

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB2 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6505-10 (2007) Article DOI: 10.1016/j.bmcl.2007.09.089 BindingDB Entry DOI: 10.7270/Q2S75H5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity of compound was determined against Opioid receptor delta 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027063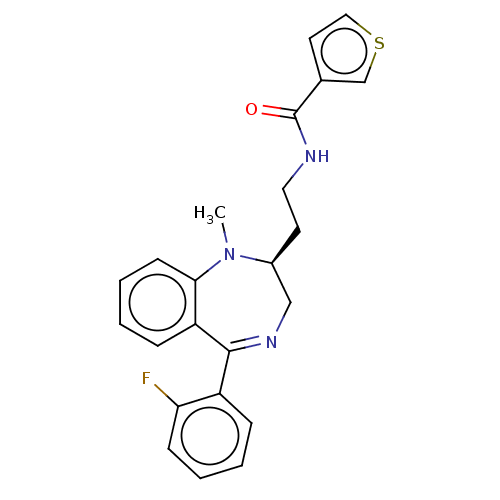 (CHEMBL2111838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50505384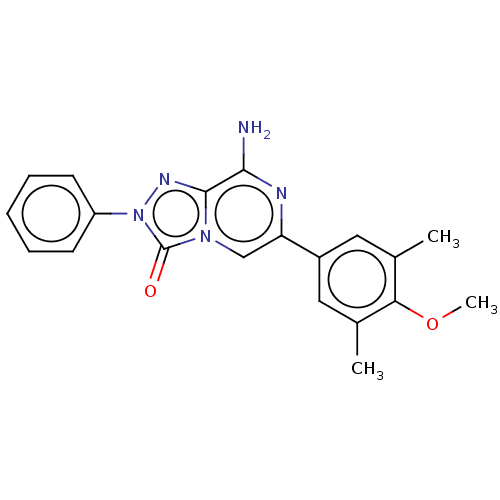 (CHEMBL4457393) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cell membranes by radioligand competition assay | J Med Chem 62: 8511-8531 (2019) Article DOI: 10.1021/acs.jmedchem.9b00778 BindingDB Entry DOI: 10.7270/Q2ST7T4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027063 (CHEMBL2111838) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50067499 ((6aR,10aR)-3(1,1-dimethylheptyl)-9-hydroxymethyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ di Pisa Curated by ChEMBL | Assay Description Displacement of [3H]CP-55940 from human recombinant CB1 receptor expressed in HEK293 cells | Bioorg Med Chem Lett 17: 6505-10 (2007) Article DOI: 10.1016/j.bmcl.2007.09.089 BindingDB Entry DOI: 10.7270/Q2S75H5H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50027064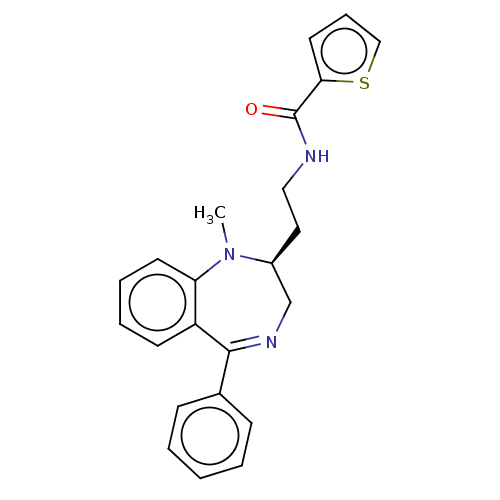 (CHEMBL2111836) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50513286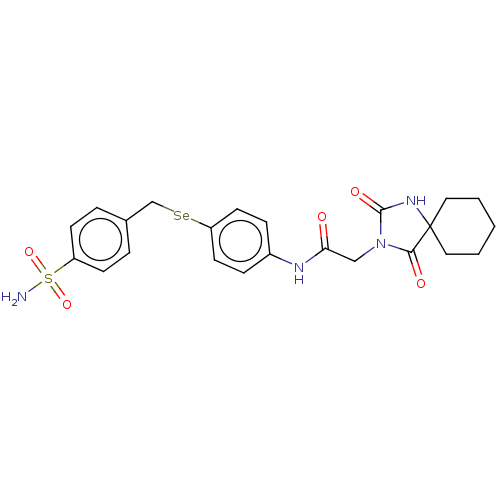 (CHEMBL4470319) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | Eur J Med Chem 177: 188-197 (2019) Article DOI: 10.1016/j.ejmech.2019.05.058 BindingDB Entry DOI: 10.7270/Q2T156ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50341392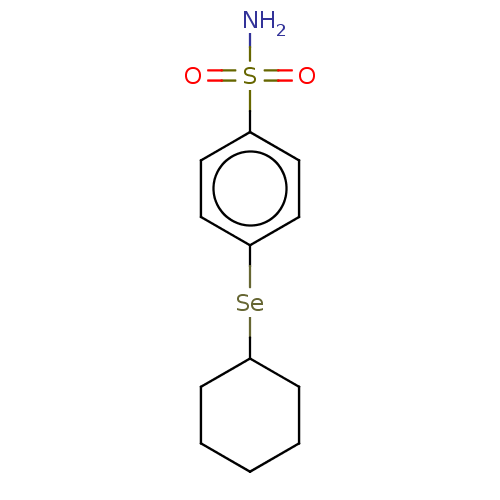 (CHEMBL4171524) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay | ACS Med Chem Lett 9: 462-467 (2018) Article DOI: 10.1021/acsmedchemlett.8b00076 BindingDB Entry DOI: 10.7270/Q2319ZFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21864 ((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor delta 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50341395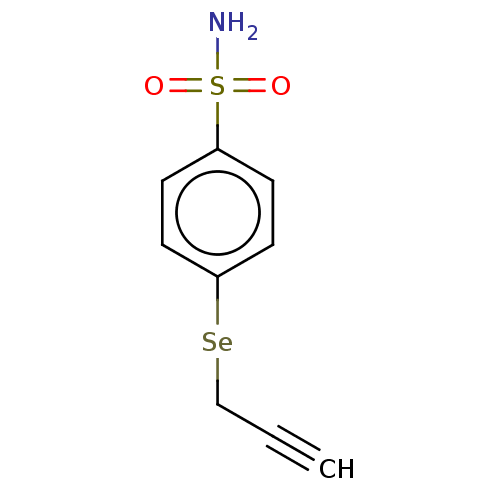 (CHEMBL4175369) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay | ACS Med Chem Lett 9: 462-467 (2018) Article DOI: 10.1021/acsmedchemlett.8b00076 BindingDB Entry DOI: 10.7270/Q2319ZFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131967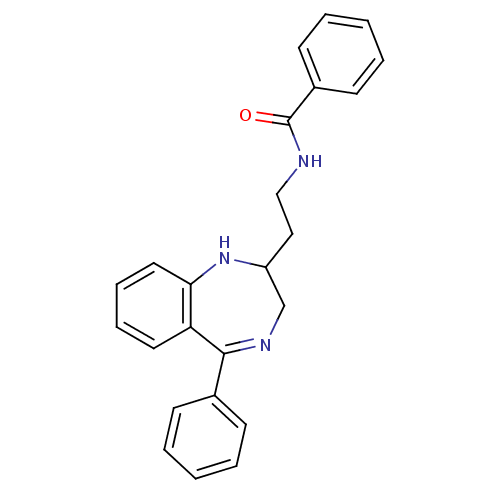 (CHEMBL123898 | N-[2-(5-Phenyl-2,3-dihydro-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131967 (CHEMBL123898 | N-[2-(5-Phenyl-2,3-dihydro-1H-benzo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50341389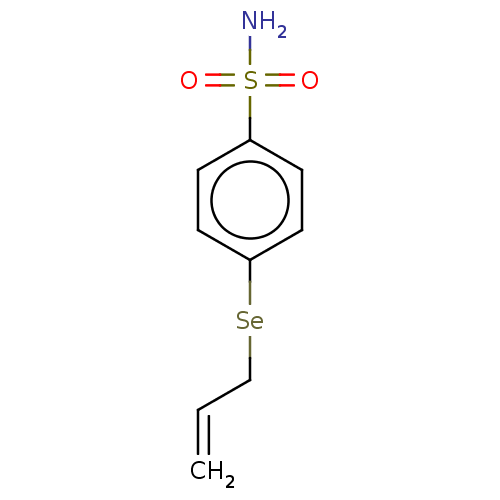 (CHEMBL4164747) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay | ACS Med Chem Lett 9: 