

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM26113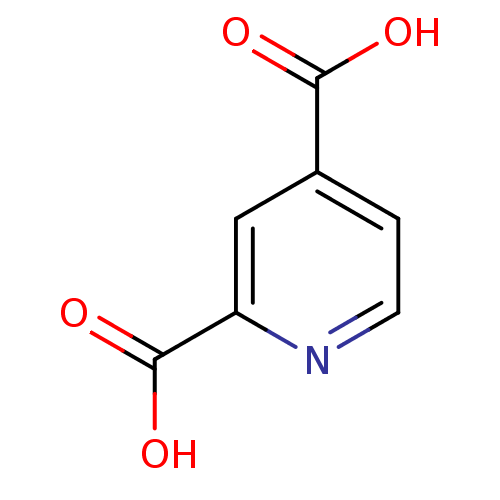 (2,4 PDCA | cid_10365 | pyridine carboxylate, 6a | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151392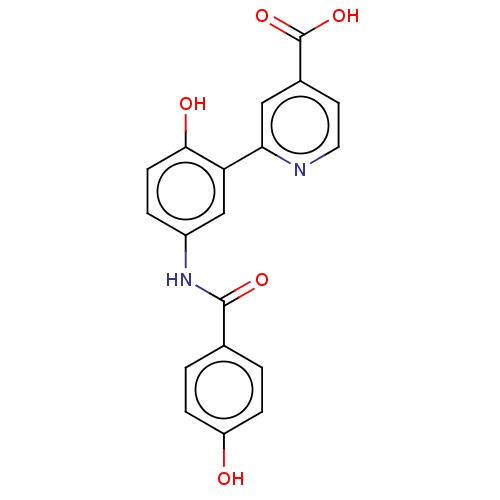 (CHEMBL3775262) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4C (Homo sapiens (Human)) | BDBM50151399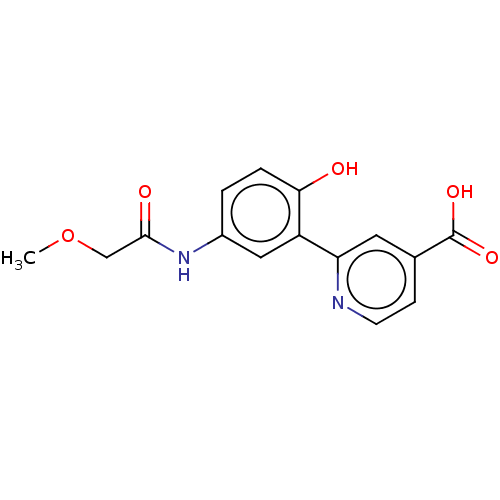 (CHEMBL3775380) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | Article PubMed | 683 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of N-terminal His6-tagged KDM4C (1 to 352 residues) (unknown origin) expressed in Escherichia coli Rosetta 2(DE3)pLysS using A... | J Med Chem 59: 1580-98 (2016) Article DOI: 10.1021/acs.jmedchem.5b01527 BindingDB Entry DOI: 10.7270/Q2P84DRX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM50395076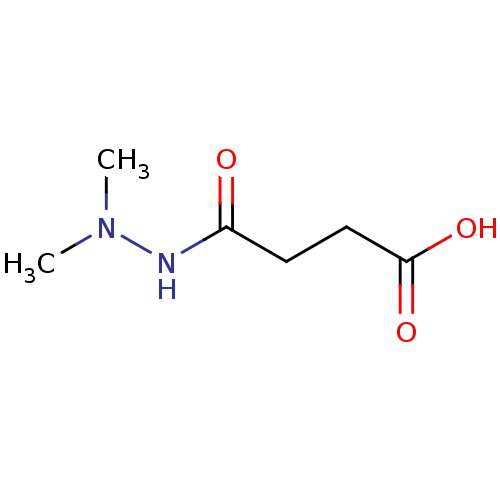 (CHEMBL2164243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Competitive inhibition of human KDM2A expressed in Escherichia coli using 2-oxoglutarate by enzyme kinetic assay | J Med Chem 55: 6639-43 (2012) Article DOI: 10.1021/jm300677j BindingDB Entry DOI: 10.7270/Q2JH3N9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 