

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50034908 ((2R,3S)-3-((R)-6-Amino-purin-9-yl)-nonan-2-ol | (2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against Human erythrocyte adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50011575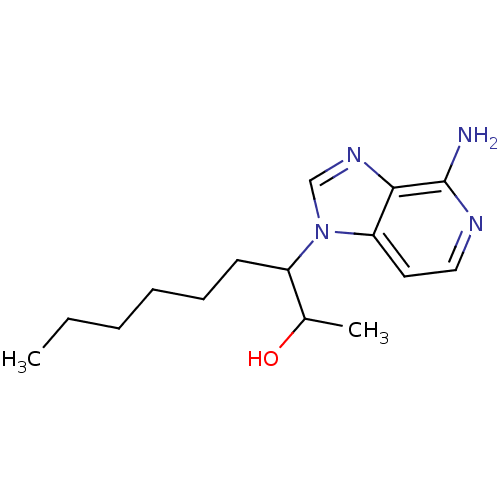 (3-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-nonan-2-ol ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against Human erythrocyte adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50034908 ((2R,3S)-3-((R)-6-Amino-purin-9-yl)-nonan-2-ol | (2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against calf intestine adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50011575 (3-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-nonan-2-ol ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against calf intestine adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50011591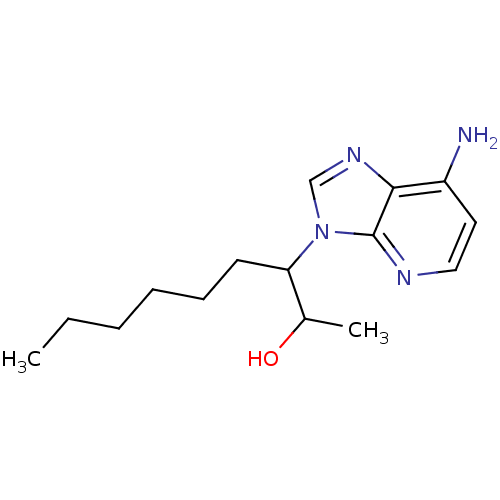 (3-(7-Amino-imidazo[4,5-b]pyridin-3-yl)-nonan-2-ol ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against Human erythrocyte adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50011591 (3-(7-Amino-imidazo[4,5-b]pyridin-3-yl)-nonan-2-ol ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against calf intestine adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50011565 (3-(4-Amino-benzoimidazol-1-yl)-nonan-2-ol | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against Human erythrocyte adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50011565 (3-(4-Amino-benzoimidazol-1-yl)-nonan-2-ol | CHEMBL...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 7.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against calf intestine adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Homo sapiens (Human)) | BDBM50011566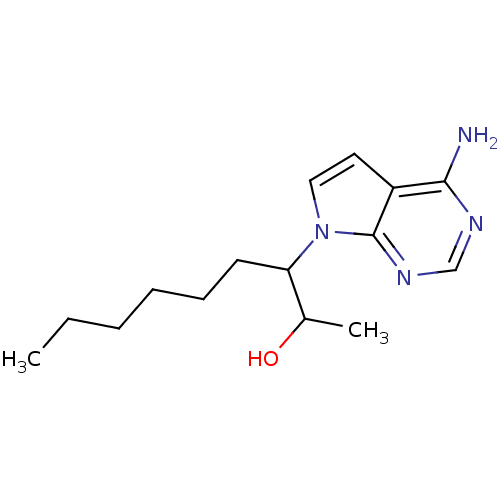 (3-(4-Amino-pyrrolo[2,3-d]pyrimidin-7-yl)-nonan-2-o...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against Human erythrocyte adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine deaminase (Bos taurus (bovine)) | BDBM50011566 (3-(4-Amino-pyrrolo[2,3-d]pyrimidin-7-yl)-nonan-2-o...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Camerino Curated by ChEMBL | Assay Description Inhibition constant (Ki) against calf intestine adenosine deaminase | J Med Chem 31: 390-3 (1988) BindingDB Entry DOI: 10.7270/Q2X067M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089969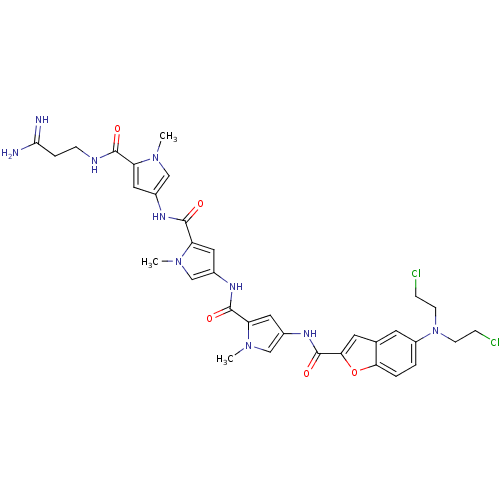 (5-[Bis-(2-chloro-ethyl)-amino]-benzofuran-2-carbox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089961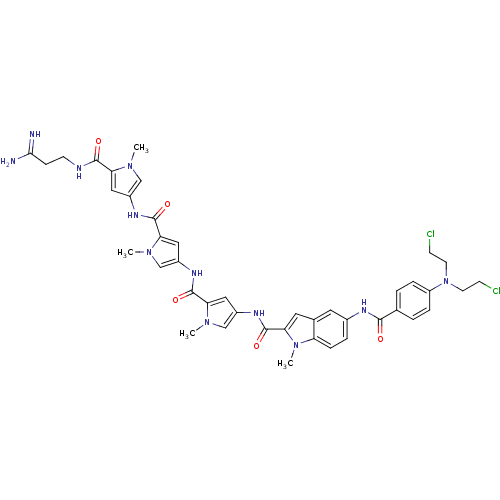 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089960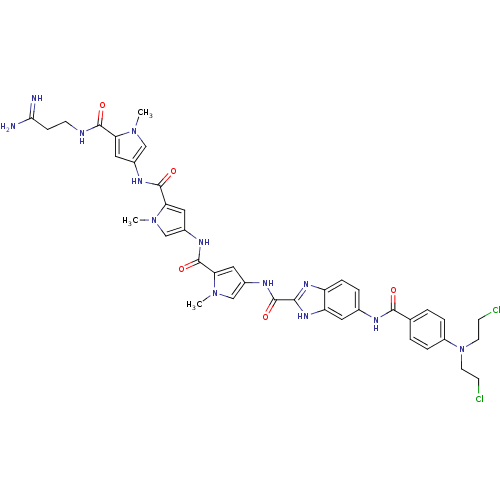 