

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139913 (US8895745, 375) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139918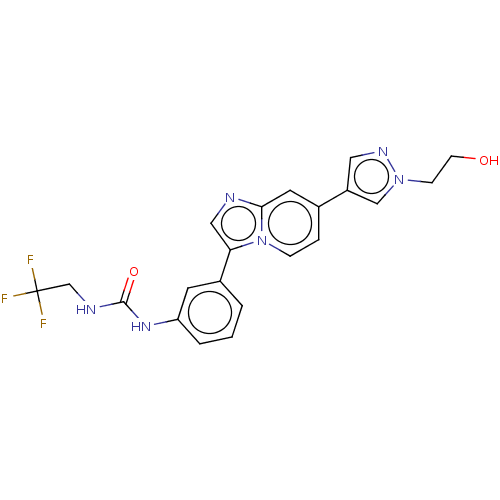 (US8895745, 401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.704 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139909 (US8895745, 310) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.780 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139914 (US8895745, 378) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein phosphatase non-receptor type 11 (Homo sapiens (Human)) | BDBM527814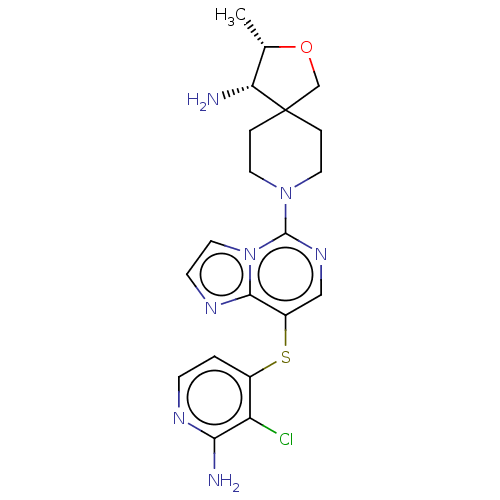 ((3S,4S)-8-(8-((2- amino-3- chloropyridin-4- yl)thi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q24X5CRG | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139920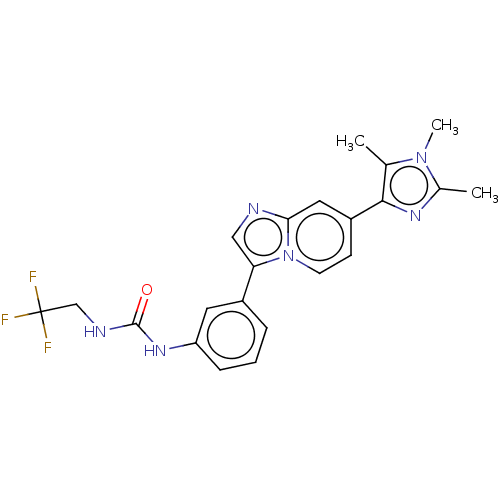 (US8895745, 412) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.24 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM13534 (CHEMBL572878 | N-[4-({4-[(3-methyl-1H-pyrazol-5-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27083 (1-(2,6-difluorophenyl)-3-{3-[5-(morpholin-4-ylmeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139924 (US8895745, 402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139915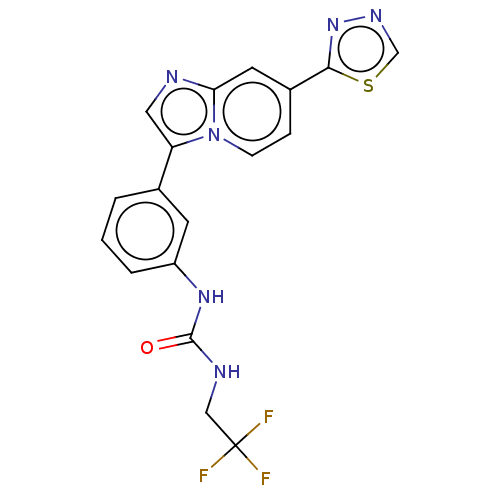 (US8895745, 384) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394015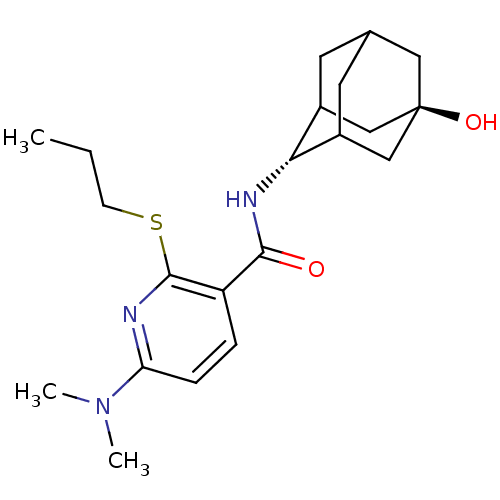 (CHEMBL2158468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139921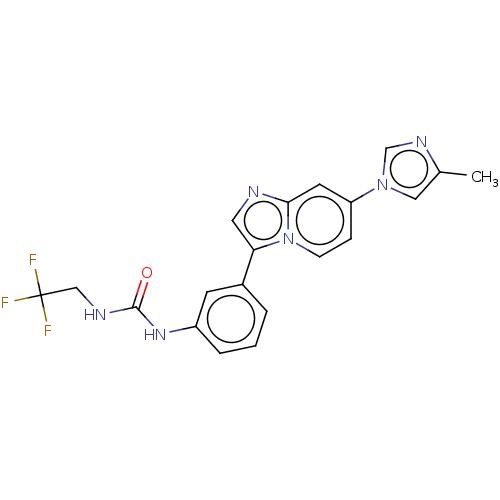 (US8895745, 416) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.18 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139922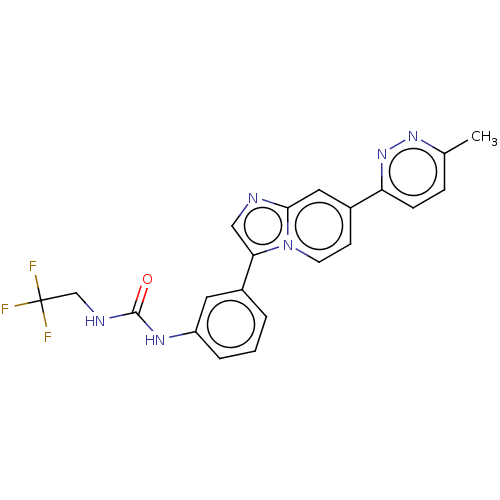 (US8895745, 421) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM27083 (1-(2,6-difluorophenyl)-3-{3-[5-(morpholin-4-ylmeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27082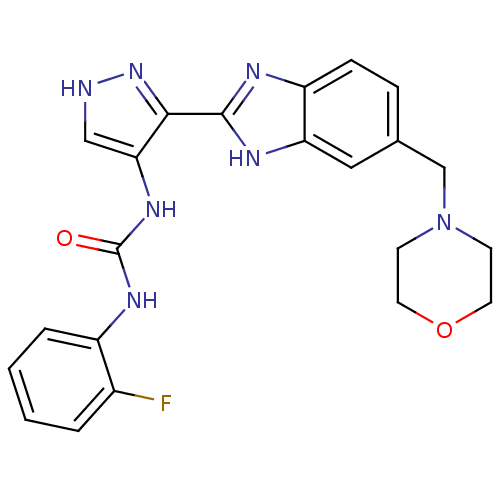 (1-(2-fluorophenyl)-3-{3-[5-(morpholin-4-ylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394013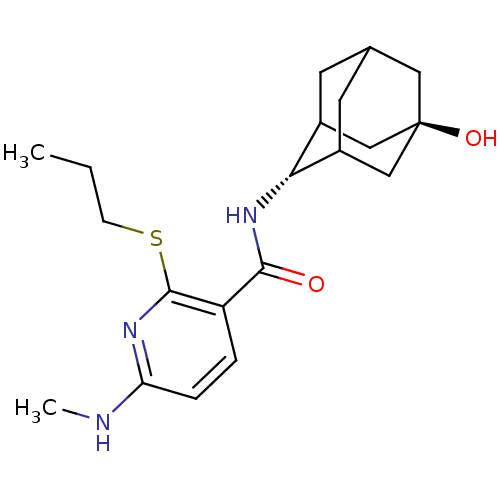 (CHEMBL2158466) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139908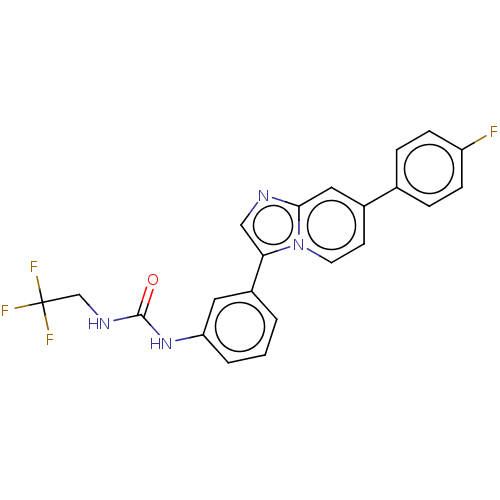 (US8895745, 59) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139923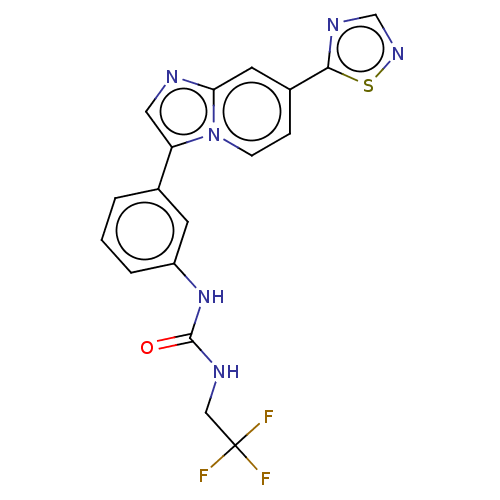 (US8895745, 422) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27079 (N-{3-[5-(morpholin-4-ylmethyl)-1H-1,3-benzodiazol-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525146 (US11168102, Example 32-31.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.53 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394014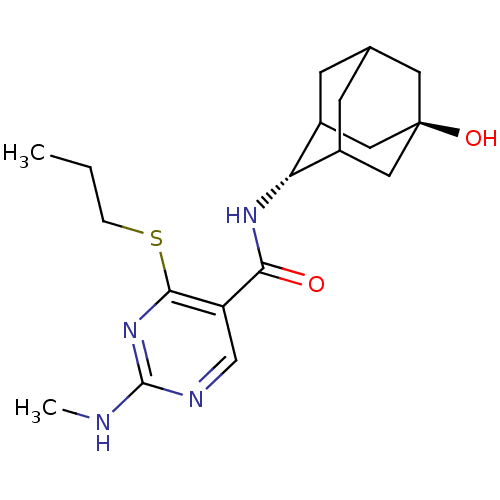 (CHEMBL2158467) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394016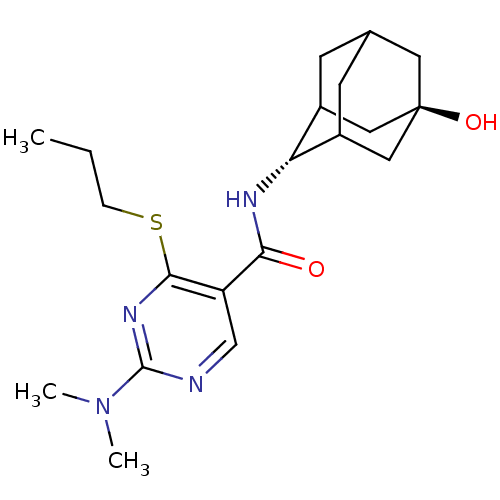 (CHEMBL2158469) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394017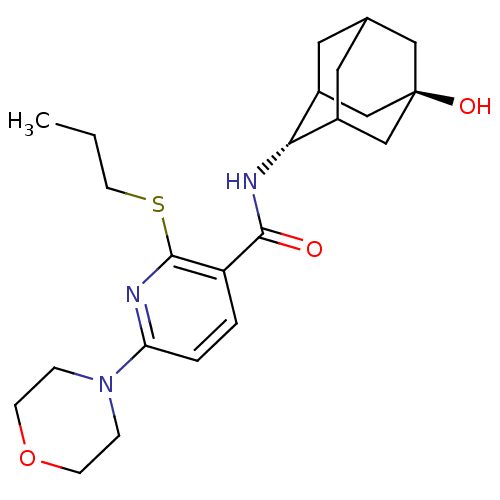 (CHEMBL2158470) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fibroblast growth factor receptor 3 (Homo sapiens (Human)) | BDBM139917 (US8895745, 399) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.74 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Astex Therapeutics Limited US Patent | Assay Description Enzymes (from Upstate) were prepared at 2x final concentration in 1x kinase assay buffer (as described below). Enzymes were then incubated with test ... | US Patent US8895745 (2014) BindingDB Entry DOI: 10.7270/Q2833QQC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525147 (US11168102, Example 32-32.