 Found 474 hits with Last Name = 'goldsmith' and Initial = 'r'
Found 474 hits with Last Name = 'goldsmith' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086681
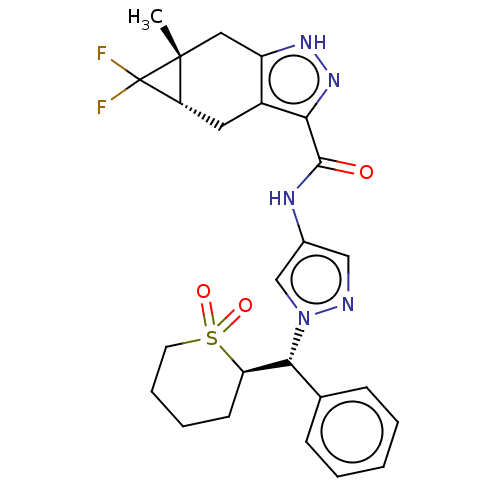
(CHEMBL3426309)Show SMILES [H][C@]12Cc3c(C[C@@]1(C)C2(F)F)[nH]nc3C(=O)Nc1cnn(c1)[C@H](c1ccccc1)[C@@]1([H])CCCCS1(=O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434810
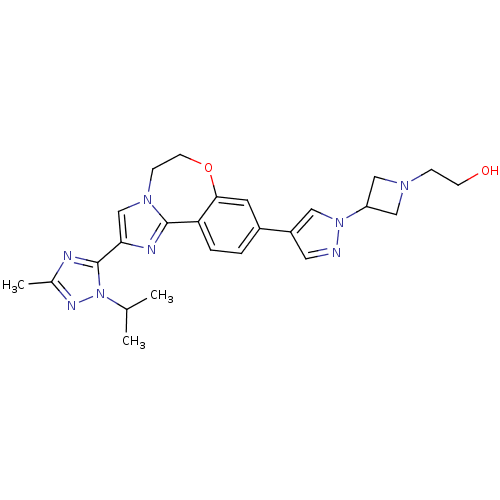
(CHEMBL2386970)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C1CN(CCO)C1 Show InChI InChI=1S/C25H30N8O2/c1-16(2)33-25(27-17(3)29-33)22-15-31-7-9-35-23-10-18(4-5-21(23)24(31)28-22)19-11-26-32(12-19)20-13-30(14-20)6-8-34/h4-5,10-12,15-16,20,34H,6-9,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50434806
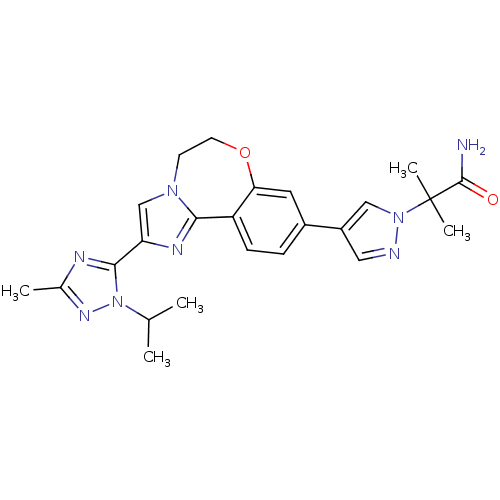
(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434807

(CHEMBL2387079)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CC(C)(C)O)c1 Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-22(24-14-26-30)19-12-28-7-8-32-20-9-16(5-6-18(20)21(28)27-19)17-10-25-29(11-17)13-23(3,4)31/h5-6,9-12,14-15,31H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434812

(CHEMBL2387086)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C21H23N7O2/c1-14(2)28-21(22-13-24-28)18-12-26-6-8-30-19-9-15(3-4-17(19)20(26)25-18)16-10-23-27(11-16)5-7-29/h3-4,9-14,29H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086604
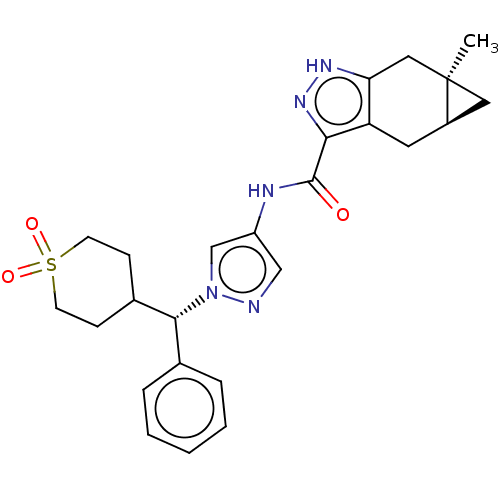
(CHEMBL3426308)Show SMILES [H][C@@]12C[C@]1(C)Cc1[nH]nc(C(=O)Nc3cnn(c3)[C@@H](C3CCS(=O)(=O)CC3)c3ccccc3)c1C2 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434814

