

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50025103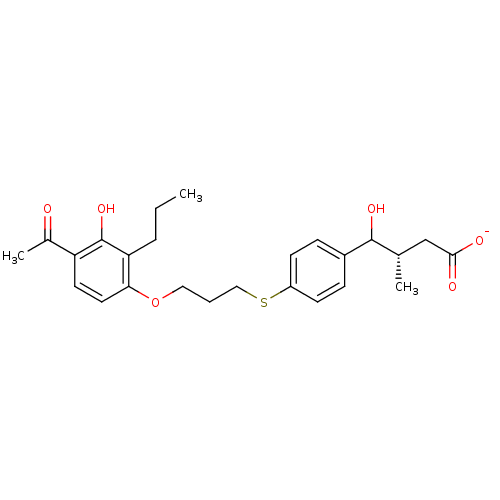 (CHEMBL41301 | Sodium; 4-{4-[3-(4-acetyl-3-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of radioligand [3H]-LTD4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1573-6 (1986) BindingDB Entry DOI: 10.7270/Q21G0K8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteinyl leukotriene receptor 1 (GUINEA PIG) | BDBM50025103 (CHEMBL41301 | Sodium; 4-{4-[3-(4-acetyl-3-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of radioligand [3H]-LTC4 binding to Cysteinyl leukotriene D4 receptor in guinea pig lung membranes | J Med Chem 29: 1573-6 (1986) BindingDB Entry DOI: 10.7270/Q21G0K8C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50029559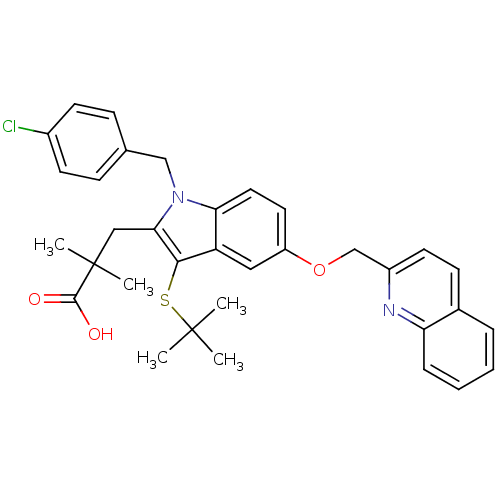 (2-[3-tert-Butylsulfanyl-1-(4-chloro-benzyl)-5-(qui...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of binding of [125I]- L- 691,831 binding to 5-lipoxygenase activating protein | Bioorg Med Chem Lett 2: 1395-1398 (1992) Article DOI: 10.1016/S0960-894X(00)80520-X BindingDB Entry DOI: 10.7270/Q2028RGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50280218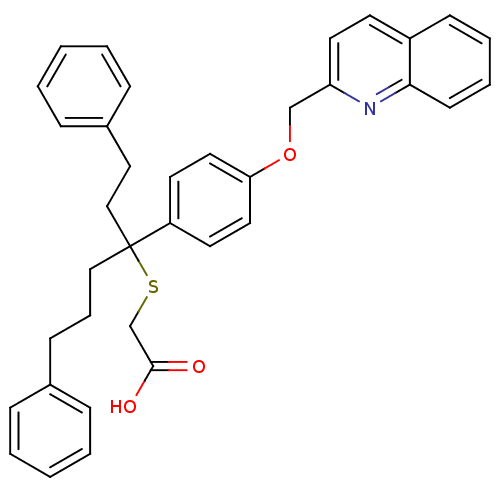 (CHEMBL281308 | {1-Phenethyl-4-phenyl-1-[4-(quinoli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of binding of [125I]- L- 691,831 binding to 5-lipoxygenase activating protein (FLAP) | Bioorg Med Chem Lett 2: 1395-1398 (1992) Article DOI: 10.1016/S0960-894X(00)80520-X BindingDB Entry DOI: 10.7270/Q2028RGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142042 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000845 (4-Methoxy-4-[3-(naphthalen-2-ylmethoxy)-phenyl]-te...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro potency against human 5-Lipoxygenase | J Med Chem 37: 512-8 (1994) BindingDB Entry DOI: 10.7270/Q2J965FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50040423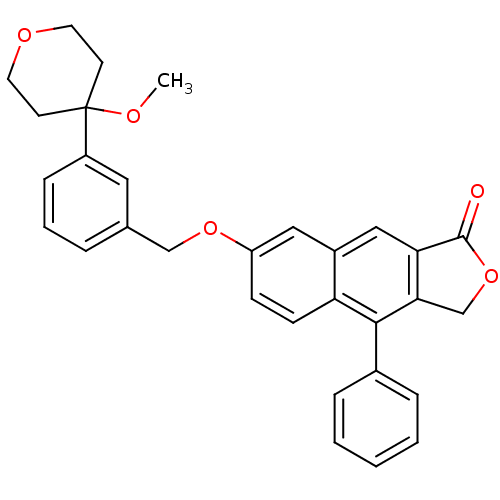 (7-(3-(4-methoxytetrahydro-2H-pyran-4-yl)benzyloxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro potency against human 5-Lipoxygenase | J Med Chem 37: 512-8 (1994) BindingDB Entry DOI: 10.7270/Q2J965FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142047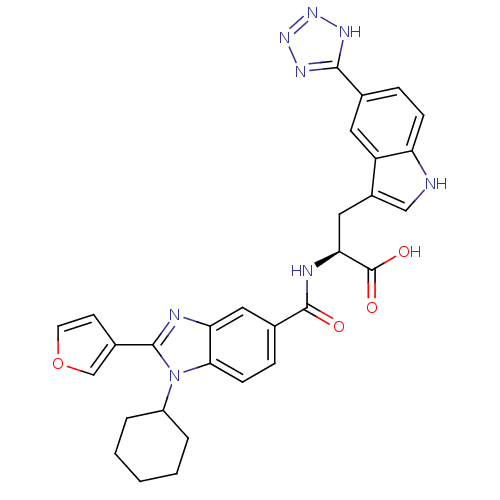 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193054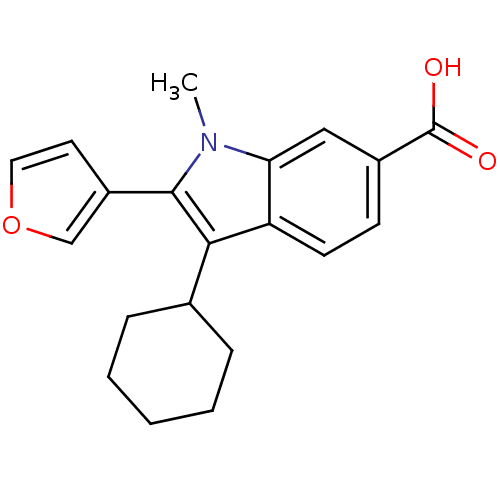 (3-cyclohexyl-2-(furan-3-yl)-1-methyl-1H-indole-6-c...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142043 ((2S)-3-(5-(carboxymethoxy)-1H-indol-3-yl)-2-(1-cyc...