32)29-10-8-28(2)9-11-29/h3-7,12-15H,8-11H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.61 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha kinase |
Bioorg Med Chem Lett 22: 3879-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.116
BindingDB Entry DOI: 10.7270/Q2VH5PWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 14
(Homo sapiens (Human)) | BDBM50384389

(CHEMBL2031461)Show SMILES CN1CCN(CC1)c1ccc2ncn(-c3cc(ccc3C)C(=O)Nc3ccon3)c(=O)c2c1 Show InChI InChI=1S/C24H24N6O3/c1-16-3-4-17(23(31)26-22-7-12-33-27-22)13-21(16)30-15-25-20-6-5-18(14-19(20)24(30)32)29-10-8-28(2)9-11-29/h3-7,12-15H,8-11H2,1-2H3,(H,26,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of p38alpha kinase |
Bioorg Med Chem Lett 22: 3879-83 (2012)
Article DOI: 10.1016/j.bmcl.2012.04.116
BindingDB Entry DOI: 10.7270/Q2VH5PWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50392216

(CHEMBL2153191 | US8673938, 7)Show SMILES CCCSc1nc(ccc1C(=O)NC1CCCCC1)N1CCC[C@@H](CC(O)=O)C1 |r| Show InChI InChI=1S/C22H33N3O3S/c1-2-13-29-22-18(21(28)23-17-8-4-3-5-9-17)10-11-19(24-22)25-12-6-7-16(15-25)14-20(26)27/h10-11,16-17H,2-9,12-15H2,1H3,(H,23,28)(H,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal 6-His tagged full length human 11betaHSD1 incubated for 25 mins by HTRF assay |
J Med Chem 55: 10136-47 (2012)
Article DOI: 10.1021/jm301252n
BindingDB Entry DOI: 10.7270/Q2WM1FJT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data