(CHEMBL2387082)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(C)c1 Show InChI InChI=1S/C21H23N7O/c1-13(2)28-21(23-14(3)25-28)18-12-27-7-8-29-19-9-15(16-10-22-26(4)11-16)5-6-17(19)20(27)24-18/h5-6,9-13H,7-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086605
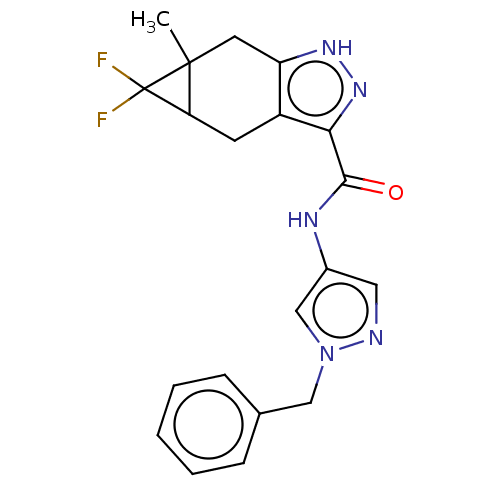
(CHEMBL3426307)Show SMILES CC12Cc3[nH]nc(C(=O)Nc4cnn(Cc5ccccc5)c4)c3CC1C2(F)F | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086659
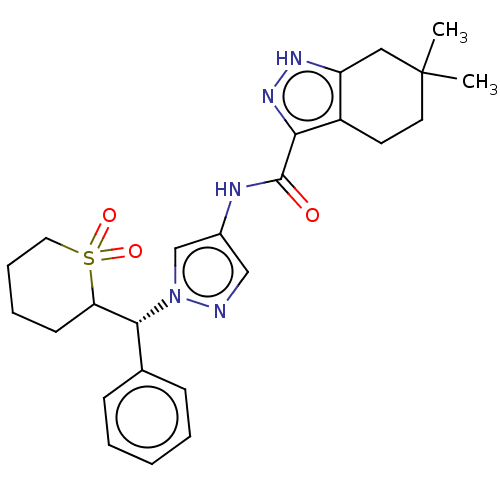
(CHEMBL3426303)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCCCS1(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C25H31N5O3S/c1-25(2)12-11-19-20(14-25)28-29-22(19)24(31)27-18-15-26-30(16-18)23(17-8-4-3-5-9-17)21-10-6-7-13-34(21,32)33/h3-5,8-9,15-16,21,23H,6-7,10-14H2,1-2H3,(H,27,31)(H,28,29)/t21?,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086659

(CHEMBL3426303)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCCCS1(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C25H31N5O3S/c1-25(2)12-11-19-20(14-25)28-29-22(19)24(31)27-18-15-26-30(16-18)23(17-8-4-3-5-9-17)21-10-6-7-13-34(21,32)33/h3-5,8-9,15-16,21,23H,6-7,10-14H2,1-2H3,(H,27,31)(H,28,29)/t21?,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086671
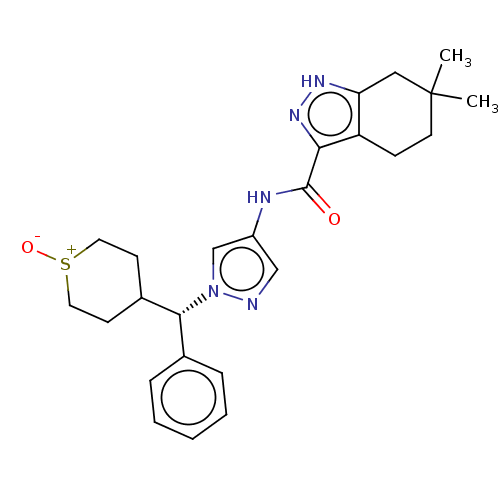
(CHEMBL3426305)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CC[S+]([O-])CC1)c1ccccc1 |r,wU:19.22,(6.24,-16.51,;7.44,-16.22,;7.85,-15.06,;8.94,-15.9,;10,-17.05,;9.51,-18.51,;8.01,-18.84,;6.96,-17.71,;7.86,-20.36,;9.28,-20.98,;10.28,-19.84,;11.81,-20,;12.53,-19,;12.44,-21.4,;13.97,-21.56,;14.72,-22.89,;16.23,-22.57,;16.38,-21.03,;14.97,-20.41,;17.71,-20.26,;17.71,-18.72,;19.04,-17.95,;19.04,-16.41,;17.7,-15.64,;17.7,-14.41,;16.37,-16.42,;16.38,-17.96,;19.05,-21.03,;20.38,-20.26,;21.72,-21.03,;21.72,-22.57,;20.38,-23.34,;19.05,-22.57,)| Show InChI InChI=1S/C25H31N5O2S/c1-25(2)11-8-20-21(14-25)28-29-22(20)24(31)27-19-15-26-30(16-19)23(17-6-4-3-5-7-17)18-9-12-33(32)13-10-18/h3-7,15-16,18,23H,8-14H2,1-2H3,(H,27,31)(H,28,29)/t18?,23-,33?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086671