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50040419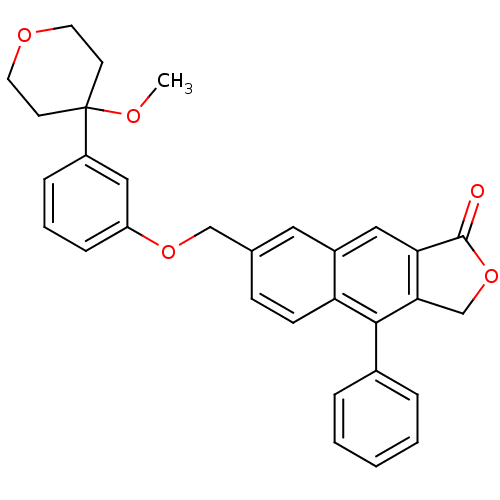 (7-[3-(4-Methoxy-tetrahydro-pyran-4-yl)-phenoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro potency against human 5-Lipoxygenase | J Med Chem 37: 512-8 (1994) BindingDB Entry DOI: 10.7270/Q2J965FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142041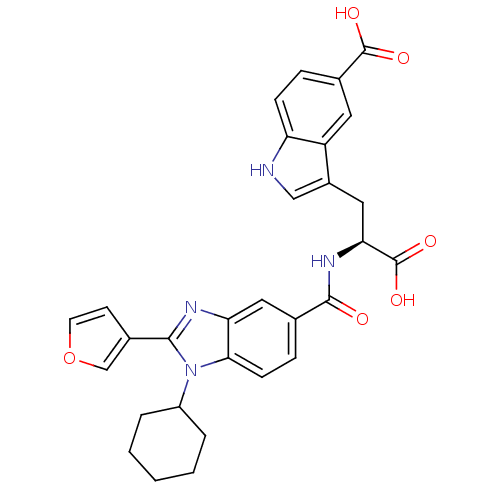 (3-{(S)-2-Carboxy-2-[(1-cyclohexyl-2-furan-3-yl-1H-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193050 (3-cyclopentyl-2-(furan-3-yl)-1-methyl-1H-indole-6-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50006805 (3-(1-(4-chlorobenzyl)-3-(tert-butylthio)-5-isoprop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of binding of [125I]- L- 691,831 binding to 5-lipoxygenase activating protein (FLAP) | Bioorg Med Chem Lett 2: 1395-1398 (1992) Article DOI: 10.1016/S0960-894X(00)80520-X BindingDB Entry DOI: 10.7270/Q2028RGQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193059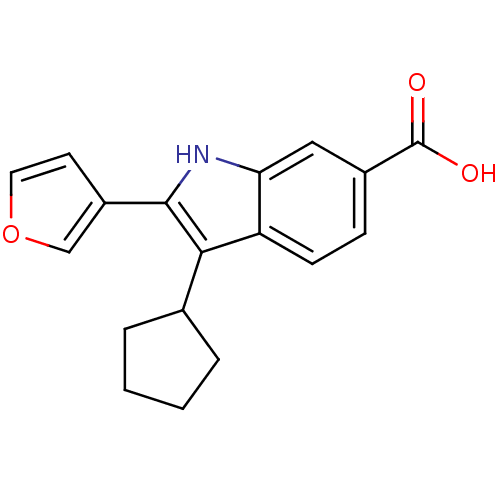 (3-cyclopentyl-2-(furan-3-yl)-1H-indole-6-carboxyli...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193060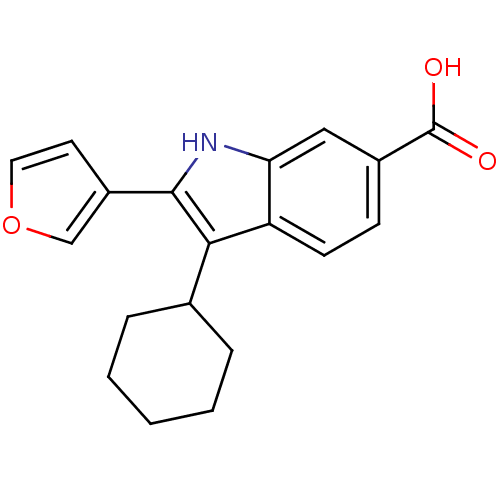 (3-cyclohexyl-2-(furan-3-yl)-1H-indole-6-carboxylic...