462-467 (2018) Article DOI: 10.1021/acsmedchemlett.8b00076 BindingDB Entry DOI: 10.7270/Q2319ZFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50341393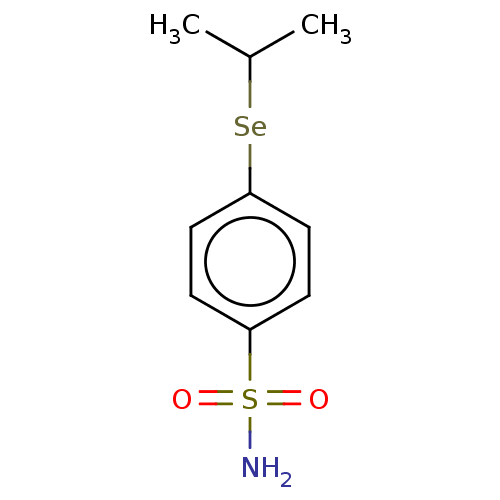 (CHEMBL4165154) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay | ACS Med Chem Lett 9: 462-467 (2018) Article DOI: 10.1021/acsmedchemlett.8b00076 BindingDB Entry DOI: 10.7270/Q2319ZFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131972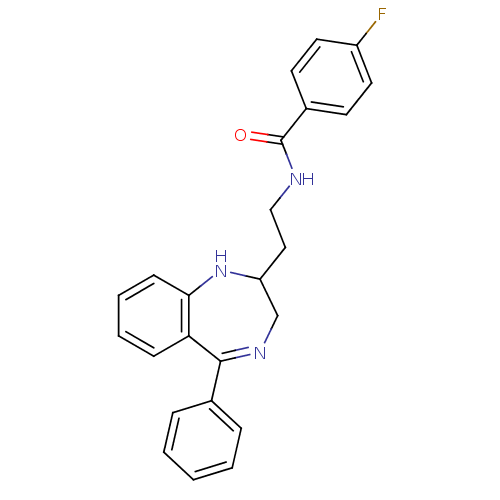 (4-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131972 (4-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50511588 (CHEMBL4453207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA7 preincubated for 15 mins by measured for 10 to 100 secs by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128147 BindingDB Entry DOI: 10.7270/Q27S7SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049802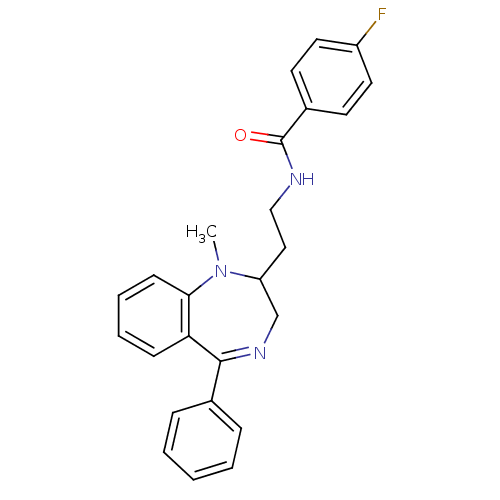 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049802 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50341388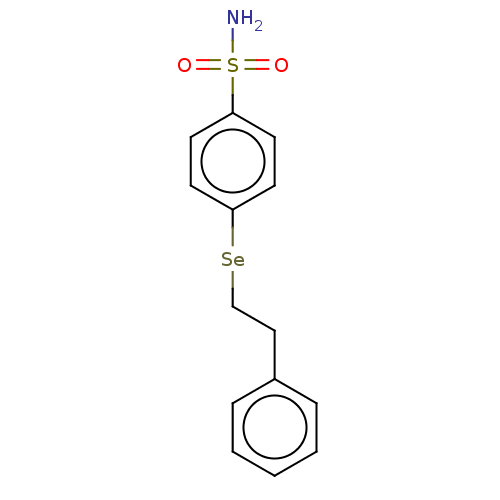 (CHEMBL4159208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay | ACS Med Chem Lett 9: 462-467 (2018) Article DOI: 10.1021/acsmedchemlett.8b00076 BindingDB Entry DOI: 10.7270/Q2319ZFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049807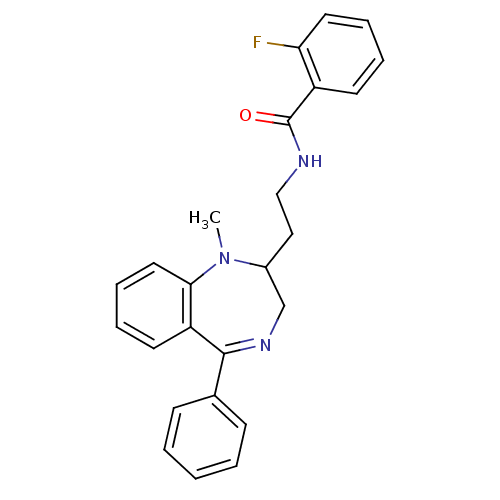 (2-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049807 (2-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131968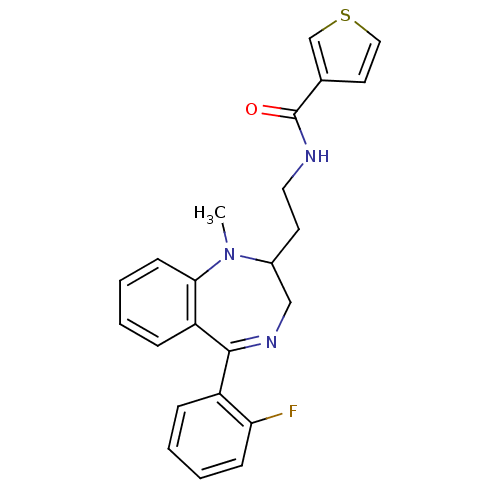 (CHEMBL127828 | Thiophene-3-carboxylic acid {2-[5-(...