

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM162123 (US9051279, 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of human MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Canis lupus familiaris) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of dog MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Mus musculus) | BDBM50229787 ((4S,5R)-Nutlin-3 | (rac)-(4,5-bis(4-chlorophenyl)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mouse MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Canis lupus familiaris) | BDBM162123 (US9051279, 106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of dog MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Mus musculus) | BDBM162123 (US9051279, 106) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Inhibition of mouse MDM2 by TR-FRET assay | J Med Chem 58: 6348-58 (2015) Article DOI: 10.1021/acs.jmedchem.5b00810 BindingDB Entry DOI: 10.7270/Q2HQ41QV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50546172 (CHEMBL4762397) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423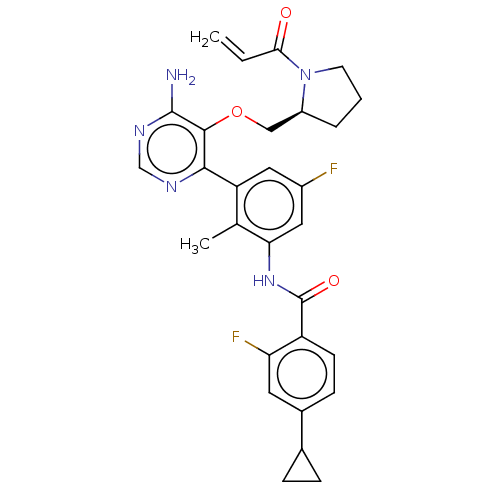 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259423 (US10457647, Example 22 | US11180460, Example 22 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <0.100 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 183 (Homo sapiens (Human)) | BDBM50011716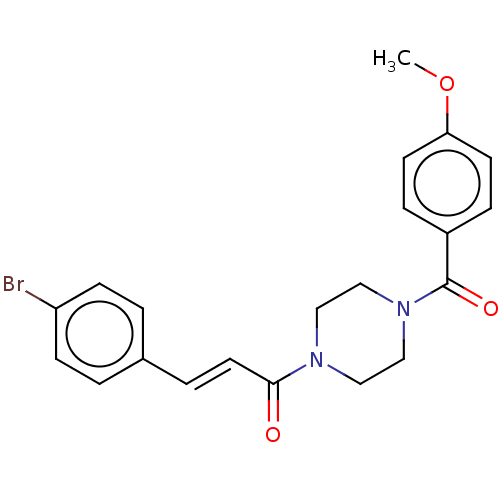 (CHEMBL3262896) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at EBI2 receptor in human U937 cells assessed as inhibition of 7-alpha,25-OHC-induced cell migration after 3 hrs by flow cytometr... | J Med Chem 57: 3358-68 (2014) Article DOI: 10.1021/jm4019355 BindingDB Entry DOI: 10.7270/Q2BG2QJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427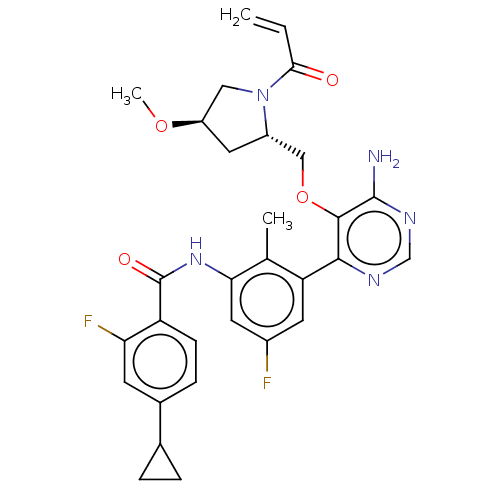 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259427 (US10457647, Example 26 | US11180460, Example 26 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425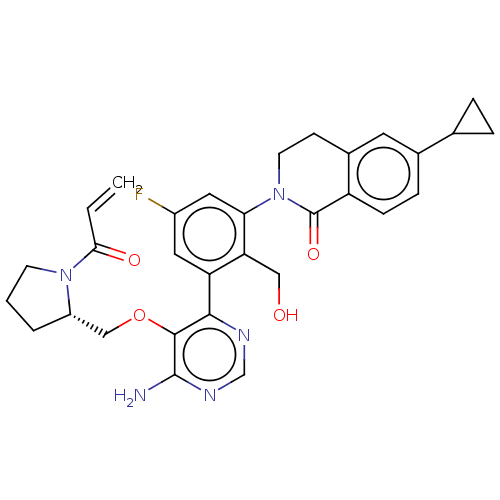 