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50162092 (3-Cyclohexyl-1-methyl-2-phenyl-1H-indole-6-carboxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175297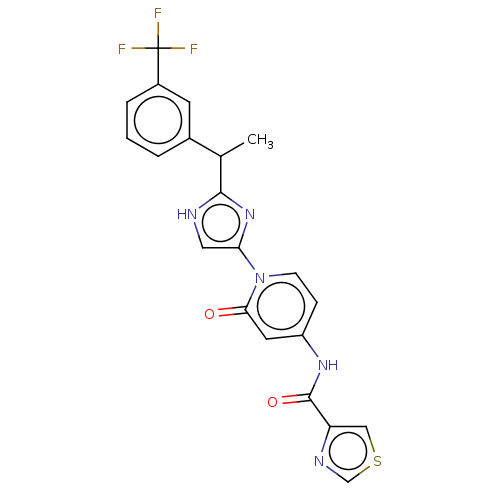 (CHEMBL3810245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142056 ((S)-3-(5-Carbamoyl-1H-indol-3-yl)-2-[(1-cyclohexyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193057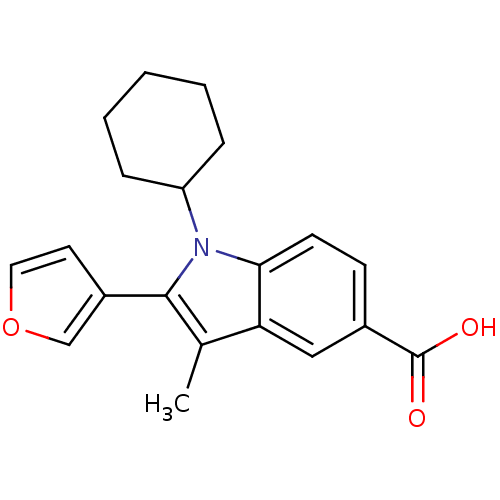 (1-cyclohexyl-2-(furan-3-yl)-3-methyl-1H-indole-5-c...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50040422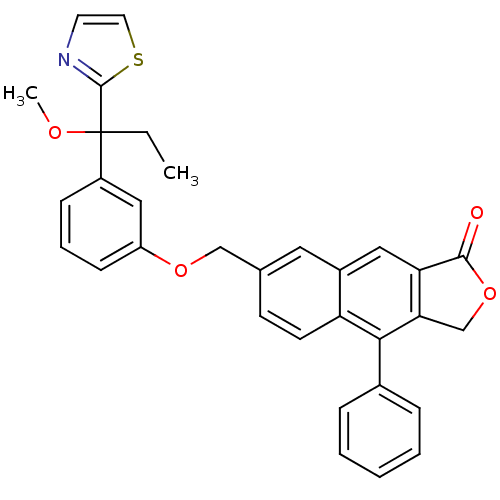 (7-[3-(1-Methoxy-1-thiazol-2-yl-propyl)-phenoxymeth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro potency against human 5-Lipoxygenase | J Med Chem 37: 512-8 (1994) BindingDB Entry DOI: 10.7270/Q2J965FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50162094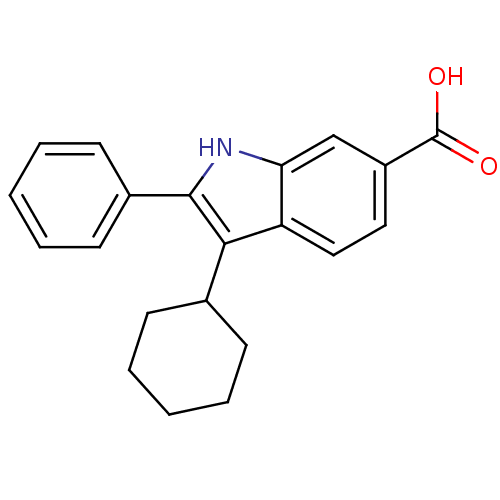 (3-Cyclohexyl-2-phenyl-1H-indole-6-carboxylic acid ...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142052 ((2S)-2-(1-cyclohexyl-2-(furan-3-yl)-1H-benzo[d]imi...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193069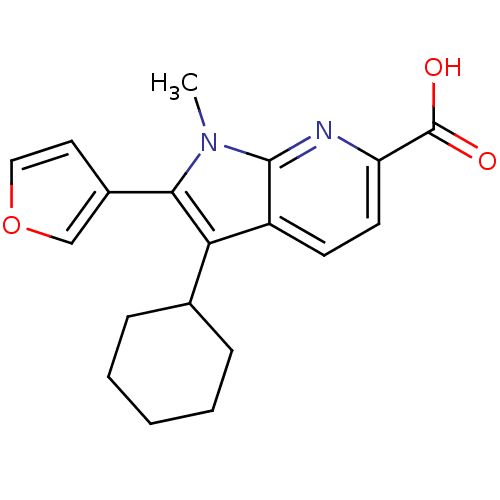 (3-cyclohexyl-2-(furan-3-yl)-1-methyl-1H-pyrrolo[2,...