 Found 278 hits with Last Name = 'ghadiri' and Initial = 'mr'
Found 278 hits with Last Name = 'ghadiri' and Initial = 'mr' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50388925
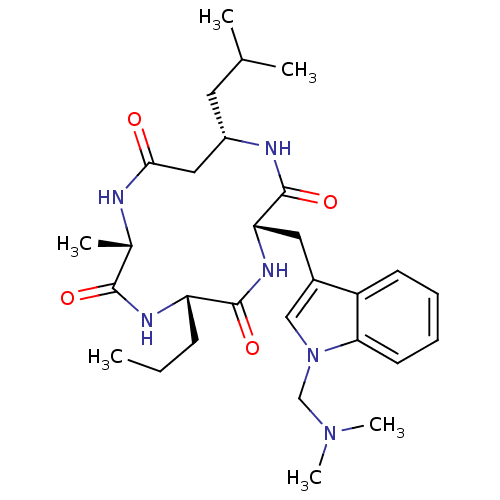
(CHEMBL2063396)Show SMILES CCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2cn(CN(C)C)c3ccccc23)NC1=O |r| Show InChI InChI=1S/C29H44N6O4/c1-7-10-23-28(38)33-24(14-20-16-35(17-34(5)6)25-12-9-8-11-22(20)25)29(39)31-21(13-18(2)3)15-26(36)30-19(4)27(37)32-23/h8-9,11-12,16,18-19,21,23-24H,7,10,13-15,17H2,1-6H3,(H,30,36)(H,31,39)(H,32,37)(H,33,38)/t19-,21-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC2 using Ac-Lys(Ac)-AMC as substrate by Lineweaver-Burk plot analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50388925

(CHEMBL2063396)Show SMILES CCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2cn(CN(C)C)c3ccccc23)NC1=O |r| Show InChI InChI=1S/C29H44N6O4/c1-7-10-23-28(38)33-24(14-20-16-35(17-34(5)6)25-12-9-8-11-22(20)25)29(39)31-21(13-18(2)3)15-26(36)30-19(4)27(37)32-23/h8-9,11-12,16,18-19,21,23-24H,7,10,13-15,17H2,1-6H3,(H,30,36)(H,31,39)(H,32,37)(H,33,38)/t19-,21-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC3/NCoR2 using Ac-Lys(Ac)-AMC as substrate by Lineweaver-Burk plot analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50388926
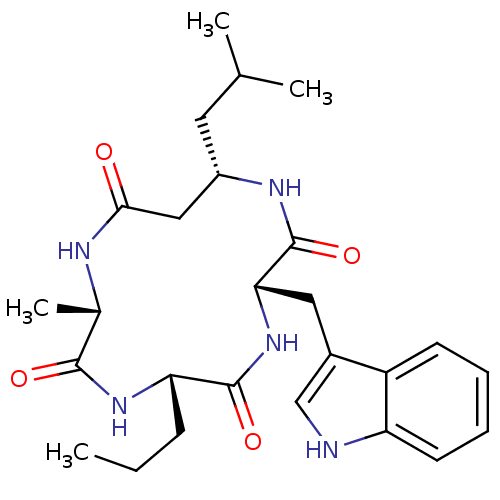
(CHEMBL2063395)Show SMILES CCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C26H37N5O4/c1-5-8-21-25(34)31-22(12-17-14-27-20-10-7-6-9-19(17)20)26(35)29-18(11-15(2)3)13-23(32)28-16(4)24(33)30-21/h6-7,9-10,14-16,18,21-22,27H,5,8,11-13H2,1-4H3,(H,28,32)(H,29,35)(H,30,33)(H,31,34)/t16-,18-,21-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC1 using Ac-Lys(Ac)-AMC as substrate by Lineweaver-Burk plot analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50388925