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089967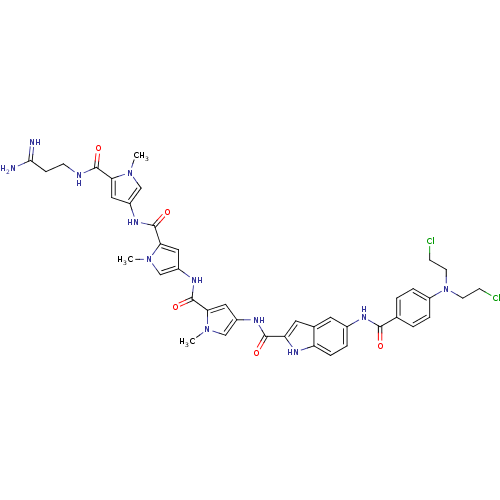 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089962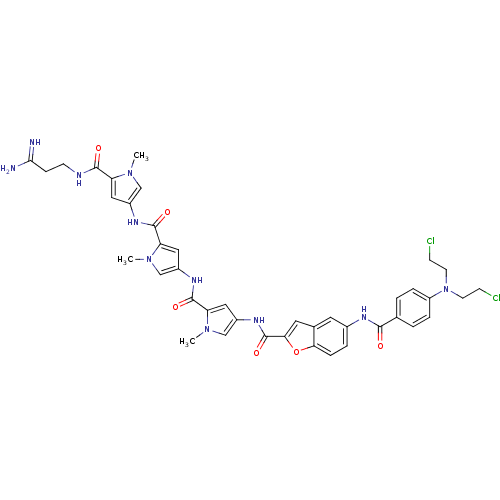 (5-{4-[Bis-(2-chloro-ethyl)-amino]-benzoylamino}-be...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089978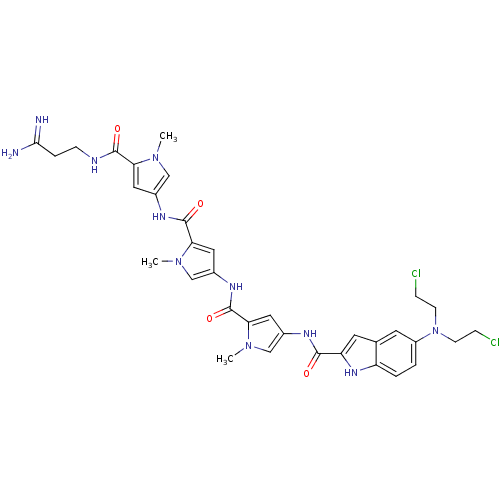 (5-[Bis-(2-chloro-ethyl)-amino]-1H-indole-2-carboxy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50089971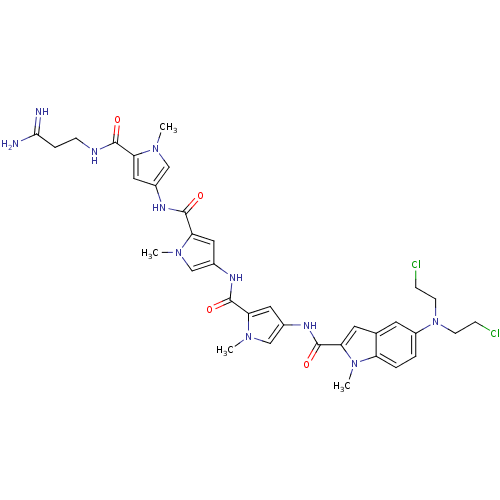 (5-[Bis-(2-chloro-ethyl)-amino]-1-methyl-1H-indole-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase HRas (Homo sapiens (Human)) | BDBM50055659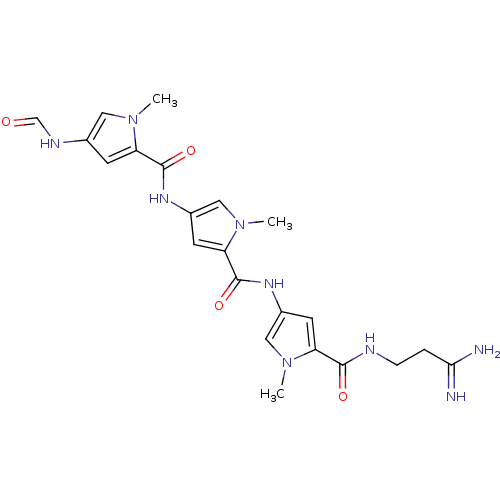 (2N-(3,3-aminoiminopropyl)-4-[4-(4-formamido-1-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Ferrara Curated by ChEMBL | Assay Description Tested for 50% inhibition of generation of Human Ha-ras polymerase chain reaction(PCR) products | J Med Chem 43: 2675-84 (2000) BindingDB Entry DOI: 10.7270/Q2959J79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50008936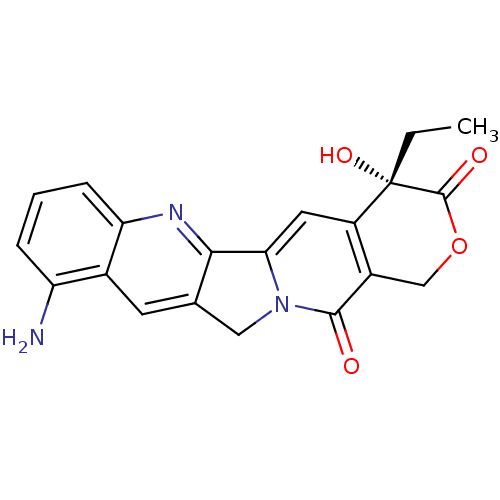 ((S)-10-Amino-4-ethyl-4-hydroxy-1,12-dihydro-4H-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291417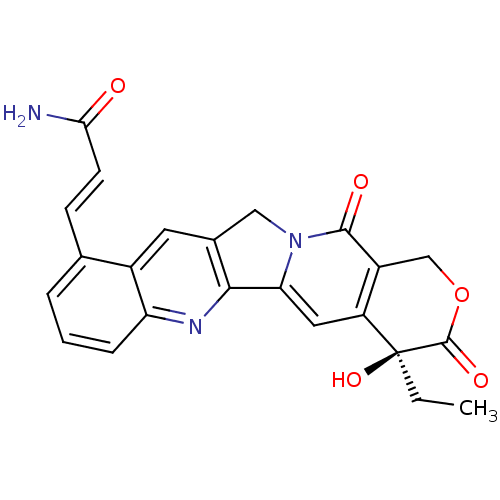 ((E)-3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291415 ((S)-4-Ethyl-4-hydroxy-10-vinyl-1,12-dihydro-4H-2-o...