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142045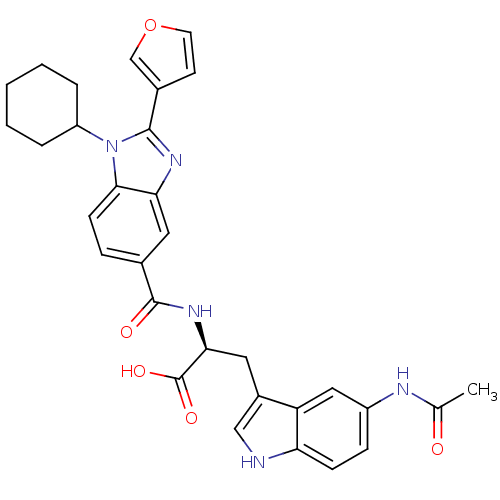 ((S)-3-(5-Acetylamino-1H-indol-3-yl)-2-[(1-cyclohex...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193063 (3-cyclopentyl-2-(furan-3-yl)-7-hydroxy-1-methyl-1H...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193055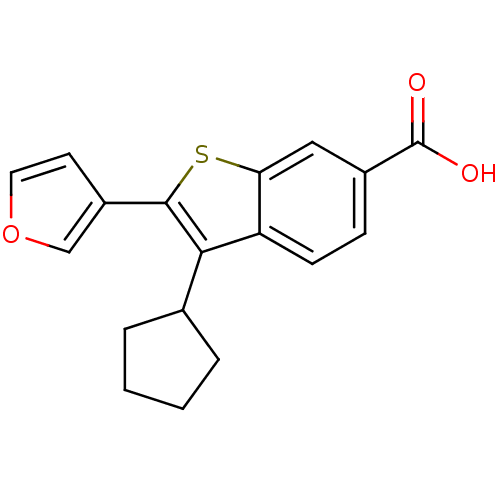 (3-cyclopentyl-2-(furan-3-yl)benzo[b]thiophene-6-ca...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193052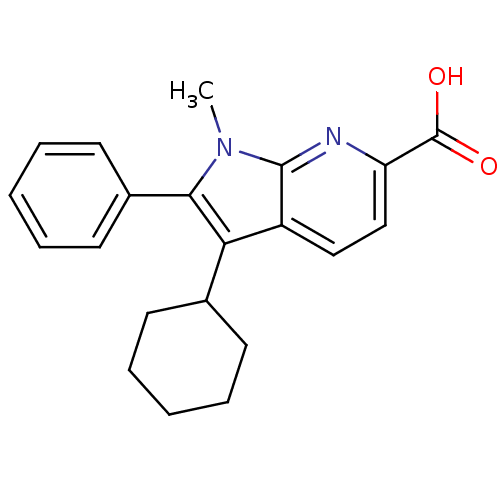 (3-cyclohexyl-1-methyl-2-phenyl-1H-pyrrolo[2,3-b]py...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193064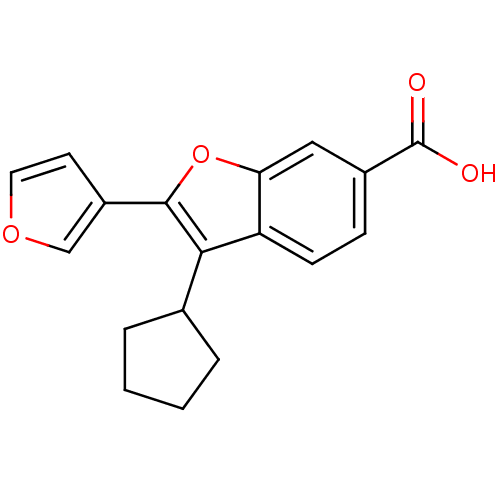 (3-cyclopentyl-2-(furan-3-yl)benzofuran-6-carboxyli...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142053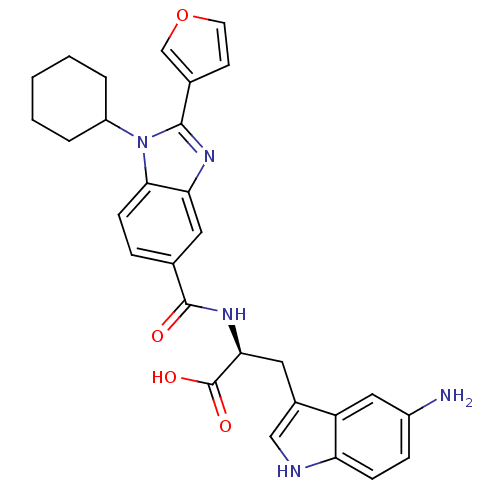 ((S)-3-(5-Amino-1H-indol-3-yl)-2-[(1-cyclohexyl-2-f...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193062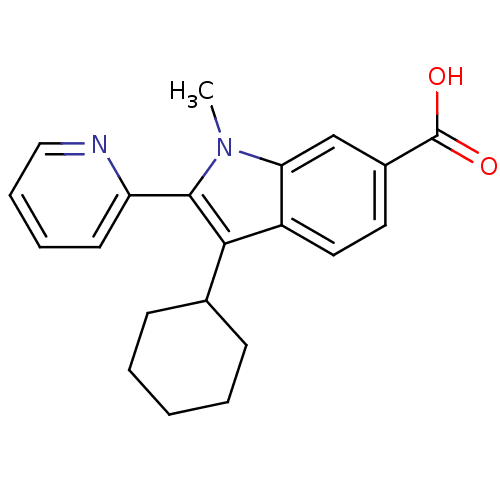 (3-cyclohexyl-1-methyl-2-(pyridin-2-yl)-1H-indole-6...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142044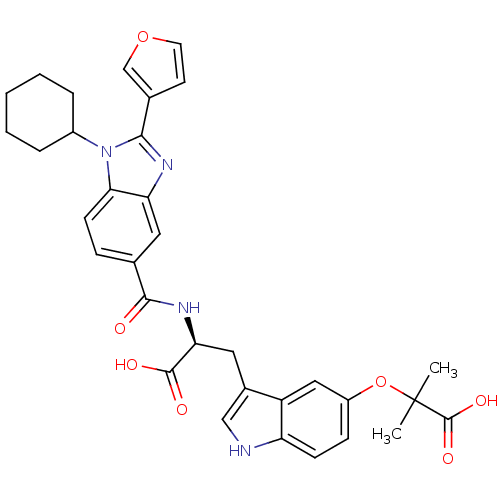 ((S)-3-[5-(1-Carboxy-1-methyl-ethoxy)-1H-indol-3-yl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142049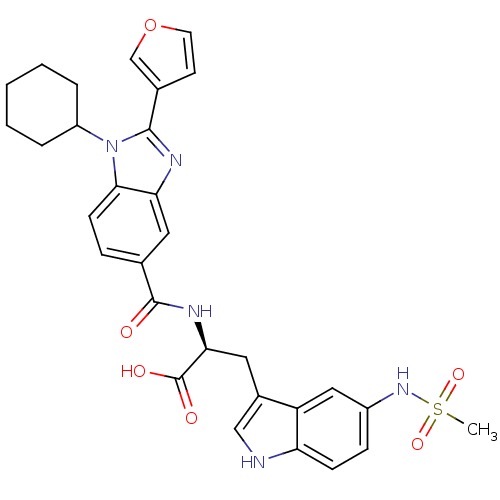 