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Homo sapiens (Human)) | BDBM27084 (1-cyclohexyl-3-{3-[5-(morpholin-4-ylmethyl)-1H-1,3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | 8.5 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27084 (1-cyclohexyl-3-{3-[5-(morpholin-4-ylmethyl)-1H-1,3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525070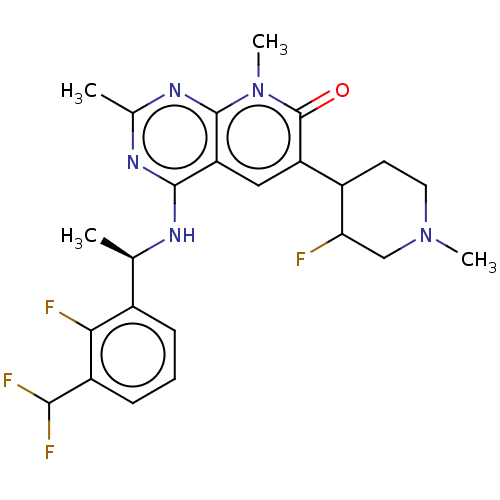 (US11168102, Example 18.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM27078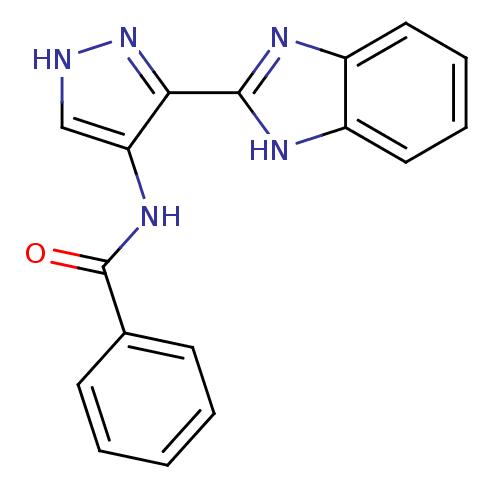 (N-[3-(1H-1,3-benzodiazol-2-yl)-1H-pyrazol-4-yl]ben...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | 7.0 | 22 |
Astex | Assay Description Assays for Aurora kinase was performed in a DELFIA format. Aurora enzyme was incubated with test compound and cross-tide substrate in the reaction bu... | J Med Chem 52: 379-88 (2009) Article DOI: 10.1021/jm800984v BindingDB Entry DOI: 10.7270/Q2P55KT7 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Mus musculus (mouse)) | BDBM50394000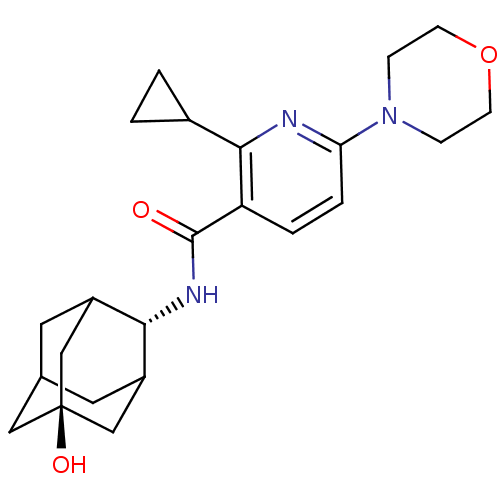 (CHEMBL2158481) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of mouse 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525148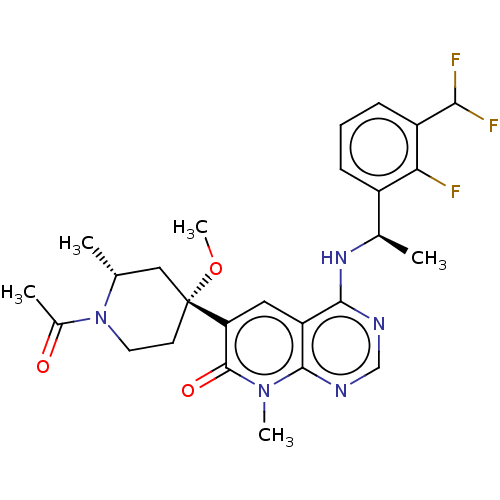 (US11168102, Example 32-51.