(CHEMBL2063396)Show SMILES CCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2cn(CN(C)C)c3ccccc23)NC1=O |r| Show InChI InChI=1S/C29H44N6O4/c1-7-10-23-28(38)33-24(14-20-16-35(17-34(5)6)25-12-9-8-11-22(20)25)29(39)31-21(13-18(2)3)15-26(36)30-19(4)27(37)32-23/h8-9,11-12,16,18-19,21,23-24H,7,10,13-15,17H2,1-6H3,(H,30,36)(H,31,39)(H,32,37)(H,33,38)/t19-,21-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Competitive inhibition of HDAC1 using Ac-Lys(Ac)-AMC as substrate by Lineweaver-Burk plot analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19151
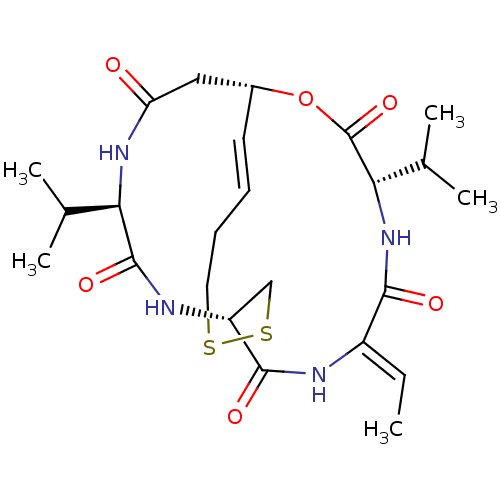
((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC1 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19151

((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC2 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302052
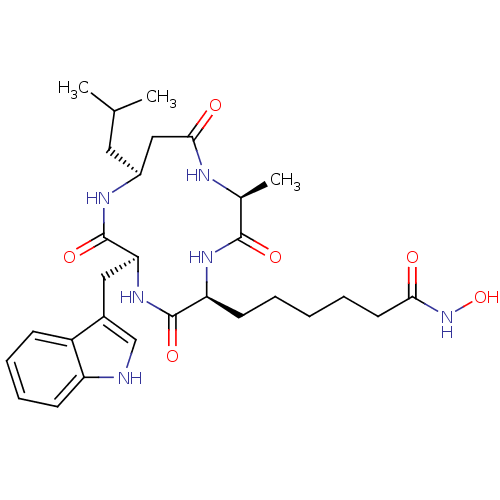
(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM19151

((1S,4S,7Z,10S,16E,21R)-7-ethylidene-4,21-bis(propa...)Show SMILES C\C=C1/NC(=O)[C@H]2CSSCC\C=C\[C@H](CC(=O)N[C@H](C(C)C)C(=O)N2)OC(=O)[C@@H](NC1=O)C(C)C |r,t:12| Show InChI InChI=1S/C24H36N4O6S2/c1-6-16-21(30)28-20(14(4)5)24(33)34-15-9-7-8-10-35-36-12-17(22(31)25-16)26-23(32)19(13(2)3)27-18(29)11-15/h6-7,9,13-15,17,19-20H,8,10-12H2,1-5H3,(H,25,31)(H,26,32)(H,27,29)(H,28,30)/b9-7+,16-6-/t15-,17-,19-,20+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC3/NCoR2 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379130
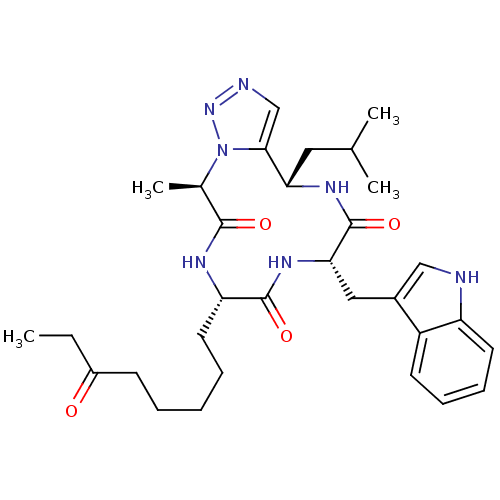
(CHEMBL2012817)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H](C)n2nncc2[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H43N7O4/c1-5-22(39)11-7-6-8-14-25-30(41)36-27(16-21-17-32-24-13-10-9-12-23(21)24)31(42)35-26(15-19(2)3)28-18-33-37-38(28)20(4)29(40)34-25/h9-10,12-13,17-20,25-27,32H,5-8,11,14-16H2,1-4H3,(H,34,40)(H,35,42)(H,36,41)/t20-,25+,26+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379130