(CHEMBL3426305)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CC[S+]([O-])CC1)c1ccccc1 |r,wU:19.22,(6.24,-16.51,;7.44,-16.22,;7.85,-15.06,;8.94,-15.9,;10,-17.05,;9.51,-18.51,;8.01,-18.84,;6.96,-17.71,;7.86,-20.36,;9.28,-20.98,;10.28,-19.84,;11.81,-20,;12.53,-19,;12.44,-21.4,;13.97,-21.56,;14.72,-22.89,;16.23,-22.57,;16.38,-21.03,;14.97,-20.41,;17.71,-20.26,;17.71,-18.72,;19.04,-17.95,;19.04,-16.41,;17.7,-15.64,;17.7,-14.41,;16.37,-16.42,;16.38,-17.96,;19.05,-21.03,;20.38,-20.26,;21.72,-21.03,;21.72,-22.57,;20.38,-23.34,;19.05,-22.57,)| Show InChI InChI=1S/C25H31N5O2S/c1-25(2)11-8-20-21(14-25)28-29-22(20)24(31)27-19-15-26-30(16-19)23(17-6-4-3-5-7-17)18-9-12-33(32)13-10-18/h3-7,15-16,18,23H,8-14H2,1-2H3,(H,27,31)(H,28,29)/t18?,23-,33?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086676
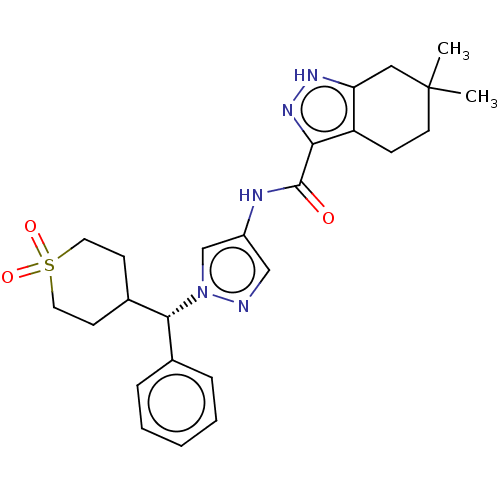
(CHEMBL3426304)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCS(=O)(=O)CC1)c1ccccc1 |r| Show InChI InChI=1S/C25H31N5O3S/c1-25(2)11-8-20-21(14-25)28-29-22(20)24(31)27-19-15-26-30(16-19)23(17-6-4-3-5-7-17)18-9-12-34(32,33)13-10-18/h3-7,15-16,18,23H,8-14H2,1-2H3,(H,27,31)(H,28,29)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434808
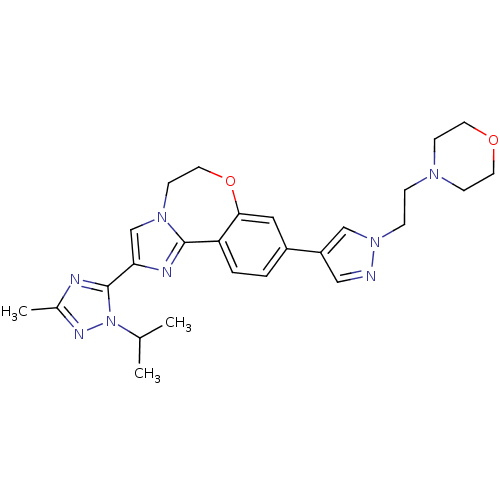
(CHEMBL2386972)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C26H32N8O2/c1-18(2)34-26(28-19(3)30-34)23-17-32-10-13-36-24-14-20(4-5-22(24)25(32)29-23)21-15-27-33(16-21)7-6-31-8-11-35-12-9-31/h4-5,14-18H,6-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434817

(CHEMBL2387081)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N7O/c1-12(2)26-19(20-11-23-26)16-10-25-5-6-27-17-7-13(14-8-21-22-9-14)3-4-15(17)18(25)24-16/h3-4,7-12H,5-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434809

(CHEMBL2386971)Show SMILES C[C@H](O)C(=O)N1CC(C1)n1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1nc(C)nn1C(C)C |r| Show InChI InChI=1S/C26H30N8O3/c1-15(2)34-25(28-17(4)30-34)22-14-31-7-8-37-23-9-18(5-6-21(23)24(31)29-22)19-10-27-33(11-19)20-12-32(13-20)26(36)16(3)35/h5-6,9-11,14-16,20,35H,7-8,12-13H2,1-4H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM14714
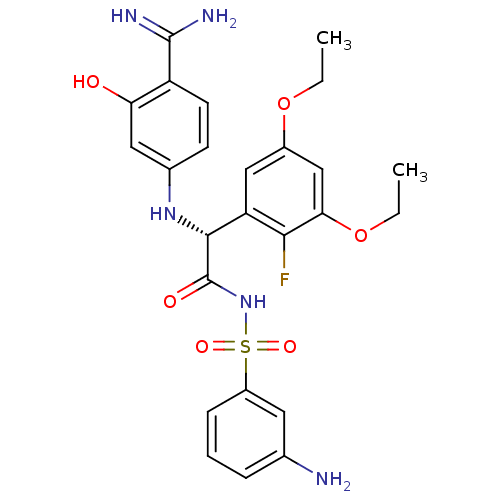
((2R)-N-[(3-aminobenzene)sulfonyl]-2-[(4-carbamimid...)Show SMILES CCOc1cc(OCC)c(F)c(c1)[C@@H](Nc1ccc(C(N)=N)c(O)c1)C(=O)NS(=O)(=O)c1cccc(N)c1 |r| Show InChI InChI=1S/C25H28FN5O6S/c1-3-36-16-12-19(22(26)21(13-16)37-4-2)23(30-15-8-9-18(24(28)29)20(32)11-15)25(33)31-38(34,35)17-7-5-6-14(27)10-17/h5-13,23,30,32H,3-4,27H2,1-2H3,(H3,28,29)(H,31,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
| MMDB
PDB
Article
PubMed
| 0.350 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.8 | 22 |
Genentech
| Assay Description
The enzyme reactions were initiated by the addition of substrate, and the color developed from the release of p-nitroanilide from each chromogenic su... |
J Biol Chem 280: 9160-9 (2005)
Article DOI: 10.1074/jbc.M409068200
BindingDB Entry DOI: 10.7270/Q2JM27W3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434813