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.77 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394011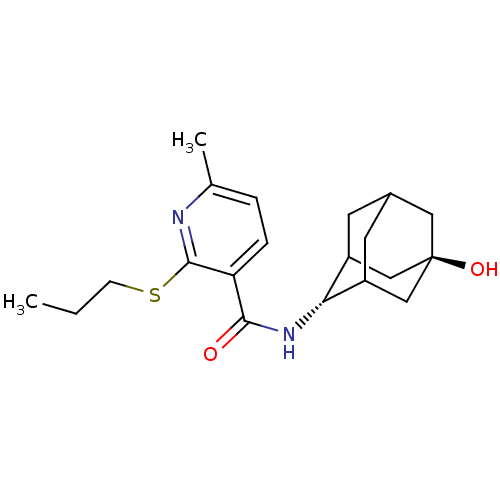 (CHEMBL2158464) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525132 (US11168102, Example 57.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.65 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525055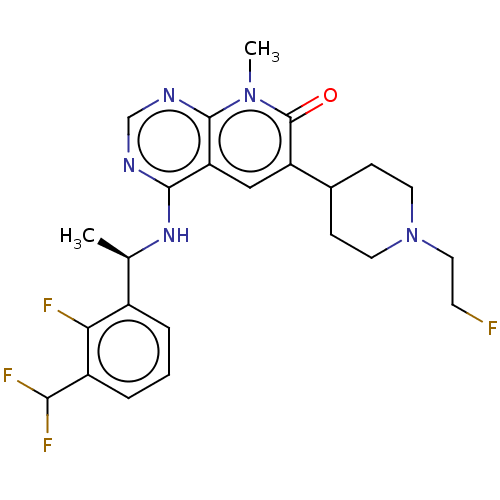 (US11168102, Example 8-4.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.66 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM525128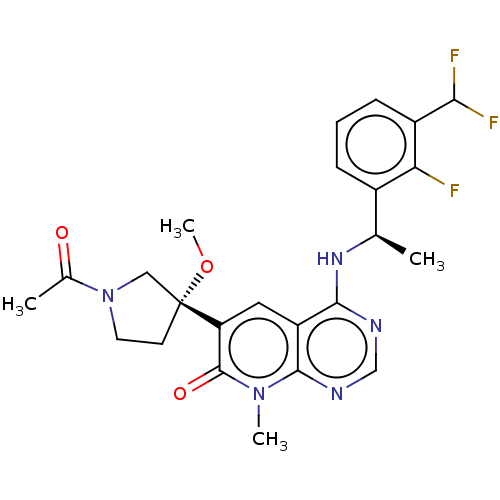 (US11168102, Example 32-19.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.82 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 11-beta-hydroxysteroid dehydrogenase 1 (Homo sapiens (Human)) | BDBM50394010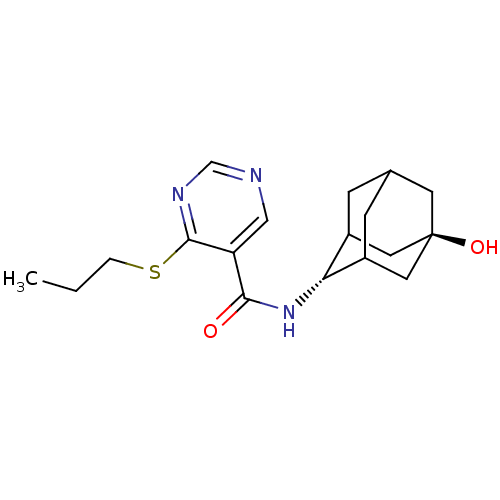 (CHEMBL2158007) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of human 11betaHSD1 by HTRF assay | Bioorg Med Chem Lett 22: 6756-61 (2012) Article DOI: 10.1016/j.bmcl.2012.08.070 BindingDB Entry