(CHEMBL2012817)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H](C)n2nncc2[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H43N7O4/c1-5-22(39)11-7-6-8-14-25-30(41)36-27(16-21-17-32-24-13-10-9-12-23(21)24)31(42)35-26(15-19(2)3)28-18-33-37-38(28)20(4)29(40)34-25/h9-10,12-13,17-20,25-27,32H,5-8,11,14-16H2,1-4H3,(H,34,40)(H,35,42)(H,36,41)/t20-,25+,26+,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379131
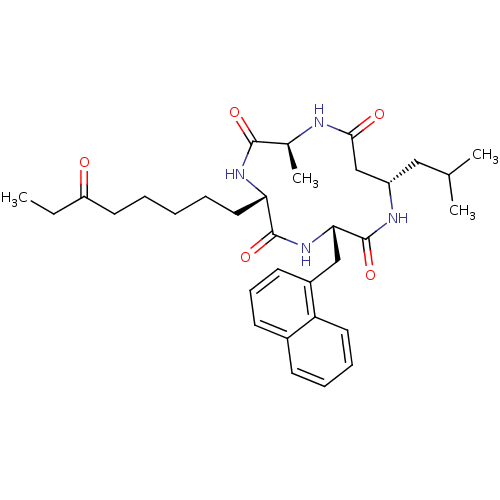
(CHEMBL2012815)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2cccc3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H46N4O5/c1-5-26(38)15-7-6-8-17-28-32(41)37-29(19-24-14-11-13-23-12-9-10-16-27(23)24)33(42)35-25(18-21(2)3)20-30(39)34-22(4)31(40)36-28/h9-14,16,21-22,25,28-29H,5-8,15,17-20H2,1-4H3,(H,34,39)(H,35,42)(H,36,40)(H,37,41)/t22-,25-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379131

(CHEMBL2012815)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2cccc3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H46N4O5/c1-5-26(38)15-7-6-8-17-28-32(41)37-29(19-24-14-11-13-23-12-9-10-16-27(23)24)33(42)35-25(18-21(2)3)20-30(39)34-22(4)31(40)36-28/h9-14,16,21-22,25,28-29H,5-8,15,17-20H2,1-4H3,(H,34,39)(H,35,42)(H,36,40)(H,37,41)/t22-,25-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302059
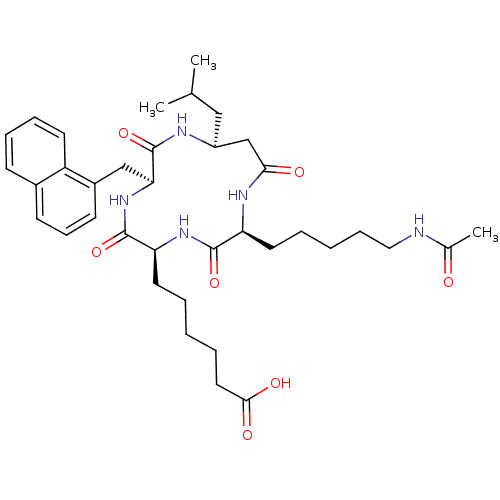
(6-((2S,5S,8S,11S)-2-(5-acetamidopentyl)-11-isobuty...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](CCCCCNC(C)=O)C(=O)N[C@@H](CCCCCC(O)=O)C(=O)N[C@@H](Cc2cccc3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C37H53N5O7/c1-24(2)21-28-23-33(44)40-30(17-7-5-11-20-38-25(3)43)35(47)41-31(18-6-4-8-19-34(45)46)36(48)42-32(37(49)39-28)22-27-15-12-14-26-13-9-10-16-29(26)27/h9-10,12-16,24,28,30-32H,4-8,11,17-23H2,1-3H3,(H,38,43)(H,39,49)(H,40,44)(H,41,47)(H,42,48)(H,45,46)/t28-,30-,31-,32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302073
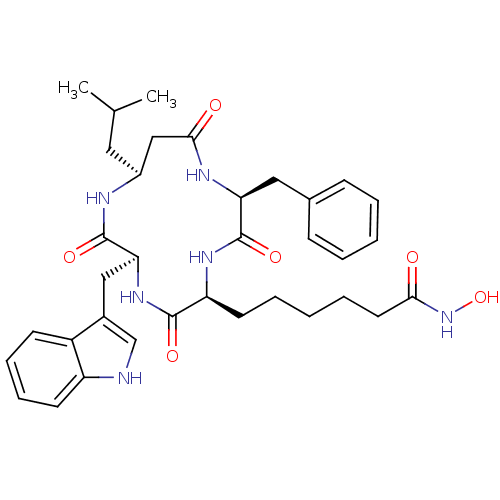
(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-2-benz...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C35H46N6O6/c1-22(2)17-25-20-32(43)38-29(18-23-11-5-3-6-12-23)35(46)39-28(15-7-4-8-16-31(42)41-47)33(44)40-30(34(45)37-25)19-24-21-36-27-14-10-9-13-26(24)27/h3,5-6,9-14,21-22,25,28-30,36,47H,4,7-8,15-20H2,1-2H3,(H,37,45)(H,38,43)(H,39,46)(H,40,44)(H,41,42)/t25-,28-,29-,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50379130