(CHEMBL2387083)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C22H25N7O2/c1-14(2)29-22(24-15(3)26-29)19-13-27-7-9-31-20-10-16(4-5-18(20)21(27)25-19)17-11-23-28(12-17)6-8-30/h4-5,10-14,30H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363990
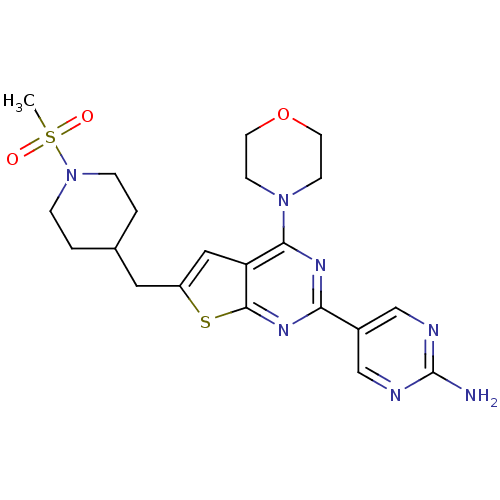
(CHEMBL1949915)Show SMILES CS(=O)(=O)N1CCC(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)28-4-2-14(3-5-28)10-16-11-17-19(27-6-8-31-9-7-27)25-18(26-20(17)32-16)15-12-23-21(22)24-13-15/h11-14H,2-10H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086680
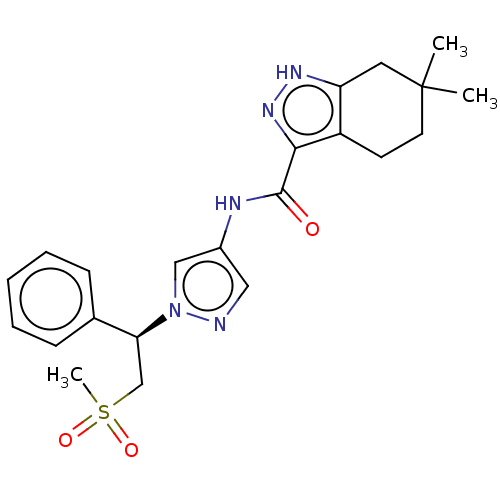
(CHEMBL3426306)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](CS(C)(=O)=O)c1ccccc1 |r| Show InChI InChI=1S/C22H27N5O3S/c1-22(2)10-9-17-18(11-22)25-26-20(17)21(28)24-16-12-23-27(13-16)19(14-31(3,29)30)15-7-5-4-6-8-15/h4-8,12-13,19H,9-11,14H2,1-3H3,(H,24,28)(H,25,26)/t19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347092
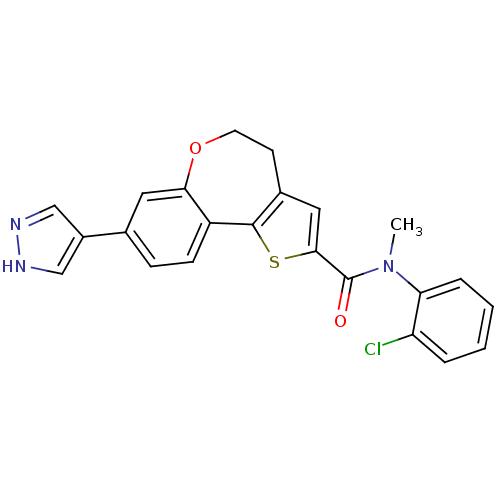
(CHEMBL1796757)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)-c1cn[nH]c1)c1ccccc1Cl Show InChI InChI=1S/C23H18ClN3O2S/c1-27(19-5-3-2-4-18(19)24)23(28)21-11-15-8-9-29-20-10-14(16-12-25-26-13-16)6-7-17(20)22(15)30-21/h2-7,10-13H,8-9H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086669
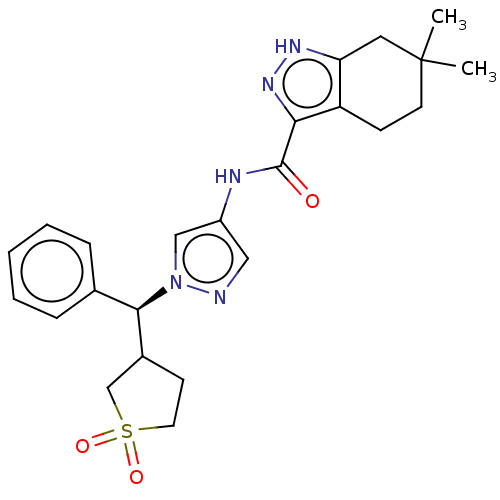
(CHEMBL3426301)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCS(=O)(=O)C1)c1ccccc1 |r| Show InChI InChI=1S/C24H29N5O3S/c1-24(2)10-8-19-20(12-24)27-28-21(19)23(30)26-18-13-25-29(14-18)22(16-6-4-3-5-7-16)17-9-11-33(31,32)15-17/h3-7,13-14,17,22H,8-12,15H2,1-2H3,(H,26,30)(H,27,28)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086669

(CHEMBL3426301)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCS(=O)(=O)C1)c1ccccc1 |r| Show InChI InChI=1S/C24H29N5O3S/c1-24(2)10-8-19-20(12-24)27-28-21(19)23(30)26-18-13-25-29(14-18)22(16-6-4-3-5-7-16)17-9-11-33(31,32)15-17/h3-7,13-14,17,22H,8-12,15H2,1-2H3,(H,26,30)(H,27,28)/t17?,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419761