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291418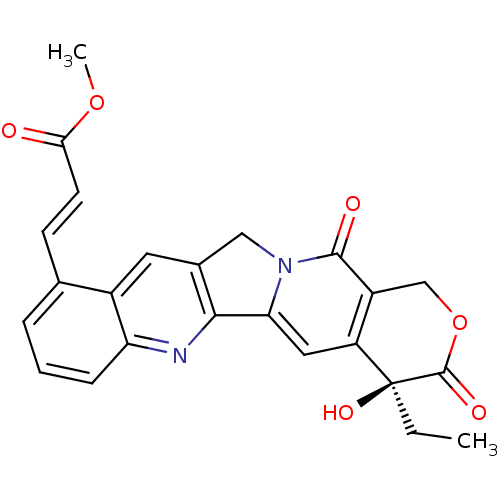 ((E)-3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291416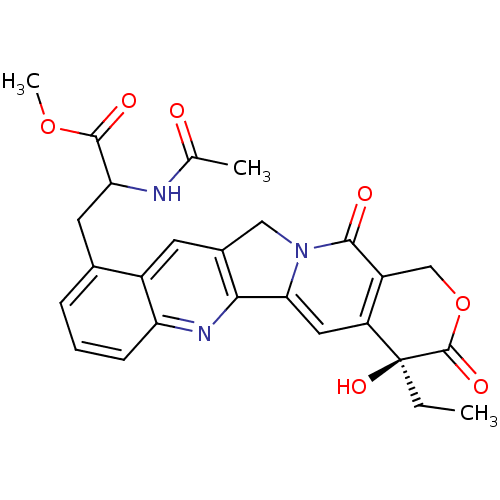 (2-Acetylamino-3-((S)-4-ethyl-4-hydroxy-3,13-dioxo-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291412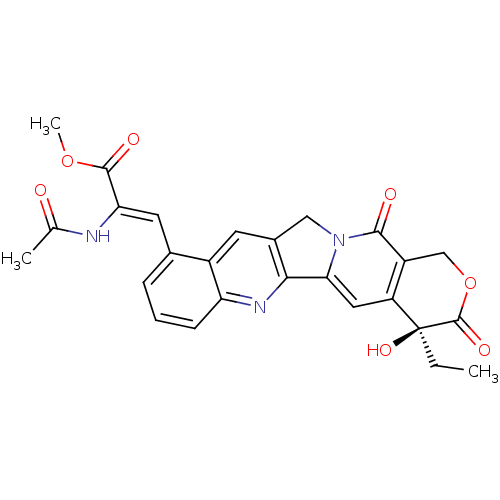 ((Z)-2-Acetylamino-3-((S)-4-ethyl-4-hydroxy-3,13-di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291410 (3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-tetr...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 4.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291411 ((S)-4-Ethyl-4-hydroxy-10-((E)-styryl)-1,12-dihydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291413 ((E)-3-((S)-4-Ethyl-4-hydroxy-3,13-dioxo-3,4,12,13-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 1 (Homo sapiens (Human)) | BDBM50291414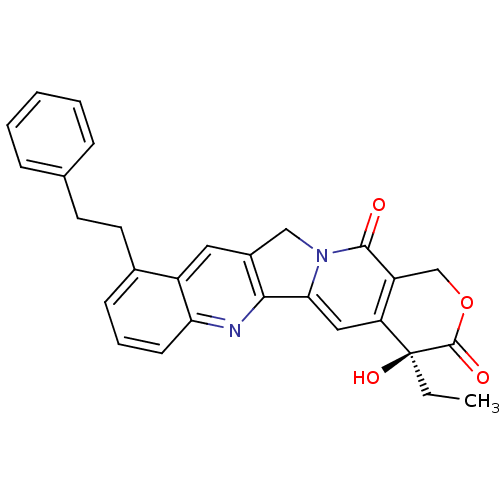 ((S)-4-Ethyl-4-hydroxy-10-phenethyl-1,12-dihydro-4H...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit 50% relaxation of 250 ng of SV40 DNA obtained with 0.5 I.U topoisomerase I at 37 degrees C for 30 min | Bioorg Med Chem Lett 7: 847-850 (1997) Article DOI: 10.1016/S0960-894X(97)00105-4 BindingDB Entry DOI: 10.7270/Q2T43T3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||