(CHEMBL2012817)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H](C)n2nncc2[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H43N7O4/c1-5-22(39)11-7-6-8-14-25-30(41)36-27(16-21-17-32-24-13-10-9-12-23(21)24)31(42)35-26(15-19(2)3)28-18-33-37-38(28)20(4)29(40)34-25/h9-10,12-13,17-20,25-27,32H,5-8,11,14-16H2,1-4H3,(H,34,40)(H,35,42)(H,36,41)/t20-,25+,26+,27+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50379130

(CHEMBL2012817)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H](C)n2nncc2[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H43N7O4/c1-5-22(39)11-7-6-8-14-25-30(41)36-27(16-21-17-32-24-13-10-9-12-23(21)24)31(42)35-26(15-19(2)3)28-18-33-37-38(28)20(4)29(40)34-25/h9-10,12-13,17-20,25-27,32H,5-8,11,14-16H2,1-4H3,(H,34,40)(H,35,42)(H,36,41)/t20-,25+,26+,27+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632
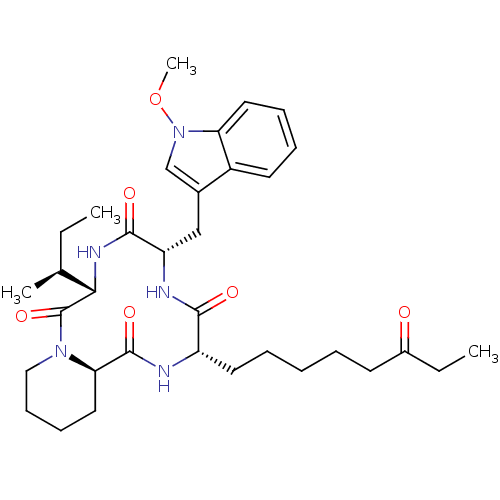
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Lys(Ac)-7-amino-4-methylcoumarin as substrate after 30 mins by trypsin-coupled fluorogenic assay |
ACS Med Chem Lett 3: 749-753 (2012)
Article DOI: 10.1021/ml300162r
BindingDB Entry DOI: 10.7270/Q29P32R4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379129
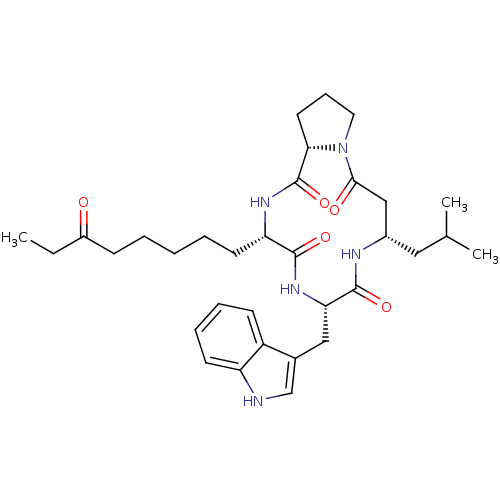
(CHEMBL2012814)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H47N5O5/c1-4-24(39)11-6-5-7-14-27-31(41)37-28(18-22-20-34-26-13-9-8-12-25(22)26)32(42)35-23(17-21(2)3)19-30(40)38-16-10-15-29(38)33(43)36-27/h8-9,12-13,20-21,23,27-29,34H,4-7,10-11,14-19H2,1-3H3,(H,35,42)(H,36,43)(H,37,41)/t23-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379129