(CHEMBL1949919)Show SMILES CS(=O)(=O)c1cccc(c1)-c1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C21H20N6O3S2/c1-32(28,29)15-4-2-3-13(9-15)17-10-16-19(27-5-7-30-8-6-27)25-18(26-20(16)31-17)14-11-23-21(22)24-12-14/h2-4,9-12H,5-8H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419769
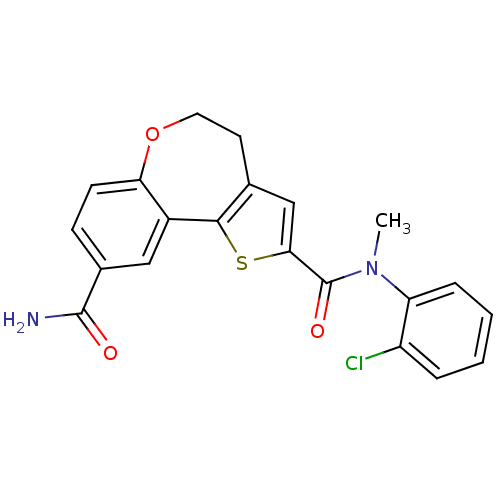
(CHEMBL1950034)Show SMILES CN(C(=O)c1cc2CCOc3ccc(cc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-7-6-13(20(23)25)10-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434811
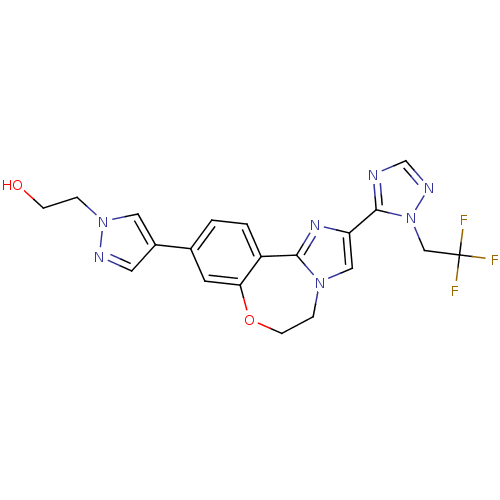
(CHEMBL2387087)Show SMILES OCCn1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1CC(F)(F)F Show InChI InChI=1S/C20H18F3N7O2/c21-20(22,23)11-30-19(24-12-26-30)16-10-28-4-6-32-17-7-13(1-2-15(17)18(28)27-16)14-8-25-29(9-14)3-5-31/h1-2,7-10,12,31H,3-6,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419759
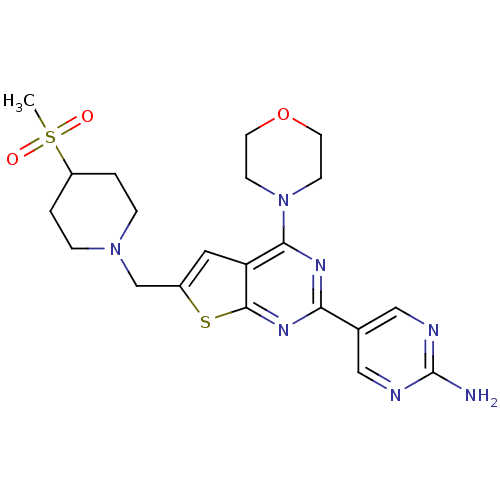
(CHEMBL1949916)Show SMILES CS(=O)(=O)C1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C21H27N7O3S2/c1-33(29,30)16-2-4-27(5-3-16)13-15-10-17-19(28-6-8-31-9-7-28)25-18(26-20(17)32-15)14-11-23-21(22)24-12-14/h10-12,16H,2-9,13H2,1H3,(H2,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347090
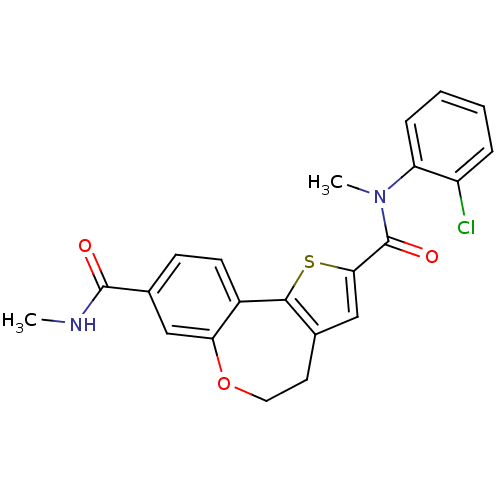
(CHEMBL1796276)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-24-21(26)14-7-8-15-18(11-14)28-10-9-13-12-19(29-20(13)15)22(27)25(2)17-6-4-3-5-16(17)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419770
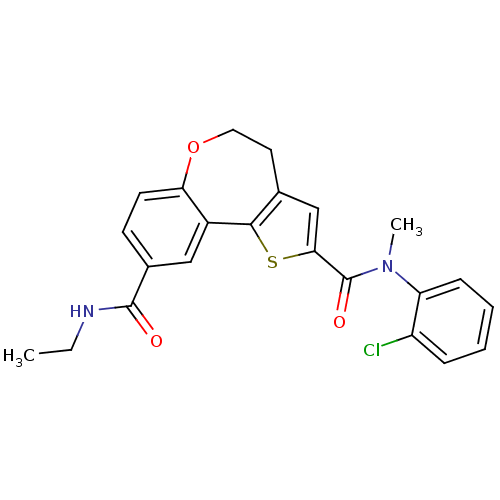
(CHEMBL1950035)Show SMILES CCNC(=O)c1ccc2OCCc3cc(sc3-c2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C23H21ClN2O3S/c1-3-25-22(27)15-8-9-19-16(12-15)21-14(10-11-29-19)13-20(30-21)23(28)26(2)18-7-5-4-6-17(18)24/h4-9,12-13H,3,10-11H2,1-2H3,(H,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419754
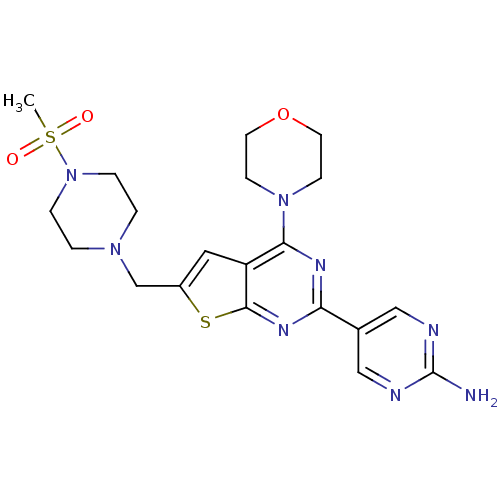
(CHEMBL1949910)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C20H26N8O3S2/c1-33(29,30)28-4-2-26(3-5-28)13-15-10-16-18(27-6-8-31-9-7-27)24-17(25-19(16)32-15)14-11-22-20(21)23-12-14/h10-12H,2-9,13H2,1H3,(H2,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087
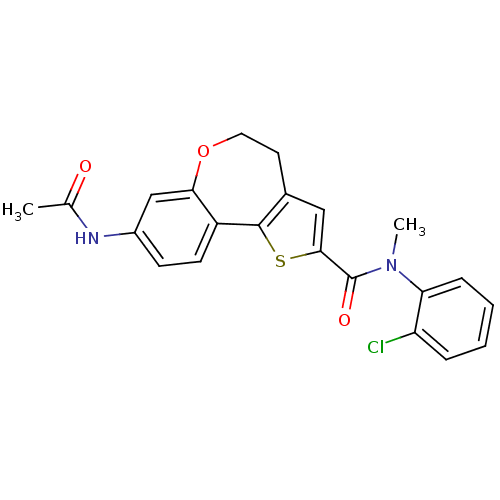
(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347089
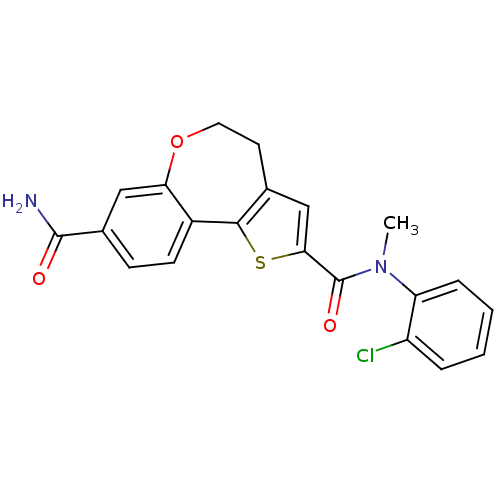
(CHEMBL1796275)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C(N)=O)c1ccccc1Cl Show InChI InChI=1S/C21H17ClN2O3S/c1-24(16-5-3-2-4-15(16)22)21(26)18-11-12-8-9-27-17-10-13(20(23)25)6-7-14(17)19(12)28-18/h2-7,10-11H,8-9H2,1H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347088
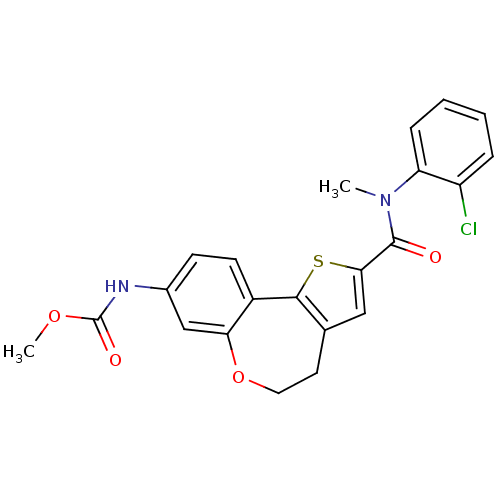
(CHEMBL1796274)Show SMILES COC(=O)Nc1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O4S/c1-25(17-6-4-3-5-16(17)23)21(26)19-11-13-9-10-29-18-12-14(24-22(27)28-2)7-8-15(18)20(13)30-19/h3-8,11-12H,9-10H2,1-2H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347087