2A (Homo sapiens (Human)) | BDBM50395076 (CHEMBL2164243) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Mixed type inhibition of human KDM2A expressed in Escherichia coli assessed inhibition constant for compound-enzyme-substrate complex using methyl ly... | J Med Chem 55: 6639-43 (2012) Article DOI: 10.1021/jm300677j BindingDB Entry DOI: 10.7270/Q2JH3N9S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM420298 (CVD-0006356 | PF-00835231 | PF-0835231 | US1152494...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PDB UniChem | n/a | n/a | 0.650 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM50097721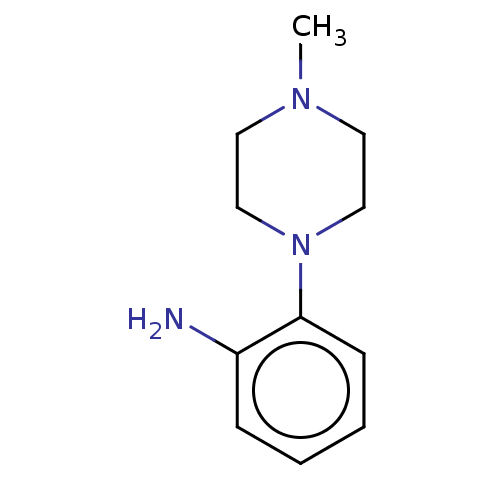 (CHEMBL1879790 | EN300-11843) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496902 (CVD-0018409 | PF-07321332 | US11351149, Example 13...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase EHMT2 (Homo sapiens (Human)) | BDBM50505576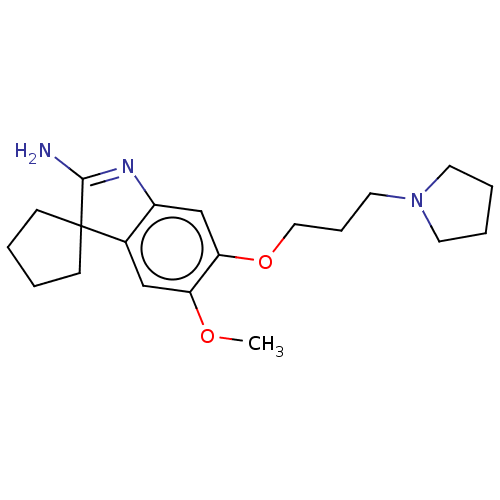 (CHEMBL4458252) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford Curated by ChEMBL | Assay Description Inhibition of G9a (unknown origin) by scintillation proximity Assay | J Med Chem 62: 9008-9025 (2019) Article DOI: 10.1021/acs.jmedchem.9b00562 BindingDB Entry DOI: 10.7270/Q27S7S2S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153099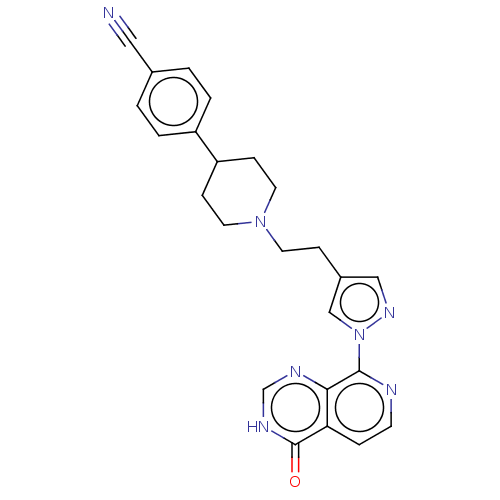 (CHEMBL3775814) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5A [1-801] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153101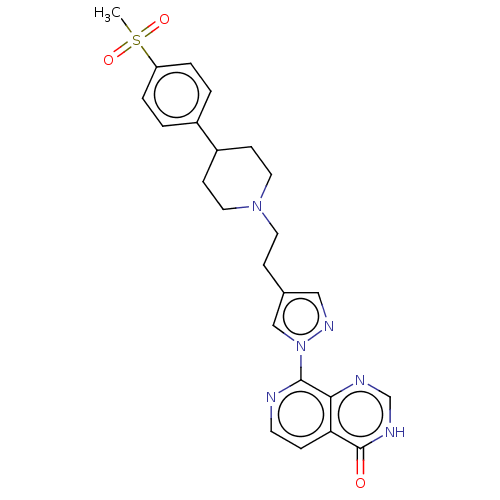 (CHEMBL3774665) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153161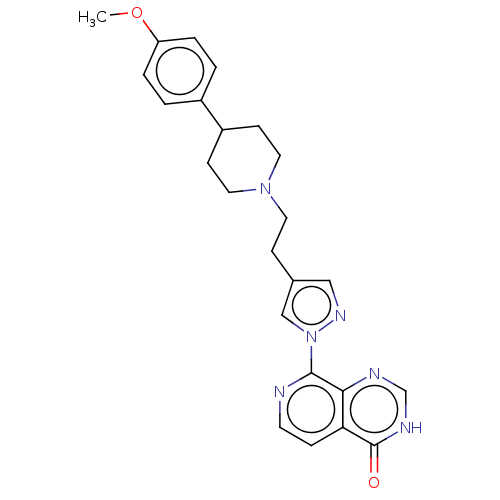 (CHEMBL3775545) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50151919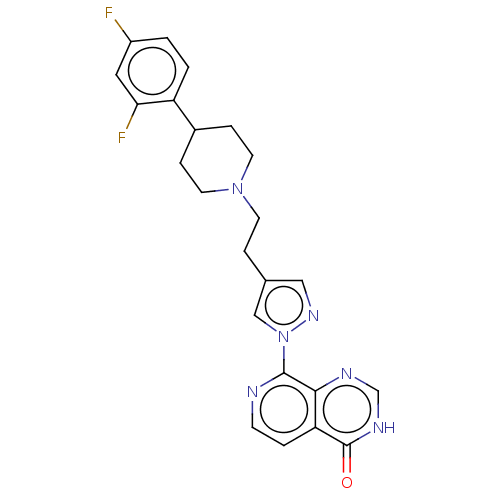 (CHEMBL3775121) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM6B (unknown origin) using biotin-H3K27me3 (21 to 44 residues) as substrate preincubated for 15 mins followed by substrate addition m... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153181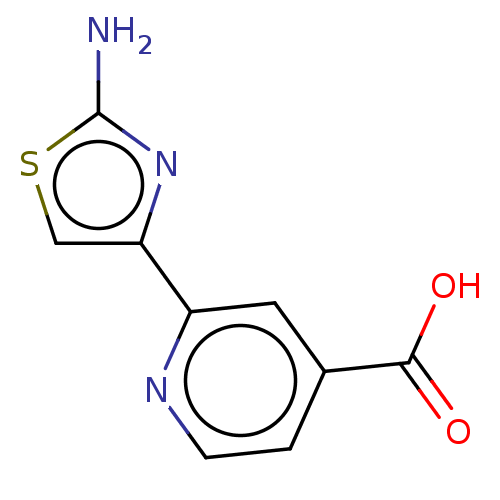 (CHEMBL3774692) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153181 (CHEMBL3774692) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C [1-765] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153092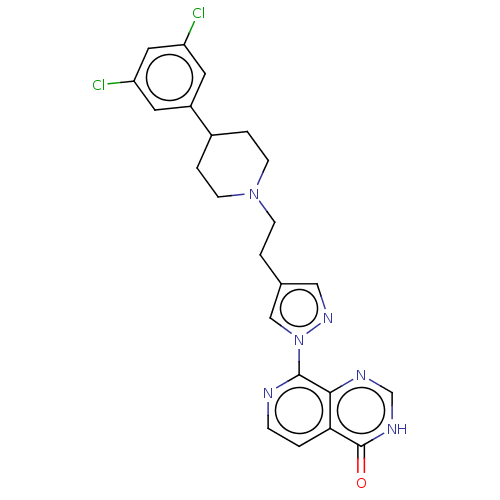 (CHEMBL3775894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50152029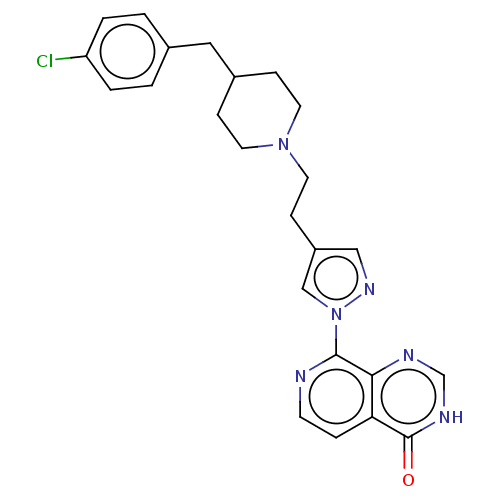 (CHEMBL3775899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153094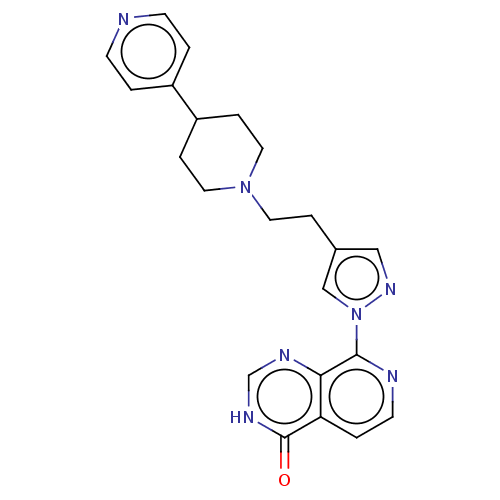 (CHEMBL3775277) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153075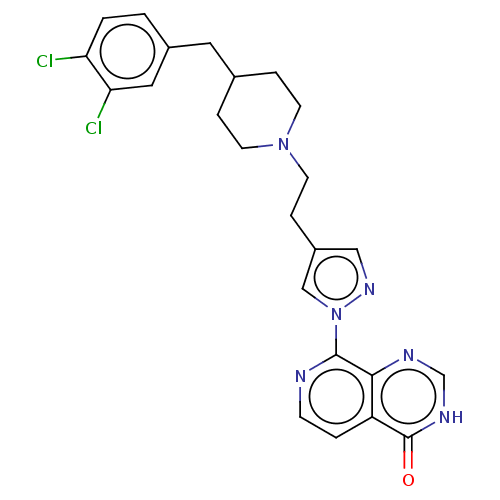 (CHEMBL3774940) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153076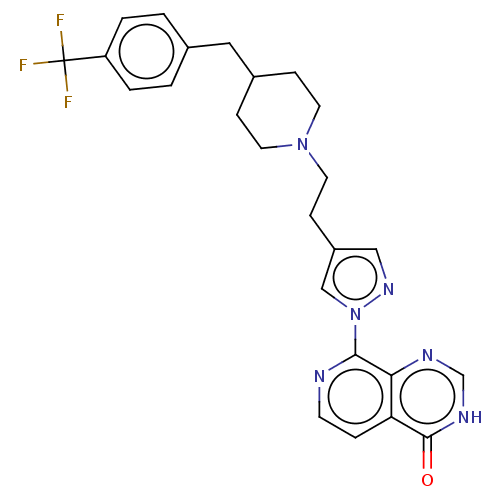 (CHEMBL3775516) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5D [1-775] (Homo sapiens (Human)) | BDBM191598 (2-(((2-((2-(dimethylamino)ethyl)(ethyl)amino)-2-ox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153073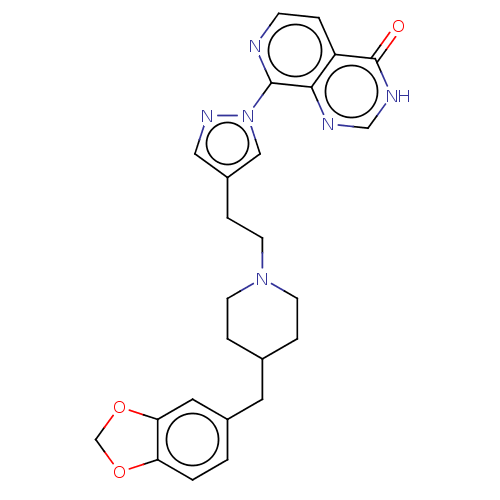 (CHEMBL3775451) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153077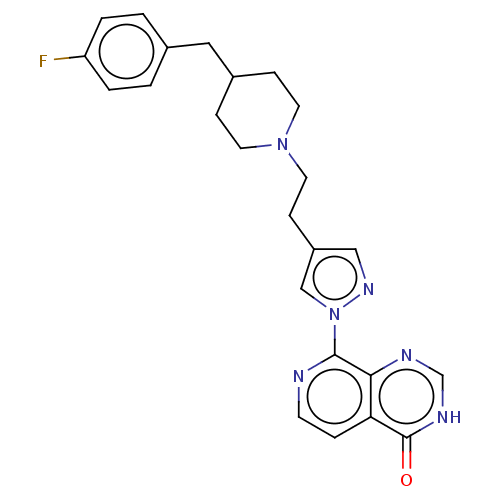 (CHEMBL3775040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496478 (LOR-NOR-30067bb9-11) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a | |
Israel Institution of Biological Research | Assay Description The assay was performed according to the published procedure. Briefly, compounds were seeded into assay-ready plates (Greiner 384PP, cat# 781280) usi... | bioRxiv 2021: (2021) BindingDB