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259425 (US10457647, Example 24 | US11180460, Example 24 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50546180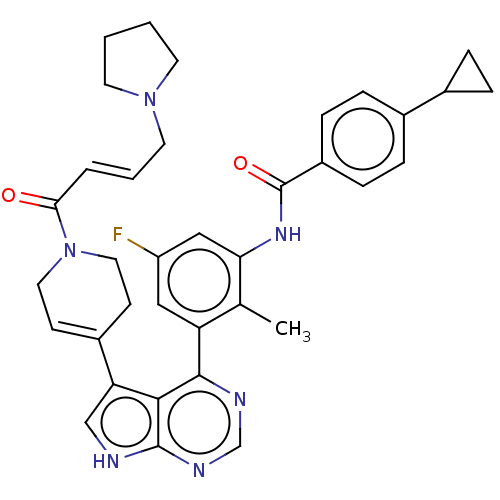 (CHEMBL4749522) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase Mdm2 (Homo sapiens (Human)) | BDBM162123 (US9051279, 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB US Patent | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibition of p53-Hdm2 and p53-Hdm4 interactions is measured by time resolved fluorescence energy transfer (TR-FRET). Fluorescence energy transfe... | US Patent US9051279 (2015) BindingDB Entry DOI: 10.7270/Q2DV1HMM | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50514642 (CHEMBL4593663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50514642 (CHEMBL4593663) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay | J Med Chem 63: 5102-5118 (2020) Article DOI: 10.1021/acs.jmedchem.9b01916 BindingDB Entry DOI: 10.7270/Q2GB27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259415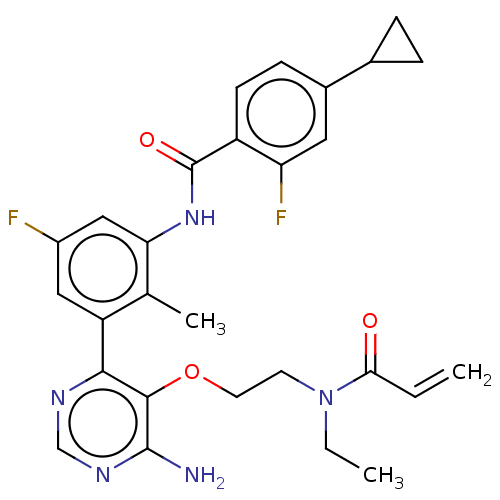 (US10457647, Example 14 | US11180460, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full-length human recombinant BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate measured after 60 mins by caliper assay | J Med Chem 63: 5102-5118 (2020) Article DOI: 10.1021/acs.jmedchem.9b01916 BindingDB Entry DOI: 10.7270/Q2GB27FW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259415 (US10457647, Example 14 | US11180460, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259416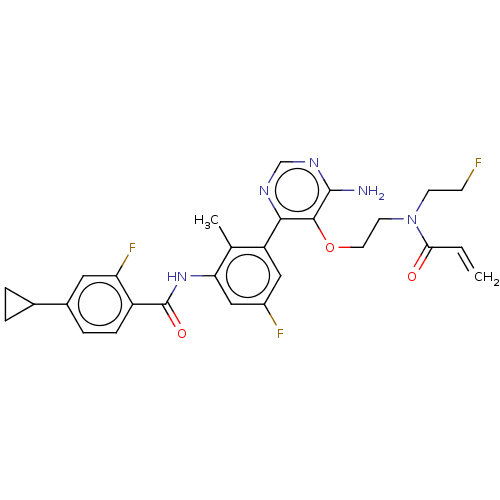 (US10457647, Example 15 | US11180460, Example 15 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259434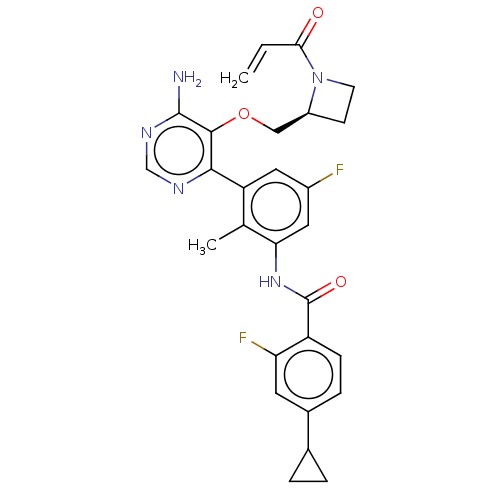 (US10457647, Example 33 | US11180460, Example 33 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259429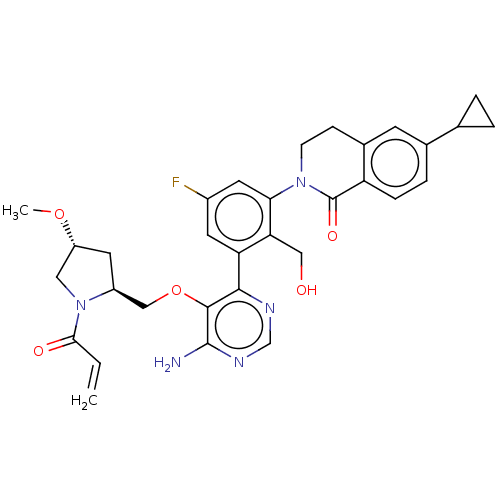 (US10457647, Example 28 | US11180460, Example 28 