(CHEMBL1796273)Show SMILES CN(C(=O)c1cc2CCOc3cc(NC(C)=O)ccc3-c2s1)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-13(26)24-15-7-8-16-19(12-15)28-10-9-14-11-20(29-21(14)16)22(27)25(2)18-6-4-3-5-17(18)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347076

(CHEMBL1796761)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccc(cc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-8-6-16(11-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419758

(CHEMBL1949914)Show SMILES Nc1ncc(cn1)-c1nc(N2CCOCC2)c2cc(CC3CCN(CC(F)(F)F)CC3)sc2n1 Show InChI InChI=1S/C22H26F3N7OS/c23-22(24,25)13-31-3-1-14(2-4-31)9-16-10-17-19(32-5-7-33-8-6-32)29-18(30-20(17)34-16)15-11-27-21(26)28-12-15/h10-12,14H,1-9,13H2,(H2,26,27,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419773
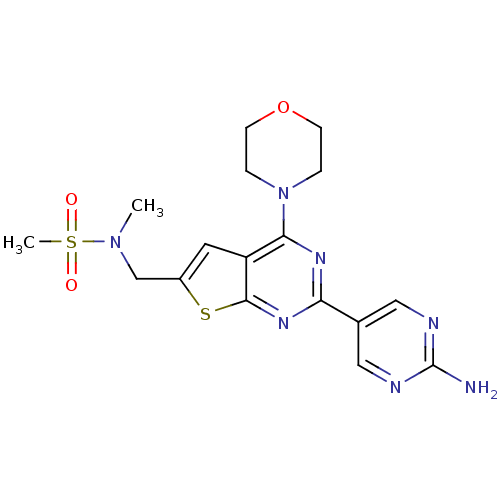
(CHEMBL1949917)Show SMILES CN(Cc1cc2c(nc(nc2s1)-c1cnc(N)nc1)N1CCOCC1)S(C)(=O)=O Show InChI InChI=1S/C17H21N7O3S2/c1-23(29(2,25)26)10-12-7-13-15(24-3-5-27-6-4-24)21-14(22-16(13)28-12)11-8-19-17(18)20-9-11/h7-9H,3-6,10H2,1-2H3,(H2,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419760
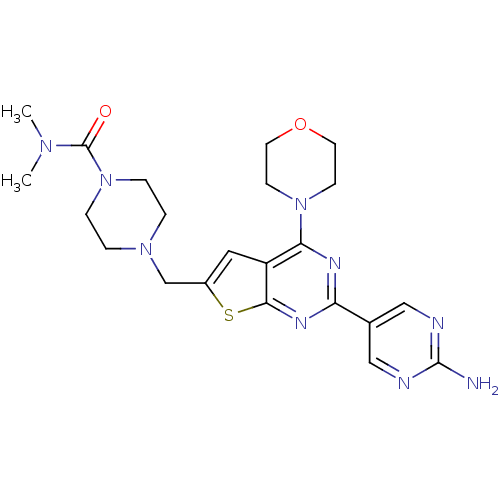
(CHEMBL1949918)Show SMILES CN(C)C(=O)N1CCN(Cc2cc3c(nc(nc3s2)-c2cnc(N)nc2)N2CCOCC2)CC1 Show InChI InChI=1S/C22H29N9O2S/c1-28(2)22(32)31-5-3-29(4-6-31)14-16-11-17-19(30-7-9-33-10-8-30)26-18(27-20(17)34-16)15-12-24-21(23)25-13-15/h11-13H,3-10,14H2,1-2H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347095