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50341394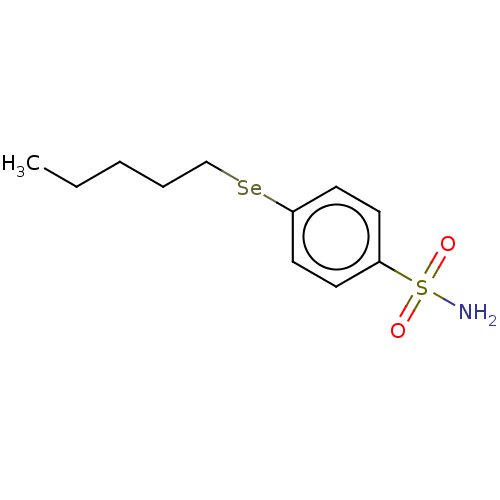 (CHEMBL4171921) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 7 after 15 mins by stopped flow carbon dioxide hydration assay | ACS Med Chem Lett 9: 462-467 (2018) Article DOI: 10.1021/acsmedchemlett.8b00076 BindingDB Entry DOI: 10.7270/Q2319ZFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515558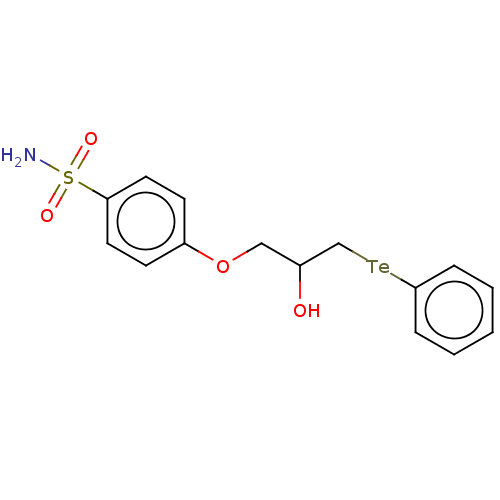 (CHEMBL4535036) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111586 BindingDB Entry DOI: 10.7270/Q2Z03CJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027064 (CHEMBL2111836) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027064 (CHEMBL2111836) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM50230195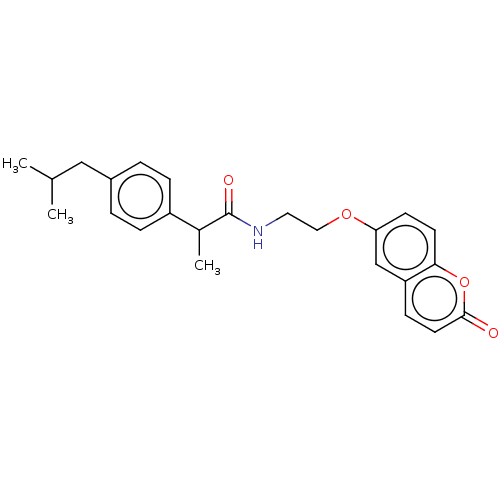 (CHEMBL4100903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human CA4 preincubated for 15 mins by stopped-flow CO2 hydration assay | J Med Chem 60: 1159-1170 (2017) Article DOI: 10.1021/acs.jmedchem.6b01607 BindingDB Entry DOI: 10.7270/Q2PC34NX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515303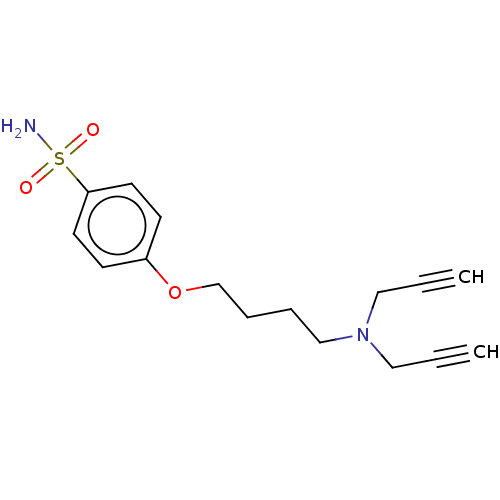 (CHEMBL4435346) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 