Entry DOI: 10.7270/Q2MS3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50153092 (CHEMBL3775894) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged KDM4B (1 to 500 residues) expressed in baculovirus infected sf9 cells using biotin-H3K9me3 as s... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50151917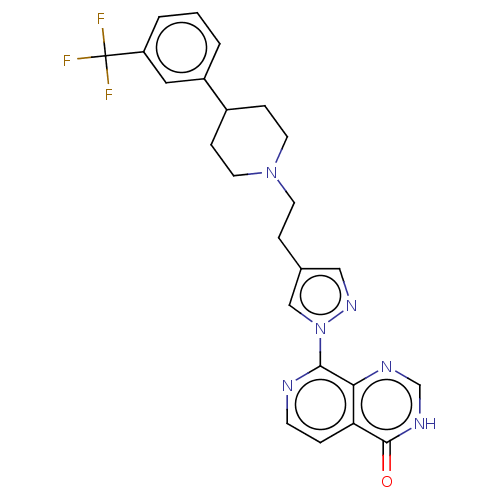 (CHEMBL3775548) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM6B (unknown origin) using biotin-H3K27me3 (21 to 44 residues) as substrate preincubated for 15 mins followed by substrate addition m... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153069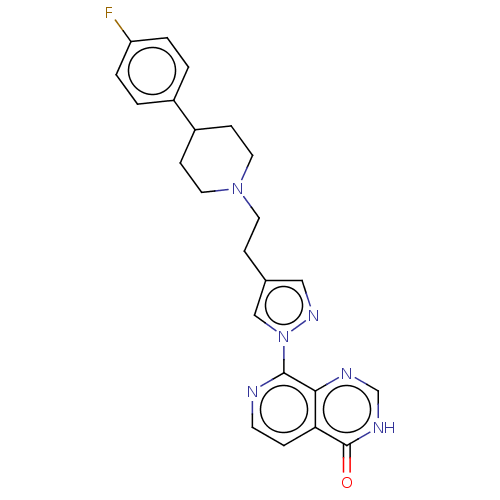 (CHEMBL3775953) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM627791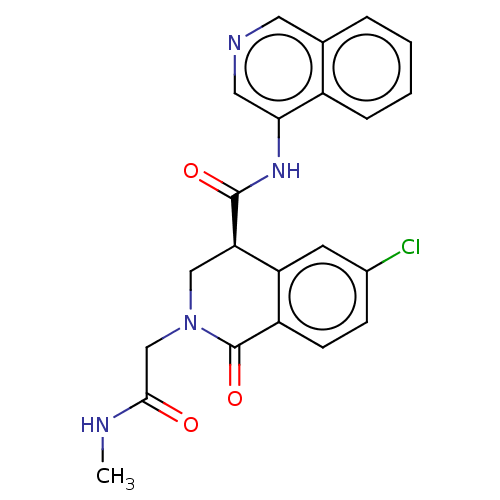 (CVD-0019230) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM495599 (CVD-0016335 | STE-KUL-d79e3d6a-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
Israel Institution of Biological Research | Assay Description Compounds were seeded into assay-ready plates (Greiner 384 low volume, cat 784900) using an Echo 555 acoustic dispenser, and DMSO was back-filled for... | bioRxiv 2021: (2021) BindingDB