(CHEMBL1796760)Show SMILES CNC(=O)c1ccc(N(C)C(=O)c2cc3CCOc4cc(ccc4-c3s2)C(=O)NC)c(Cl)c1 Show InChI InChI=1S/C24H22ClN3O4S/c1-26-22(29)14-5-7-18(17(25)10-14)28(3)24(31)20-12-13-8-9-32-19-11-15(23(30)27-2)4-6-16(19)21(13)33-20/h4-7,10-12H,8-9H2,1-3H3,(H,26,29)(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50347090

(CHEMBL1796276)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1ccccc1Cl Show InChI InChI=1S/C22H19ClN2O3S/c1-24-21(26)14-7-8-15-18(11-14)28-10-9-13-12-19(29-20(13)15)22(27)25(2)17-6-4-3-5-16(17)23/h3-8,11-12H,9-10H2,1-2H3,(H,24,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant PI3Kalpha assessed as inhibition of PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 21: 4054-8 (2011)
Article DOI: 10.1016/j.bmcl.2011.04.124
BindingDB Entry DOI: 10.7270/Q29G5N57 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086667
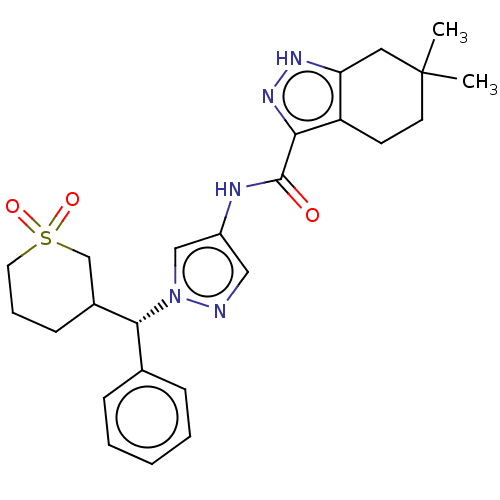
(CHEMBL3426302)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCCS(=O)(=O)C1)c1ccccc1 |r| Show InChI InChI=1S/C25H31N5O3S/c1-25(2)11-10-20-21(13-25)28-29-22(20)24(31)27-19-14-26-30(15-19)23(17-7-4-3-5-8-17)18-9-6-12-34(32,33)16-18/h3-5,7-8,14-15,18,23H,6,9-13,16H2,1-2H3,(H,27,31)(H,28,29)/t18?,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM50086667