incubated for 15 mins prior to testing measured for 10 to 100 secs by phenol red-based stopped-f... | J Med Chem 62: 7233-7249 (2019) Article DOI: 10.1021/acs.jmedchem.9b00845 BindingDB Entry DOI: 10.7270/Q2D79FRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-2/gamma-2 (Homo sapiens (Human)) | BDBM50432382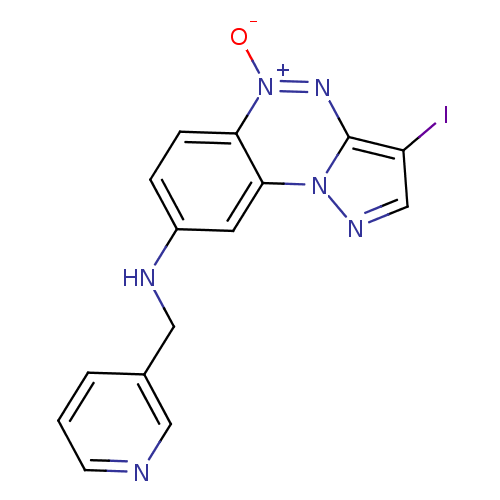 (CHEMBL2348441) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sintesi e Studio di Eterocicli Biologicamente attivi (HeteroBioLab) Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]Ro15-1788 from recombinant GABA-A alpha1beta2gamma2 receptor benzodiazepine binding site (unknown origin) after 1 hr | Bioorg Med Chem 21: 2186-98 (2013) Article DOI: 10.1016/j.bmc.2013.02.027 BindingDB Entry DOI: 10.7270/Q2KD208C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131969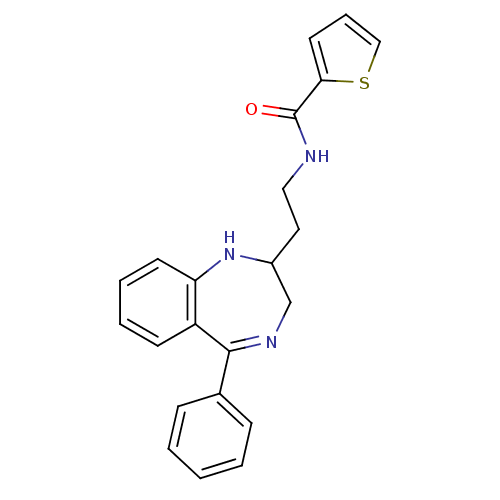 (CHEMBL126688 | Thiophene-2-carboxylic acid [2-(5-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131969 (CHEMBL126688 | Thiophene-2-carboxylic acid [2-(5-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131973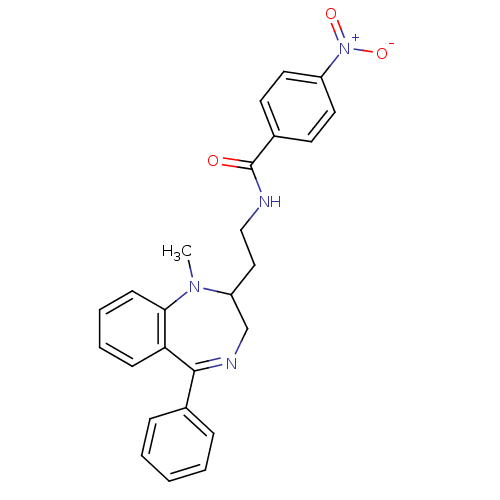 (CHEMBL338391 | N-[2-(1-Methyl-5-phenyl-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131973 (CHEMBL338391 | N-[2-(1-Methyl-5-phenyl-2,3-dihydro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding constant towards human Opioid receptor kappa 1 was reported | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049806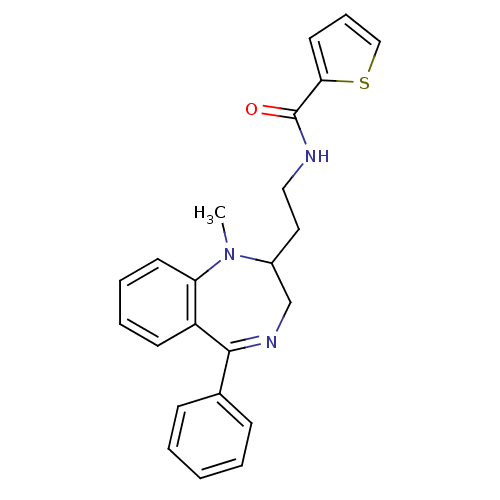 (CHEMBL127969 | Thiophene-2-carboxylic acid [2-(1-m...