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259426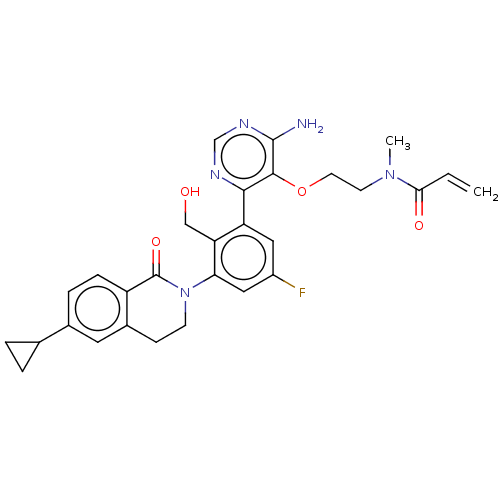 (US10457647, Example 25 | US11180460, Example 25 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259424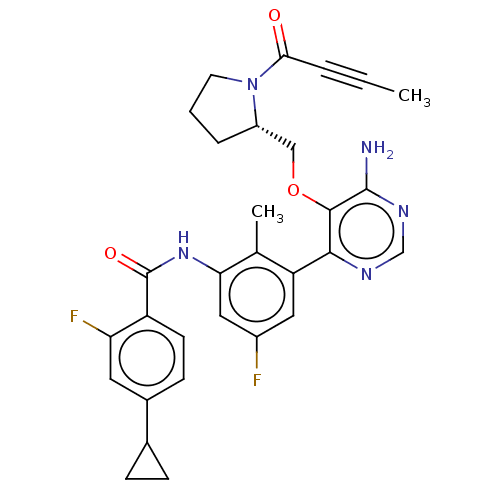 (US10457647, Example 23 | US11180460, Example 23 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259419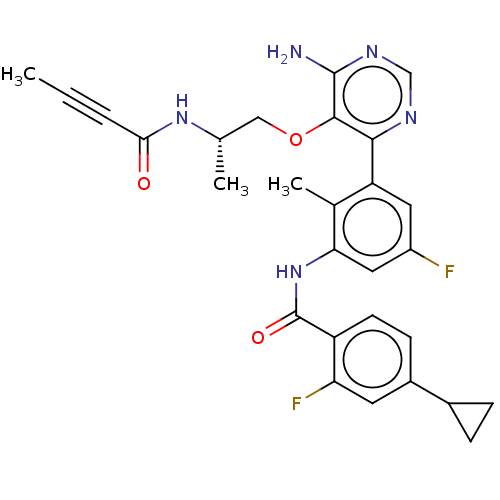 (US10457647, Example 18 | US11180460, Example 18 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259416 (US10457647, Example 15 | US11180460, Example 15 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259415 (US10457647, Example 14 | US11180460, Example 14 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259407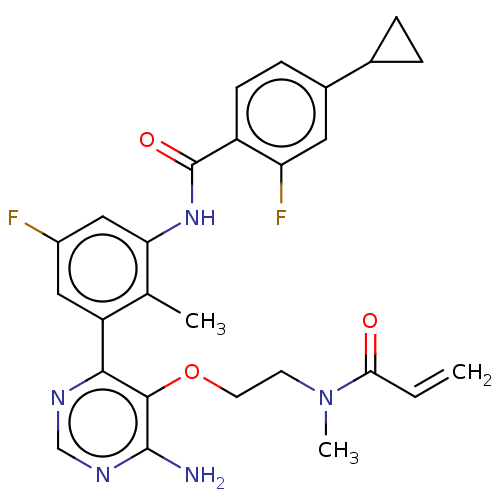 (US10457647, Example 6 | US11180460, Example 6 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259404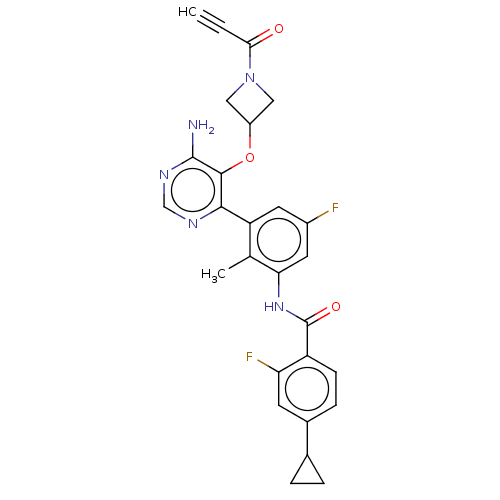 (US10457647, Example 3 | US11180460, Example 3 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | Citation and Details BindingDB Entry DOI: 10.7270/Q20R9SKR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50546179 (CHEMBL4739958) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of full length human unphosphorylated BTK using FITC-Ahx-TSELKKVVALYDYMPMNAND-NH2 as substrate incubated for 60 mins in presence of ATP at... | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytoplasmic tyrosine-protein kinase BMX (Homo sapiens (Human)) | BDBM50357312 (IBRUTINIB | PCI-32765 | US10124003, Ref. Ex. Compo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of BMX (unknown origin) | Citation and Details Article DOI: 10.1021/acsmedchemlett.9b00317 BindingDB Entry DOI: 10.7270/Q20K2D40 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259440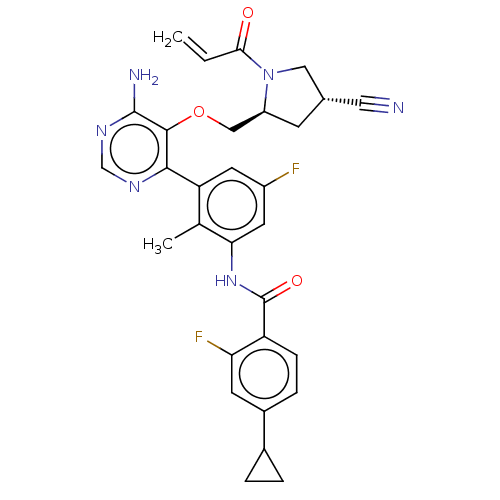 (US10457647, Example 39 | US11180460, Example 39 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259434 (US10457647, Example 33 | US11180460, Example 33 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259429 (US10457647, Example 28 | US11180460, Example 28 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2PV6Q9F | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259419 (US10457647, Example 18 | US11180460, Example 18 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259424 (US10457647, Example 23 | US11180460, Example 23 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259426 (US10457647, Example 25 | US11180460, Example 25 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259429 (US10457647, Example 28 | US11180460, Example 28 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259434 (US10457647, Example 33 | US11180460, Example 33 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259440 (US10457647, Example 39 | US11180460, Example 39 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US9512084 (2016) BindingDB Entry DOI: 10.7270/Q2ZK5FMH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092082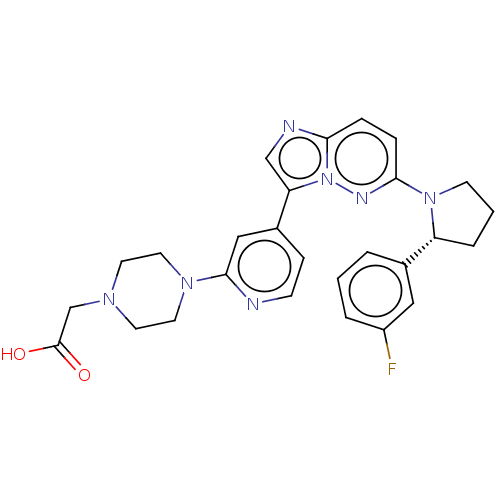 (CHEMBL3582441) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| BDNF/NT-3 growth factors receptor (Homo sapiens (Human)) | BDBM50092079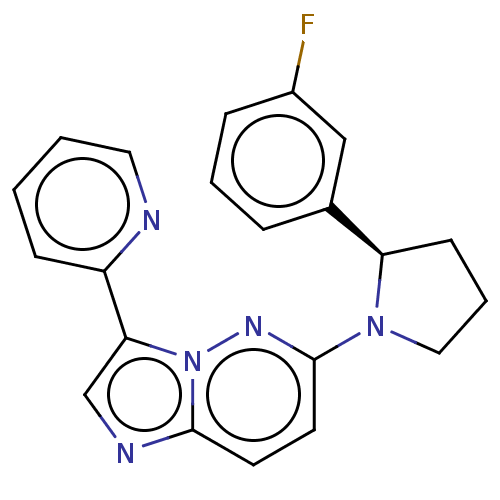 (CHEMBL3582439) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of Tel-fused TRKB (unknown origin) overexpressed in mouse BA/F3 cells assessed as inhibition of cell proliferation after 48 hrs by lucifer... | ACS Med Chem Lett 6: 562-7 (2015) Article DOI: 10.1021/acsmedchemlett.5b00050 BindingDB Entry DOI: 10.7270/Q2TM7CW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM259404 (US10457647, Example 3 | US11180460, Example 3 | US...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis AG US Patent | Assay Description The inhibitory activity of the present compounds against Btk was assessed in a biochemical enzyme assay. Assay plates in 384 well format were prepare... | US Patent US10457647 (2019) BindingDB Entry DOI: 10.7270/Q2KH0QPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 775 total ) | Next | Last >> |