DOI: 10.7270/Q23R0V0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Son of sevenless homolog 1 [564-1049] () | BDBM524861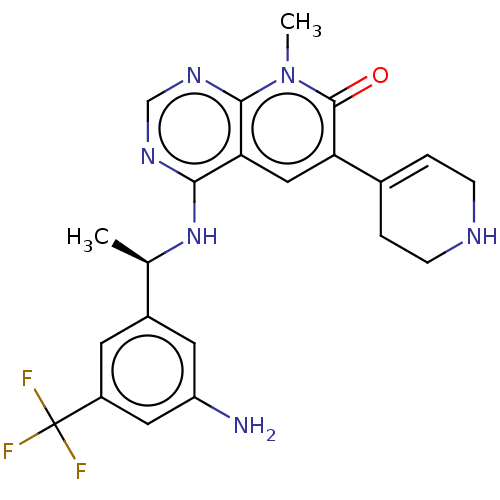 (US11168102, Example 1.) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Using a GE Biacore 8K SPR instrument, avi-tagged SOS1 catalytic domain protein was immobilized to a level of approximately 6000 response units (RU) o... | Citation and Details BindingDB Entry DOI: 10.7270/Q21V5J3D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591644 (US11566007, Example A323) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591645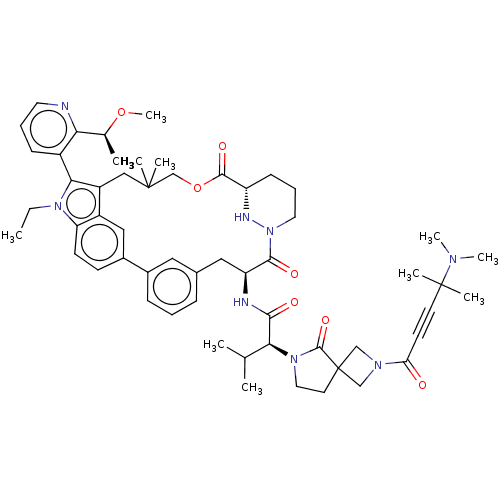 (US11566007, Example A324) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591647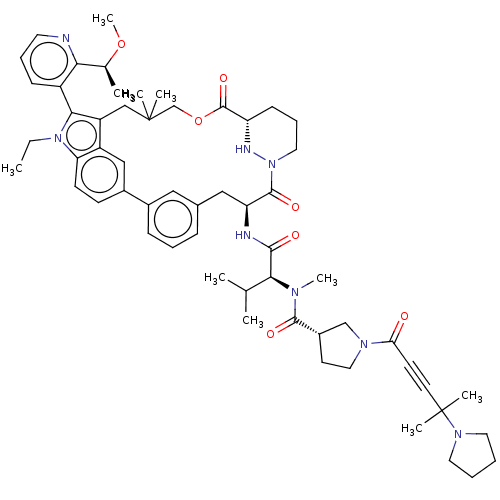 (US11566007, Example A326) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [Q61K] (Homo sapiens (Human)) | BDBM591709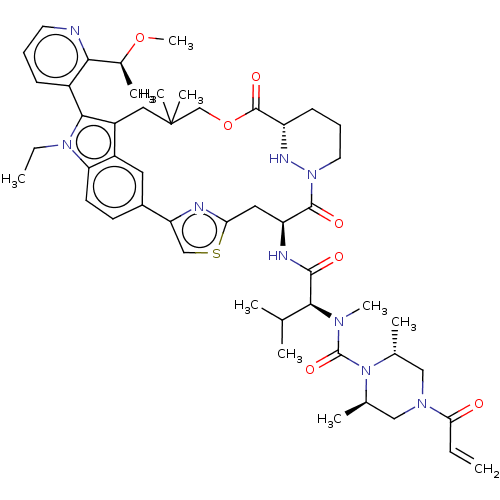 (US11566007, Example A388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591654 (US11566007, Example A333) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591657 (US11566007, Example