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049806 (CHEMBL127969 | Thiophene-2-carboxylic acid [2-(1-m...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131970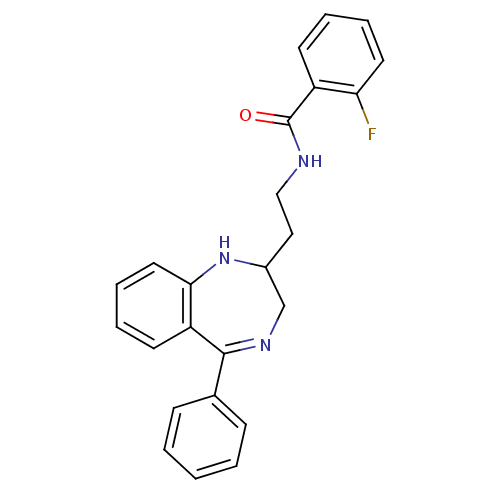 (2-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50131970 (2-Fluoro-N-[2-(5-phenyl-2,3-dihydro-1H-benzo[e][1,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 7 (Homo sapiens (Human)) | BDBM50511587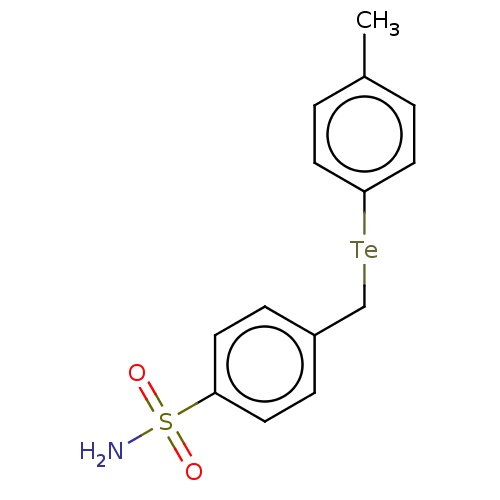 (CHEMBL4532721) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA7 preincubated for 15 mins by measured for 10 to 100 secs by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128147 BindingDB Entry DOI: 10.7270/Q27S7SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50408526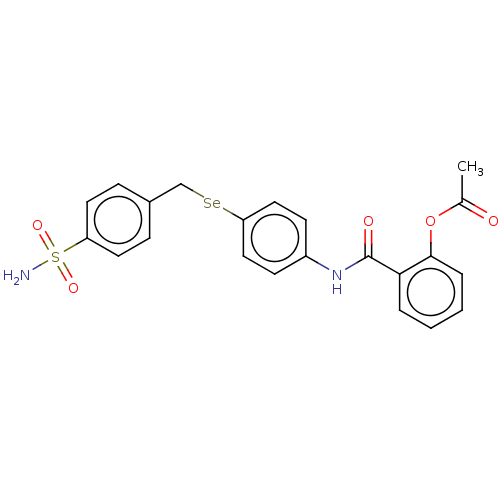 (CHEMBL4163381) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Inhibition of human carbonic anhydrase 2 incubated for 15 mins prior to testing by stopped-flow CO2 hydration assay | Eur J Med Chem 154: 210-219 (2018) Article DOI: 10.1016/j.ejmech.2018.05.026 BindingDB Entry DOI: 10.7270/Q2VX0K2M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50049808 (CHEMBL127968 | N-[2-(7-Chloro-1-methyl-5-phenyl-2,...