(CHEMBL2012814)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H47N5O5/c1-4-24(39)11-6-5-7-14-27-31(41)37-28(18-22-20-34-26-13-9-8-12-25(22)26)32(42)35-23(17-21(2)3)19-30(40)38-16-10-15-29(38)33(43)36-27/h8-9,12-13,20-21,23,27-29,34H,4-7,10-11,14-19H2,1-3H3,(H,35,42)(H,36,43)(H,37,41)/t23-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using Ac-Lys(Ac)-7-amino-4-methylcoumarin as substrate after 30 mins by trypsin-coupled fluorogenic assay |
ACS Med Chem Lett 3: 749-753 (2012)
Article DOI: 10.1021/ml300162r
BindingDB Entry DOI: 10.7270/Q29P32R4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50379129

(CHEMBL2012814)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@@H]2CCCN2C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C33H47N5O5/c1-4-24(39)11-6-5-7-14-27-31(41)37-28(18-22-20-34-26-13-9-8-12-25(22)26)32(42)35-23(17-21(2)3)19-30(40)38-16-10-15-29(38)33(43)36-27/h8-9,12-13,20-21,23,27-29,34H,4-7,10-11,14-19H2,1-3H3,(H,35,42)(H,36,43)(H,37,41)/t23-,27-,28-,29-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC8 in human HeLa cells nuclear extract using Arg-His-Lys(Ac)-Lys(Ac)-AMC as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50379135
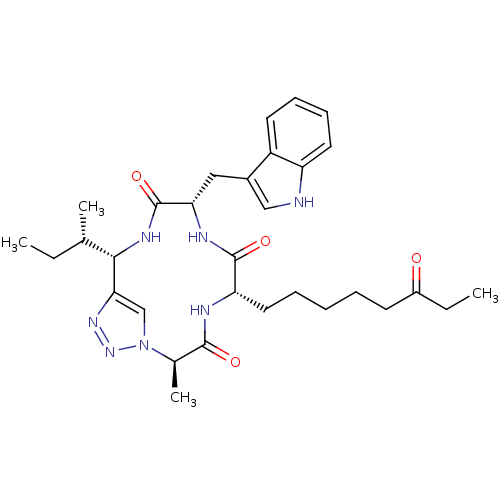
(CHEMBL2012818)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@@H](C)n2cc1nn2 |r| Show InChI InChI=1S/C31H43N7O4/c1-5-19(3)28-27-18-38(37-36-27)20(4)29(40)33-25(15-9-7-8-12-22(39)6-2)30(41)34-26(31(42)35-28)16-21-17-32-24-14-11-10-13-23(21)24/h10-11,13-14,17-20,25-26,28,32H,5-9,12,15-16H2,1-4H3,(H,33,40)(H,34,41)(H,35,42)/t19-,20+,25-,26-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC6 using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HDAC6 using Ac-Lys(Ac)-AMC as substrate after 30 mins by fluorescence analysis |
ACS Med Chem Lett 3: 505-508 (2012)
Article DOI: 10.1021/ml300081u
BindingDB Entry DOI: 10.7270/Q2JQ122X |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302053
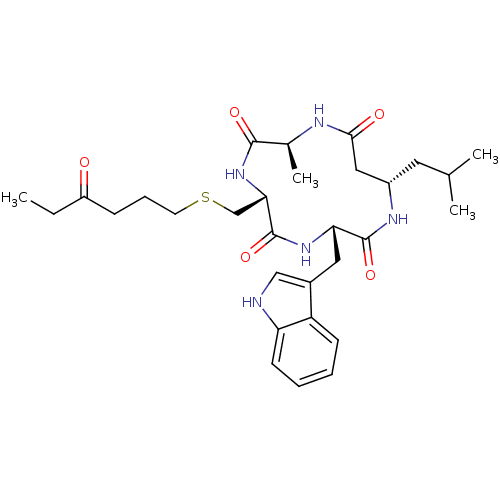
((3S,6R,9S,13S)-3-((1H-indol-3-yl)methyl)-13-isobut...)Show SMILES CCC(=O)CCCSC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C30H43N5O5S/c1-5-22(36)9-8-12-41-17-26-30(40)34-25(14-20-16-31-24-11-7-6-10-23(20)24)29(39)33-21(13-18(2)3)15-27(37)32-19(4)28(38)35-26/h6-7,10-11,16,18-19,21,25-26,31H,5,8-9,12-15,17H2,1-4H3,(H,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,21-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302053