(CHEMBL3426302)Show SMILES CC1(C)CCc2c(C1)[nH]nc2C(=O)Nc1cnn(c1)[C@@H](C1CCCS(=O)(=O)C1)c1ccccc1 |r| Show InChI InChI=1S/C25H31N5O3S/c1-25(2)11-10-20-21(13-25)28-29-22(20)24(31)27-19-14-26-30(15-19)23(17-7-4-3-5-8-17)18-9-6-12-34(32,33)16-18/h3-5,7-8,14-15,18,23H,6,9-13,16H2,1-2H3,(H,27,31)(H,28,29)/t18?,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Inc.
Curated by ChEMBL
| Assay Description
Inhibition of GST-tagged recombinant full length human ITK using AcEFPIYDFLPAKKK-NH2 as substrate after 35 mins by Morrison plot analysis |
J Med Chem 58: 3806-16 (2015)
Article DOI: 10.1021/jm501998m
BindingDB Entry DOI: 10.7270/Q28K7BTH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434815
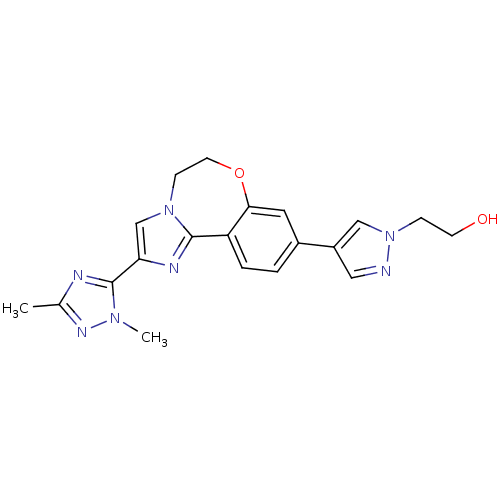
(CHEMBL2387085)Show SMILES Cc1nc(-c2cn3CCOc4cc(ccc4-c3n2)-c2cnn(CCO)c2)n(C)n1 Show InChI InChI=1S/C20H21N7O2/c1-13-22-20(25(2)24-13)17-12-26-6-8-29-18-9-14(3-4-16(18)19(26)23-17)15-10-21-27(11-15)5-7-28/h3-4,9-12,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50358203
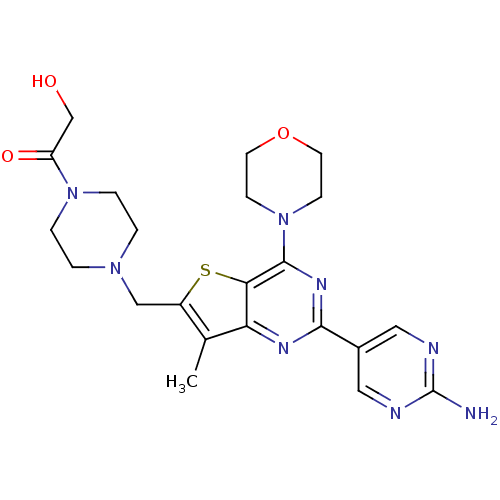
(CHEMBL1922093)Show SMILES Cc1c(CN2CCN(CC2)C(=O)CO)sc2c(nc(nc12)-c1cnc(N)nc1)N1CCOCC1 Show InChI InChI=1S/C22H28N8O3S/c1-14-16(12-28-2-4-29(5-3-28)17(32)13-31)34-19-18(14)26-20(15-10-24-22(23)25-11-15)27-21(19)30-6-8-33-9-7-30/h10-11,31H,2-9,12-13H2,1H3,(H2,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K subunit p110alpha assessed as formation of phosphatidylinositide-3-phosphate product formation after 30 mins by fluorescence polar... |
J Med Chem 54: 7579-87 (2011)
Article DOI: 10.1021/jm2009327
BindingDB Entry DOI: 10.7270/Q2SF2WMT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419771
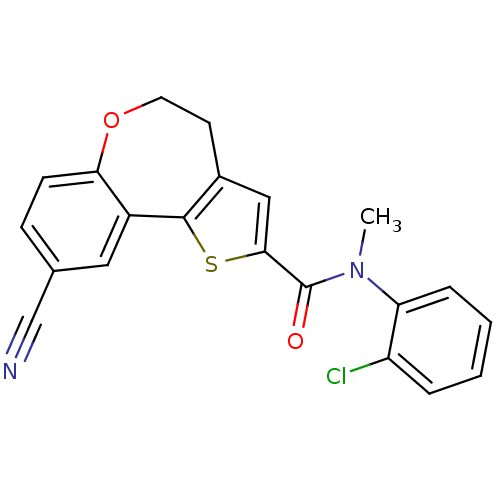
(CHEMBL1950036)Show SMILES CN(C(=O)c1cc2CCOc3ccc(cc3-c2s1)C#N)c1ccccc1Cl Show InChI InChI=1S/C21H15ClN2O2S/c1-24(17-5-3-2-4-16(17)22)21(25)19-11-14-8-9-26-18-7-6-13(12-23)10-15(18)20(14)27-19/h2-7,10-11H,8-9H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363991
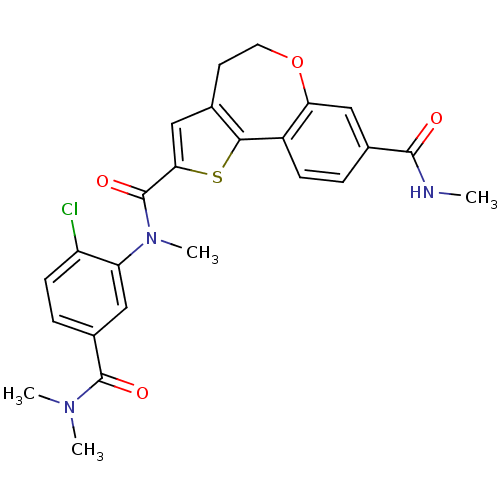
(CHEMBL1796763)Show SMILES CNC(=O)c1ccc2-c3sc(cc3CCOc2c1)C(=O)N(C)c1cc(ccc1Cl)C(=O)N(C)C Show InChI InChI=1S/C25H24ClN3O4S/c1-27-23(30)15-5-7-17-20(12-15)33-10-9-14-13-21(34-22(14)17)25(32)29(4)19-11-16(6-8-18(19)26)24(31)28(2)3/h5-8,11-13H,9-10H2,1-4H3,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419762

(CHEMBL1949920)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)-c1cc[nH]n1)c1ccccc1Cl Show InChI InChI=1S/C23H18ClN3O2S/c1-27(19-5-3-2-4-17(19)24)23(28)21-13-15-9-11-29-20-12-14(18-8-10-25-26-18)6-7-16(20)22(15)30-21/h2-8,10,12-13H,9,11H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50419766
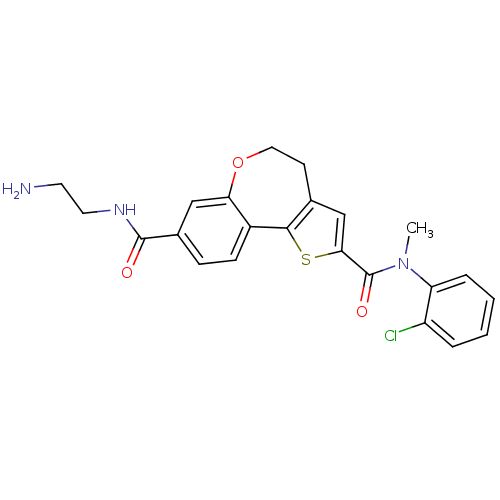
(CHEMBL1949924)Show SMILES CN(C(=O)c1cc2CCOc3cc(ccc3-c2s1)C(=O)NCCN)c1ccccc1Cl Show InChI InChI=1S/C23H22ClN3O3S/c1-27(18-5-3-2-4-17(18)24)23(29)20-13-14-8-11-30-19-12-15(22(28)26-10-9-25)6-7-16(19)21(14)31-20/h2-7,12-13H,8-11,25H2,1H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Apparent binding affinity to PI3Kalpha using PIP3 as substrate after 30 mins by fluorescence polarization assay |
J Med Chem 54: 7815-33 (2011)
Article DOI: 10.1021/jm2007084
BindingDB Entry DOI: 10.7270/Q2057GCQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data