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50469139 (CHEMBL4292479) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Department of University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 9 incubated for 15 mins prior to testing by stopped flow CO2 hydration assay | Eur J Med Chem 157: 1214-1222 (2018) Article DOI: 10.1016/j.ejmech.2018.08.096 BindingDB Entry DOI: 10.7270/Q2RR21ZR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Cavia porcellus (domestic guinea pig)) | BDBM50049802 (4-Fluoro-N-[2-(1-methyl-5-phenyl-2,3-dihydro-1H-be...) | PDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity determined against Opioid receptor kappa 1 from a native receptor in guinea pig | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515559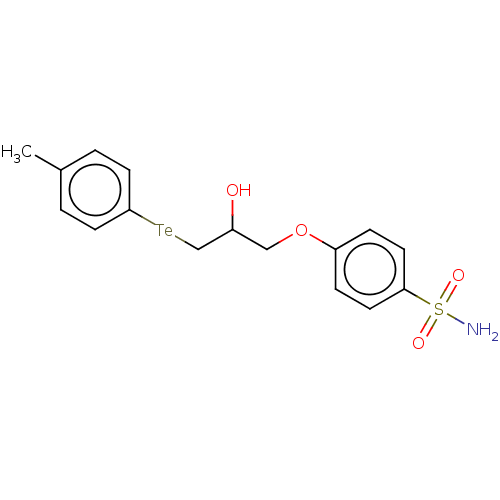 (CHEMBL4435078) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111586 BindingDB Entry DOI: 10.7270/Q2Z03CJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50505386 (CHEMBL4442908) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze Curated by ChEMBL | Assay Description Displacement of [3H]NECA from human adenosine A2A receptor expressed in CHO cell membranes by radioligand competition assay | J Med Chem 62: 8511-8531 (2019) Article DOI: 10.1021/acs.jmedchem.9b00778 BindingDB Entry DOI: 10.7270/Q2ST7T4D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515564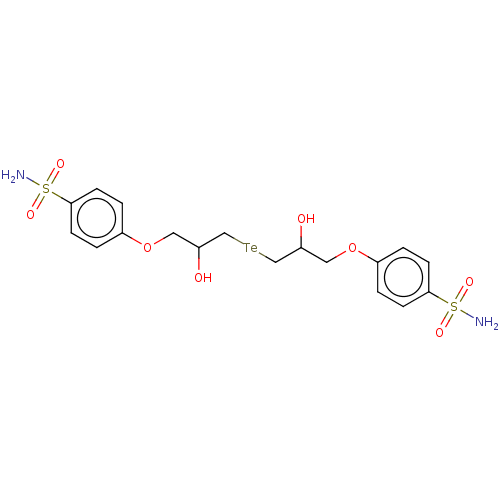 (CHEMBL4535469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111586 BindingDB Entry DOI: 10.7270/Q2Z03CJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50515563 (CHEMBL4436769) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florence Curated by ChEMBL | Assay Description Inhibition of recombinant human carbonic anhydrase 2 preincubated for 15 mins by phenol red dye based stopped flow CO2 hydration assay | Eur J Med Chem 181: (2019) Article DOI: 10.1016/j.ejmech.2019.111586 BindingDB Entry DOI: 10.7270/Q2Z03CJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50511590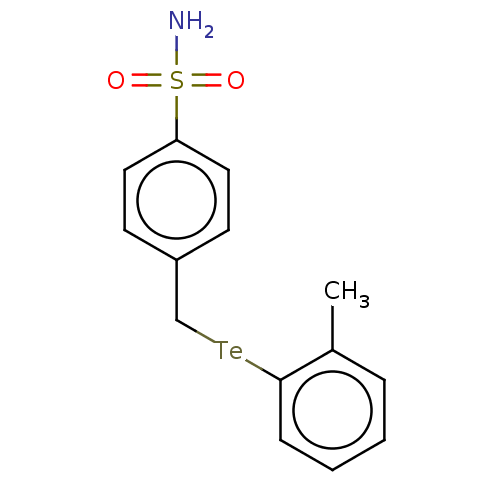 (CHEMBL4587627) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of recombinant human CA2 preincubated for 15 mins by measured for 10 to 100 secs by phenol red dye based stopped flow CO2 hydration assay | Citation and Details Article DOI: 10.1016/j.bmcl.2021.128147 BindingDB Entry DOI: 10.7270/Q27S7SJ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50027062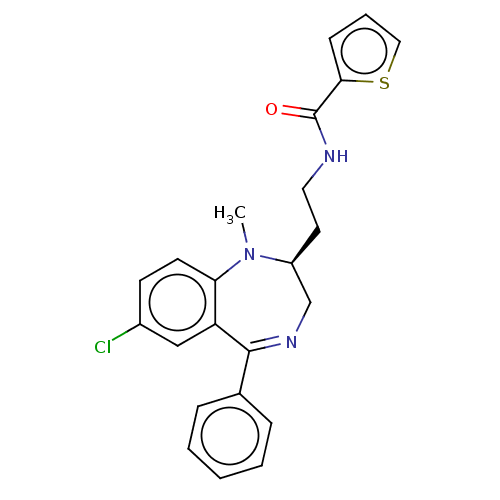 (CHEMBL2111837) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Siena Curated by ChEMBL | Assay Description Binding affinity against opioid receptor kappa 1 from human cloned receptor | J Med Chem 46: 3853-64 (2003) Article DOI: 10.1021/jm0307640 BindingDB Entry DOI: 10.7270/Q27M08QQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3509 total ) | Next | Last >> |