((3S,6R,9S,13S)-3-((1H-indol-3-yl)methyl)-13-isobut...)Show SMILES CCC(=O)CCCSC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C30H43N5O5S/c1-5-22(36)9-8-12-41-17-26-30(40)34-25(14-20-16-31-24-11-7-6-10-23(20)24)29(39)33-21(13-18(2)3)15-27(37)32-19(4)28(38)35-26/h6-7,10-11,16,18-19,21,25-26,31H,5,8-9,12-15,17H2,1-4H3,(H,32,37)(H,33,39)(H,34,40)(H,35,38)/t19-,21-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM19130

((2E,4E,6R)-7-[4-(dimethylamino)phenyl]-N-hydroxy-4...)Show SMILES CC(C=CC(=O)NO)C=C(C)C(=O)c1ccc(cc1)N(C)C |w:8.7,2.1| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-12,22H,1-4H3,(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379135

(CHEMBL2012818)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@@H](C)n2cc1nn2 |r| Show InChI InChI=1S/C31H43N7O4/c1-5-19(3)28-27-18-38(37-36-27)20(4)29(40)33-25(15-9-7-8-12-22(39)6-2)30(41)34-26(31(42)35-28)16-21-17-32-24-14-11-10-13-23(21)24/h10-11,13-14,17-20,25-26,28,32H,5-9,12,15-16H2,1-4H3,(H,33,40)(H,34,41)(H,35,42)/t19-,20+,25-,26-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302077
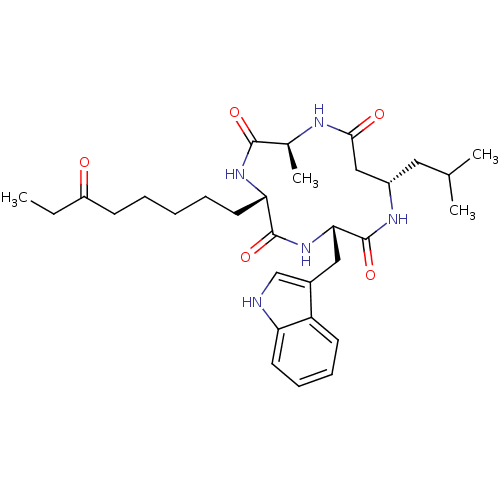
((3S,6S,9S,13S)-3-((1H-indol-3-yl)methyl)-13-isobut...)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H45N5O5/c1-5-23(37)11-7-6-8-14-26-30(40)36-27(16-21-18-32-25-13-10-9-12-24(21)25)31(41)34-22(15-19(2)3)17-28(38)33-20(4)29(39)35-26/h9-10,12-13,18-20,22,26-27,32H,5-8,11,14-17H2,1-4H3,(H,33,38)(H,34,41)(H,35,39)(H,36,40)/t20-,22-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50302077