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50085160 (CHEMBL32842 | L-674636 | {4-(4-Chloro-phenyl)-1-[4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The compound was tested for the inhibition of binding of [125I]- L- 691,831 binding to 5-lipoxygenase activating protein (FLAP) | Bioorg Med Chem Lett 2: 1395-1398 (1992) Article DOI: 10.1016/S0960-894X(00)80520-X BindingDB Entry DOI: 10.7270/Q2028RGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142050 ((S)-2-(1-cyclohexyl-2-(pyridin-2-yl)-1H-benzo[d]im...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175302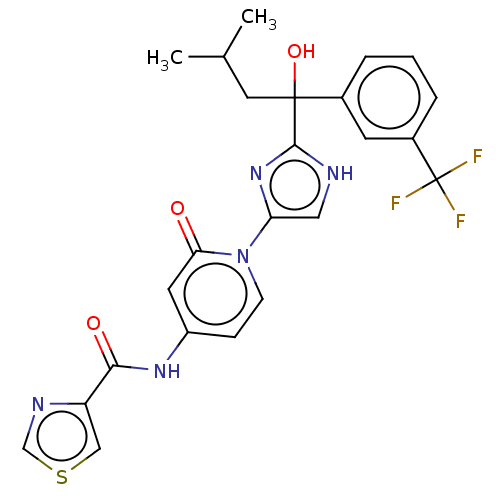 (CHEMBL3808401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50370756 (CHEMBL1789968) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142048 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000857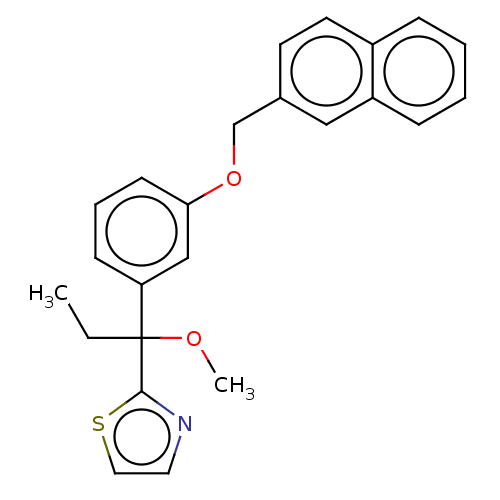 (2-(1-methoxy-1-(3-(naphthalen-2-ylmethoxy)phenyl)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro potency against human 5-Lipoxygenase | J Med Chem 37: 512-8 (1994) BindingDB Entry DOI: 10.7270/Q2J965FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142069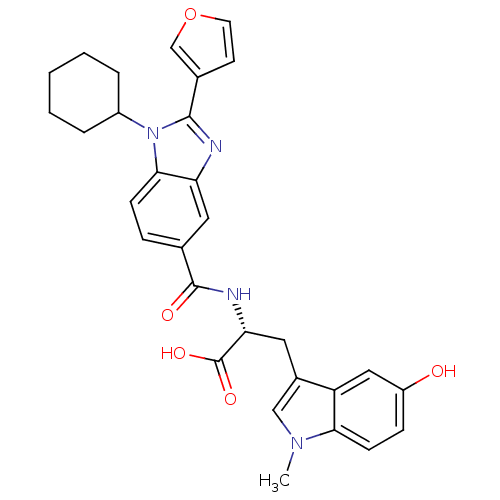 ((R)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50040418 (7-[3-(1-Methoxy-1-thiazol-2-yl-propyl)-benzyloxy]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description In vitro potency against human 5-Lipoxygenase | J Med Chem 37: 512-8 (1994) BindingDB Entry DOI: 10.7270/Q2J965FV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175303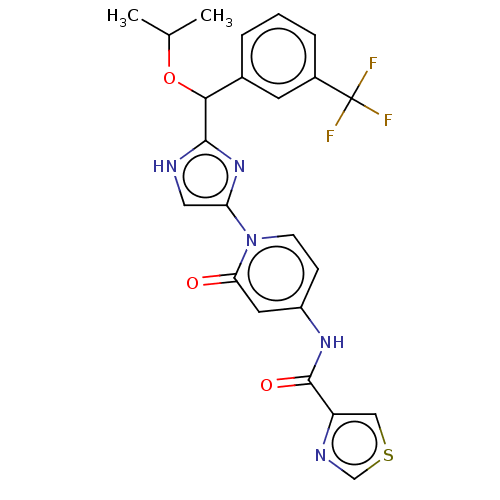 (CHEMBL3810042) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193053 (1-cyclohexyl-3-methyl-2-phenyl-1H-indole-5-carboxy...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193067 (3-cyclohexyl-2-(pyridin-2-yl)-1H-indole-6-carboxyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193051 (7-chloro-3-cyclopentyl-2-(furan-3-yl)-1-methyl-1H-...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50398047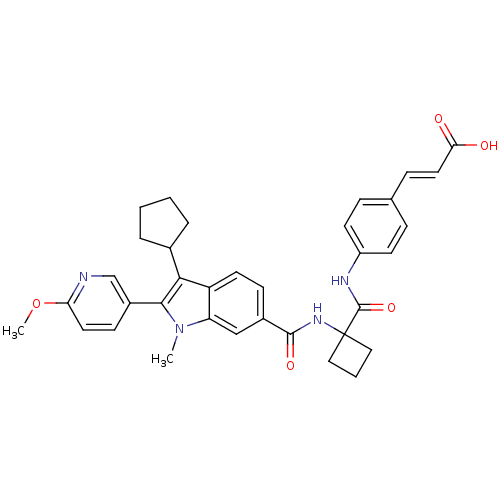 (CHEMBL2181641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 55: 7650-66 (2012) Article DOI: 10.1021/jm3006788 BindingDB Entry DOI: 10.7270/Q2ZS2XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50193066 (3-cyclopentyl-2-(furan-3-yl)-7-methoxy-1-methyl-1H...