Entry DOI: 10.7270/Q2MS3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153078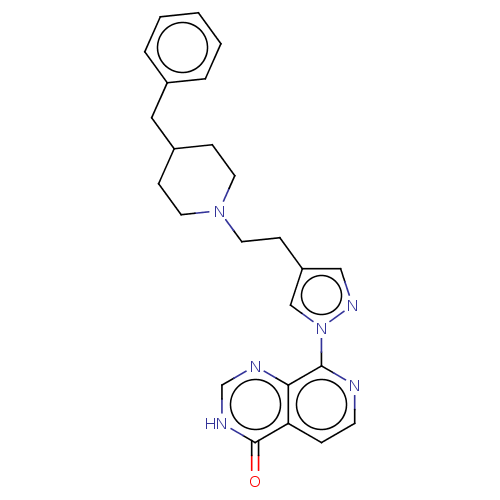 (CHEMBL3774392) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM495599 (CVD-0016335 | STE-KUL-d79e3d6a-3) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153073 (CHEMBL3775451) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153077 (CHEMBL3775040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153076 (CHEMBL3775516) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50152029 (CHEMBL3775899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153075 (CHEMBL3774940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50151923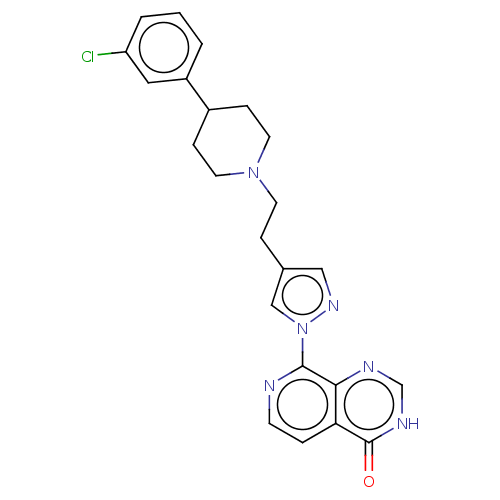 (CHEMBL3774537) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM6B (unknown origin) using biotin-H3K27me3 (21 to 44 residues) as substrate preincubated for 15 mins followed by substrate addition m... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Lysine-specific demethylase 5A [1-801] (Homo sapiens (Human)) | BDBM195612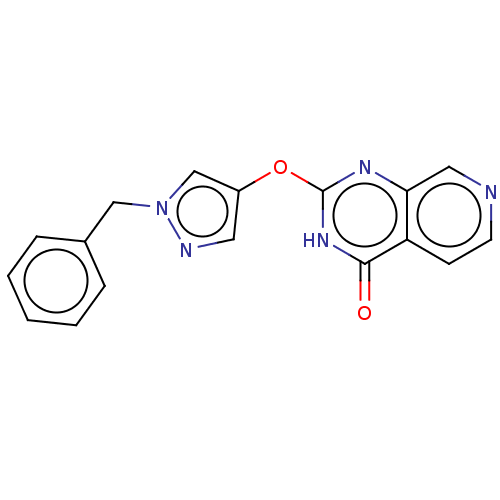 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5C (Homo sapiens (Human)) | BDBM50153099 (CHEMBL3775814) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5C (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM195612 (GSK467) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | 7.5 | n/a |
University of Oxford | Assay Description The demethylase AlphaScreen assay was performed in 384-well plate format using white proxiplates (PerkinElmer), and transfer of compound (100 nl) was... | Nat Chem Biol 12: 539-45 (2016) Article DOI: 10.1038/nchembio.2087 BindingDB Entry DOI: 10.7270/Q27P8X6K | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM496258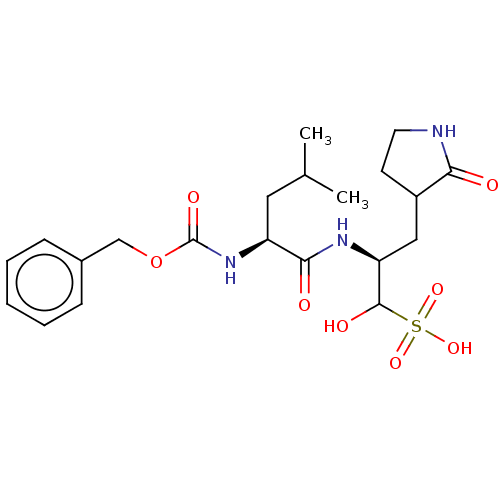 (CVD-0013146 | JOH-MSK-46727e7b-1) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a | |
Israel Institution of Biological Research | Assay Description The assay was performed according to the published procedure. Briefly, compounds were seeded into assay-ready plates (Greiner 384PP, cat# 781280) usi... | bioRxiv 2021: (2021) BindingDB Entry DOI: 10.7270/Q2MS3WV7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153093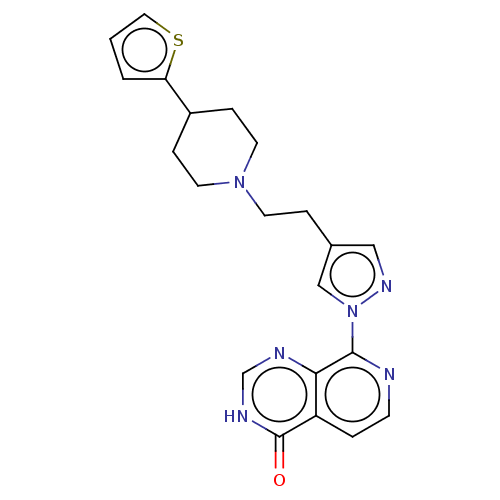 (CHEMBL3775668) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153072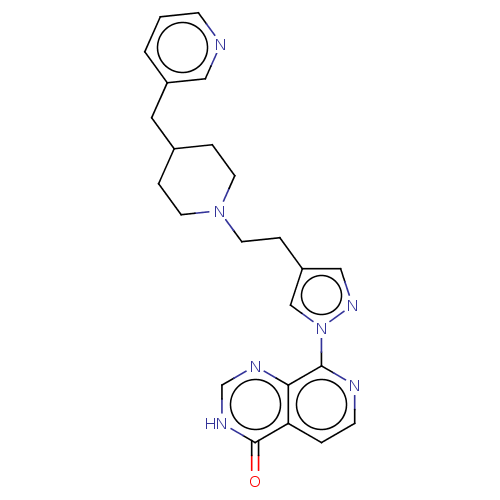 (CHEMBL3775956) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM627915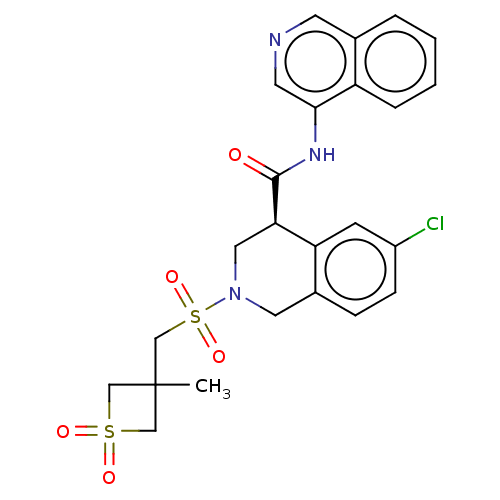 (CVD-0019273) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Replicase polyprotein 1ab (2019-nCoV) | BDBM625991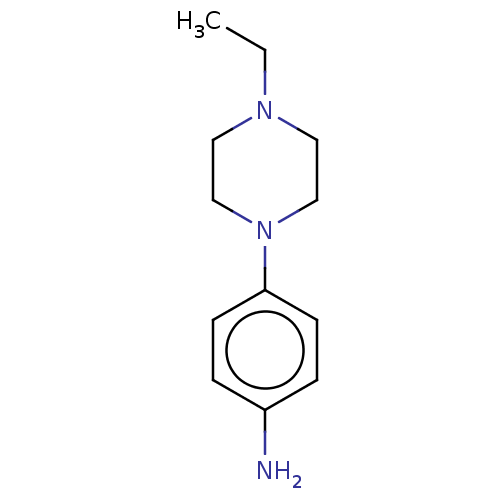 (EN300-11760) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 5B (Homo sapiens (Human)) | BDBM50153074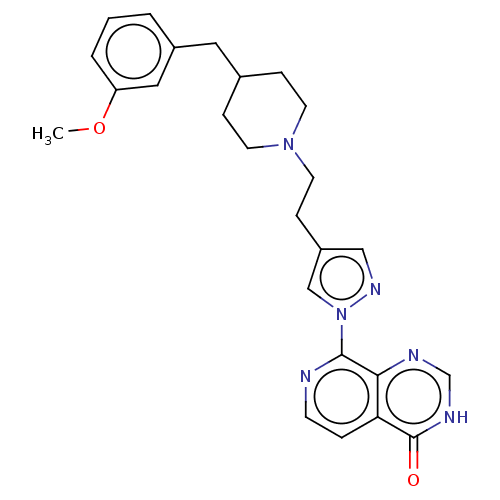 (CHEMBL3775465) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of KDM5B (unknown origin) using biotin-H3K4me3 as substrate preincubated for 15 mins followed by substrate addition measured after 20 mins... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific demethylase 4B (Homo sapiens (Human)) | BDBM50153093 (CHEMBL3775668) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
The Institute of Cancer Research Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal GST-tagged KDM4B (1 to 500 residues) expressed in baculovirus infected sf9 cells using biotin-H3K9me3 as s... | J Med Chem 59: 1388-409 (2016) Article DOI: 10.1021/acs.jmedchem.5b01635 BindingDB Entry DOI: 10.7270/Q2W097TR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5344 total ) | Next | Last >> |