((3S,6S,9S,13S)-3-((1H-indol-3-yl)methyl)-13-isobut...)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)NC(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C31H45N5O5/c1-5-23(37)11-7-6-8-14-26-30(40)36-27(16-21-18-32-25-13-10-9-12-24(21)25)31(41)34-22(15-19(2)3)17-28(38)33-20(4)29(39)35-26/h9-10,12-13,18-20,22,26-27,32H,5-8,11,14-17H2,1-4H3,(H,33,38)(H,34,41)(H,35,39)(H,36,40)/t20-,22-,26-,27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Department of Cell and Molecular Biology, The Scripps Research Institute, La Jolla, CA, USA; National Yang-Ming University, Taipei, Taiwan; National Yang-Ming University Hospital, Ilan, Taiwan.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 (unknown origin) |
Bioorg Med Chem Lett 27: 3289-3293 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.027
BindingDB Entry DOI: 10.7270/Q2Z32238 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 after 30 mins by fluorimetric assay |
J Med Chem 52: 7836-46 (2009)
Article DOI: 10.1021/jm900850t
BindingDB Entry DOI: 10.7270/Q2M046DK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50379132
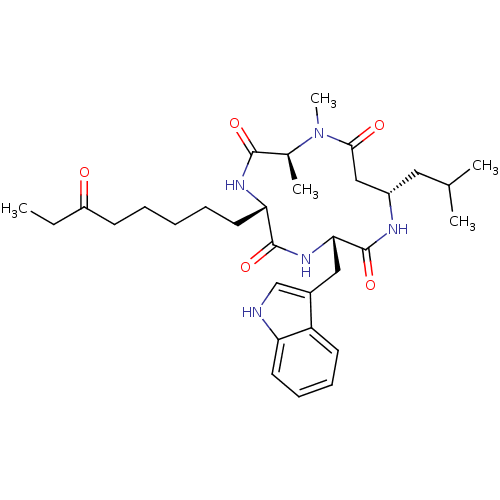
(CHEMBL2012813)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)N(C)C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C32H47N5O5/c1-6-24(38)12-8-7-9-15-27-31(41)36-28(17-22-19-33-26-14-11-10-13-25(22)26)32(42)34-23(16-20(2)3)18-29(39)37(5)21(4)30(40)35-27/h10-11,13-14,19-21,23,27-28,33H,6-9,12,15-18H2,1-5H3,(H,34,42)(H,35,40)(H,36,41)/t21-,23-,27-,28-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3/NCoR2 using Ac-Lys(Ac)-7-amino-4-methylcoumarin as substrate after 30 mins by trypsin-coupled fluorogenic assay |
ACS Med Chem Lett 3: 749-753 (2012)
Article DOI: 10.1021/ml300162r
BindingDB Entry DOI: 10.7270/Q29P32R4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50379132

(CHEMBL2012813)Show SMILES CCC(=O)CCCCC[C@@H]1NC(=O)[C@H](C)N(C)C(=O)C[C@H](CC(C)C)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC1=O |r| Show InChI InChI=1S/C32H47N5O5/c1-6-24(38)12-8-7-9-15-27-31(41)36-28(17-22-19-33-26-14-11-10-13-25(22)26)32(42)34-23(16-20(2)3)18-29(39)37(5)21(4)30(40)35-27/h10-11,13-14,19-21,23,27-28,33H,6-9,12,15-18H2,1-5H3,(H,34,42)(H,35,40)(H,36,41)/t21-,23-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using Ac-Lys(Ac)-7-amino-4-methylcoumarin as substrate after 30 mins by trypsin-coupled fluorogenic assay |
ACS Med Chem Lett 3: 749-753 (2012)
Article DOI: 10.1021/ml300162r
BindingDB Entry DOI: 10.7270/Q29P32R4 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50302073

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-2-benz...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C35H46N6O6/c1-22(2)17-25-20-32(43)38-29(18-23-11-5-3-6-12-23)35(46)39-28(15-7-4-8-16-31(42)41-47)33(44)40-30(34(45)37-25)19-24-21-36-27-14-10-9-13-26(24)27/h3,5-6,9-14,21-22,25,28-30,36,47H,4,7-8,15-20H2,1-2H3,(H,37,45)(H,38,43)(H,39,46)(H,40,44)(H,41,42)/t25-,28-,29-,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3-NCoR2 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50302052

(6-((2S,5S,8S,11S)-8-((1H-indol-3-yl)methyl)-11-iso...)Show SMILES CC(C)C[C@H]1CC(=O)N[C@@H](C)C(=O)N[C@@H](CCCCCC(=O)NO)C(=O)N[C@@H](Cc2c[nH]c3ccccc23)C(=O)N1 |r| Show InChI InChI=1S/C29H42N6O6/c1-17(2)13-20-15-26(37)31-18(3)27(38)33-23(11-5-4-6-12-25(36)35-41)28(39)34-24(29(40)32-20)14-19-16-30-22-10-8-7-9-21(19)22/h7-10,16-18,20,23-24,30,41H,4-6,11-15H2,1-3H3,(H,31,37)(H,32,40)(H,33,38)(H,34,39)(H,35,36)/t18-,20-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of HDAC6 in human HeLa cells nuclear extract using Fluor-de-Lys as substrate after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 703-707 (2011)
Article DOI: 10.1021/ml200136e
BindingDB Entry DOI: 10.7270/Q2GQ6ZRX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data