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Curated by ChEMBL | Assay Description Inhibition of HCV 1b NS5B delta21 RNA dependent RNA polymerase by SPA | Bioorg Med Chem Lett 16: 4987-93 (2006) Checked by Author Article DOI: 10.1016/j.bmcl.2006.07.074 BindingDB Entry DOI: 10.7270/Q27S7PJT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50158528 ((S)-1-cyclohexyl-2-(furan-3-yl)-N-(2-(5-hydroxy-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Inhibition of human liver CYP3A4 | Bioorg Med Chem Lett 20: 196-200 (2010) Article DOI: 10.1016/j.bmcl.2009.10.136 BindingDB Entry DOI: 10.7270/Q2TD9XFM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50398051 (CHEMBL2181777) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C19 | J Med Chem 55: 7650-66 (2012) Article DOI: 10.1021/jm3006788 BindingDB Entry DOI: 10.7270/Q2ZS2XNP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| RNA-directed RNA polymerase (Hepatitis C virus) | BDBM50142051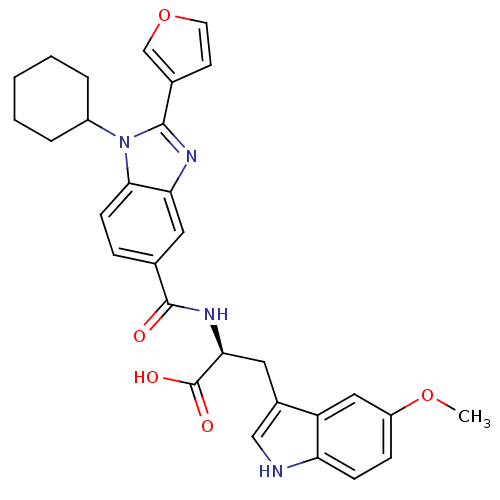 ((S)-2-[(1-Cyclohexyl-2-furan-3-yl-1H-benzoimidazol...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd Curated by ChEMBL | Assay Description Concentration required for inhibiting hepatitis C virus NS5B RNA polymerase activity. | Bioorg Med Chem Lett 14: 967-71 (2004) Article DOI: 10.1016/j.bmcl.2003.12.032 BindingDB Entry DOI: 10.7270/Q29C6WVF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 327 total ) | Next | Last >> |