A336) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591658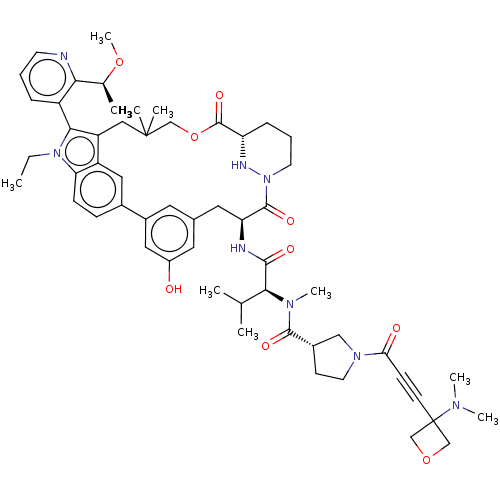 (US11566007, Example A337) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591660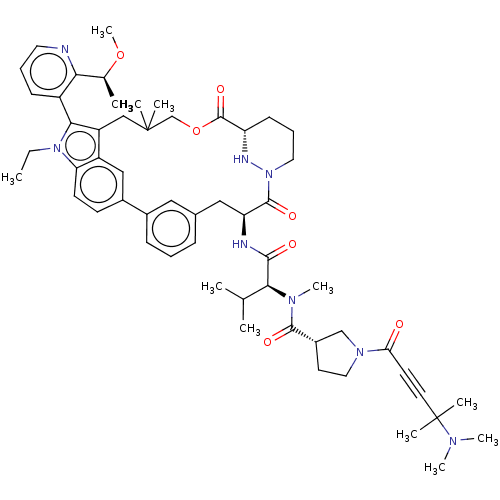 (US11566007, Example A339) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591664 (US11566007, Example A343) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591669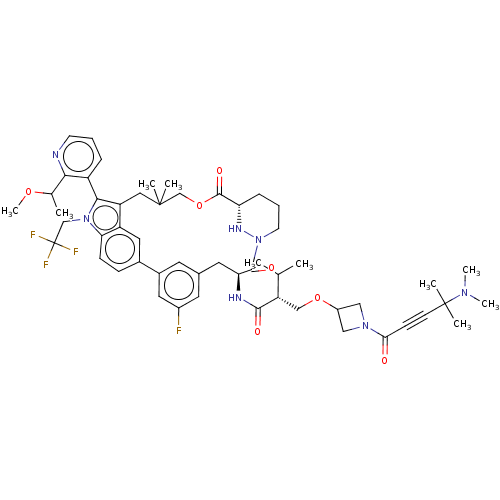 (US11566007, Example A348) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591672 (US11566007, Example A351) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase NRas [G12C] (Homo sapiens (Human)) | BDBM591686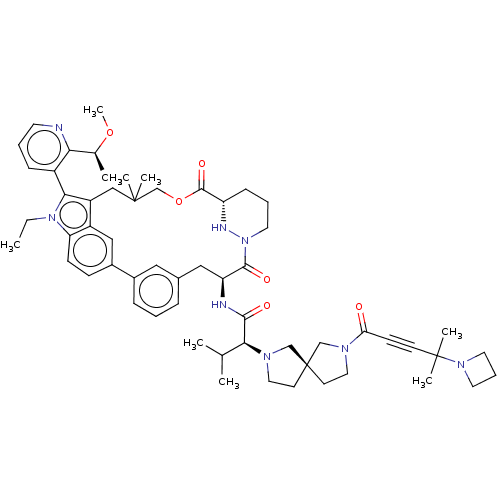 (US11566007, Example A365) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas [G13C] (Homo sapiens (Human)) | BDBM591657 (US11566007, Example A336) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description A variety of Ras proteins may be inhibited by compounds of the present invention (e.g., K-Ras, N-Ras, H-Ras, and mutants thereof at positions 12, 13 ... | Citation and Details BindingDB Entry DOI: 10.7270/Q2BP06QH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 8045 total ) | Next | Last >> |